Co(II)-Chelated Polyimines as Oxygen Reduction Reaction Catalysts in Anion Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
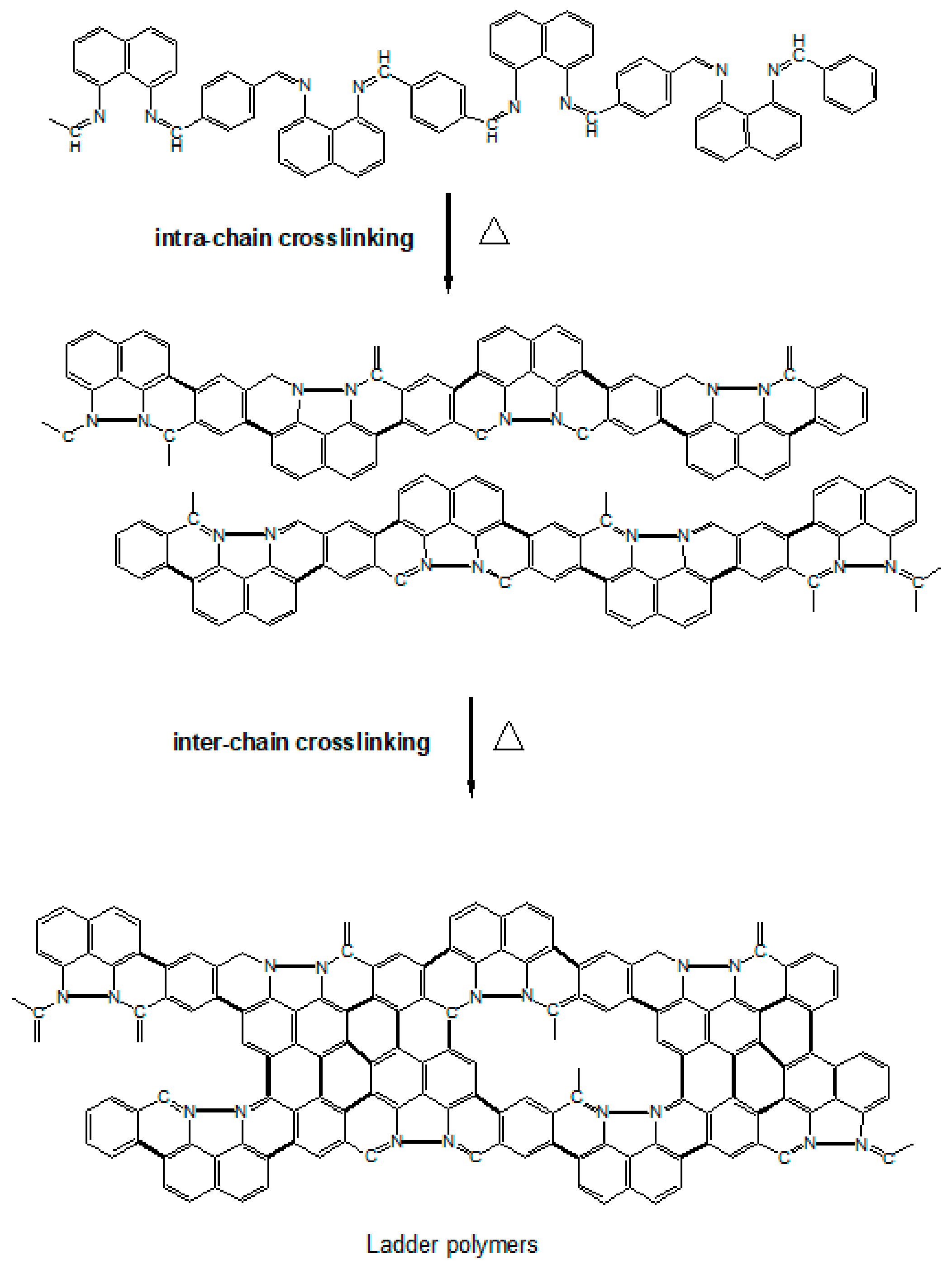
2.2. Preparation of Polynaphthalene Imine (PNIM)
2.3. Characterization
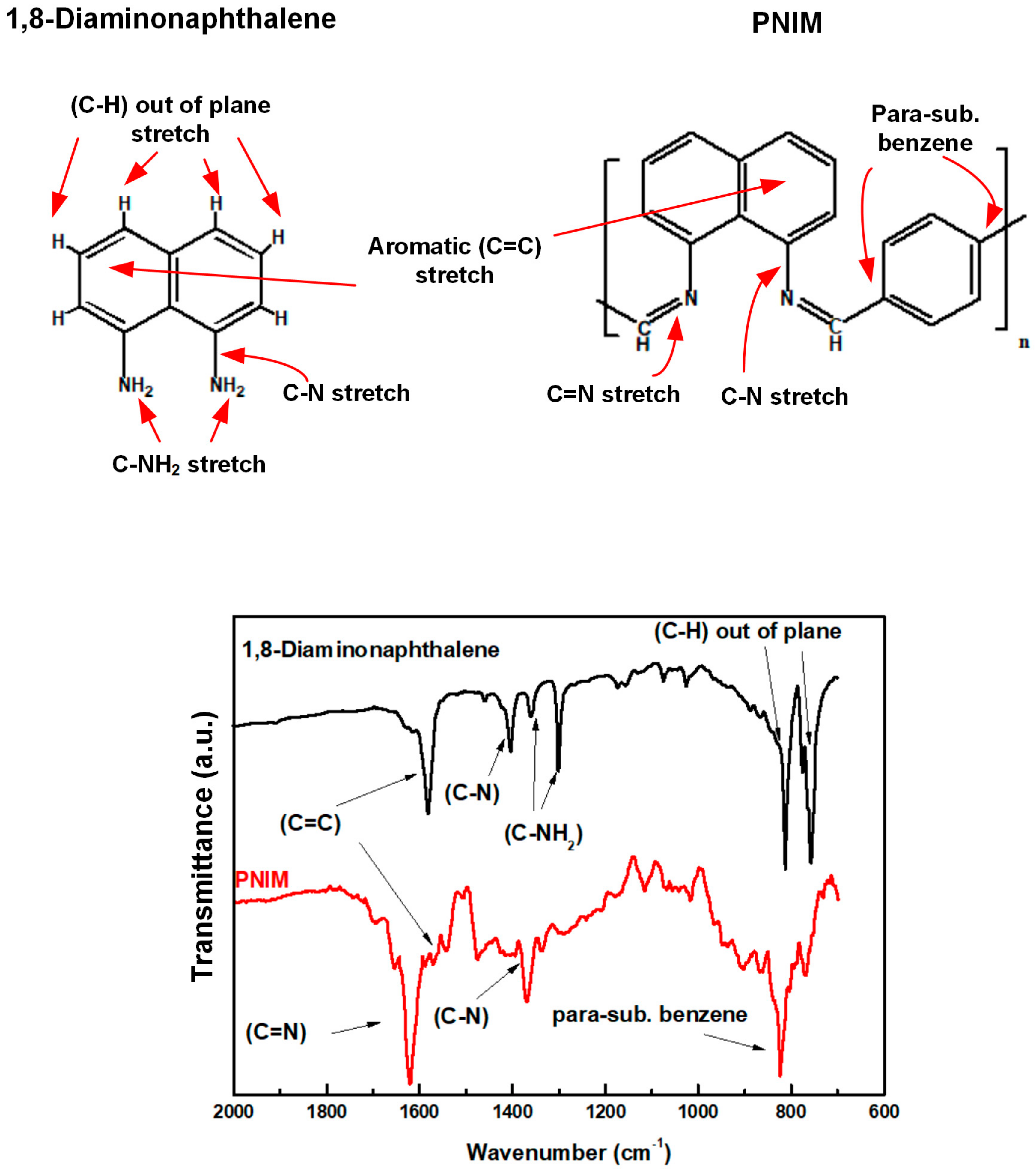
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. X-ray Diffraction Spectroscopy (XRD)
2.3.3. Raman Spectroscopy
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. BET Measurement
2.3.6. Current–Voltage Curve (C–V) and Linear Scan Voltammetry (LSV)
2.3.7. Membrane Electrode Assembly (MEA)
2.3.8. Single-Cell Test
3. Results and Discussion
3.1. FTIR Spectroscopy
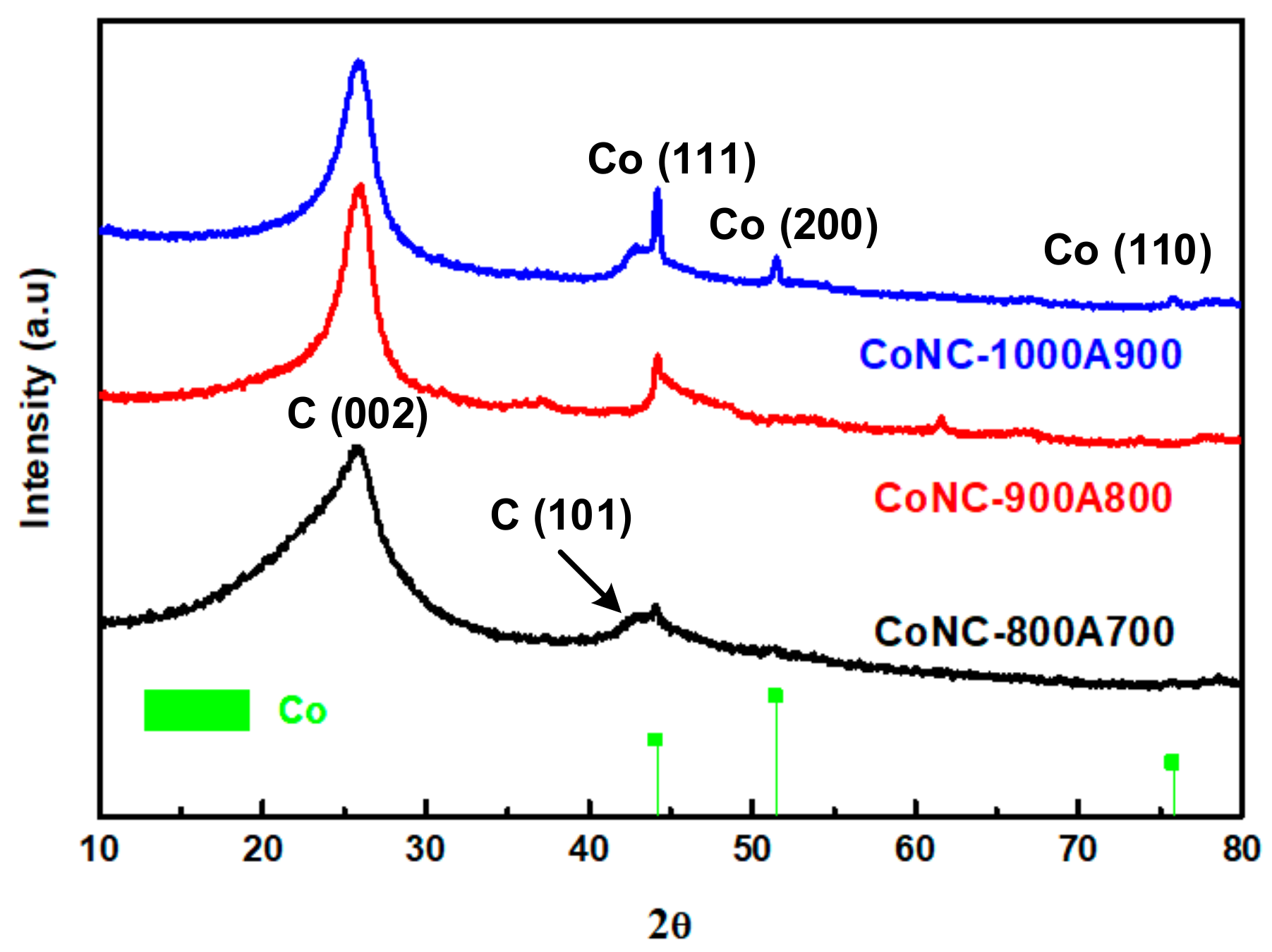
3.2. XRD Pattern
3.3. Raman Spectroscopy
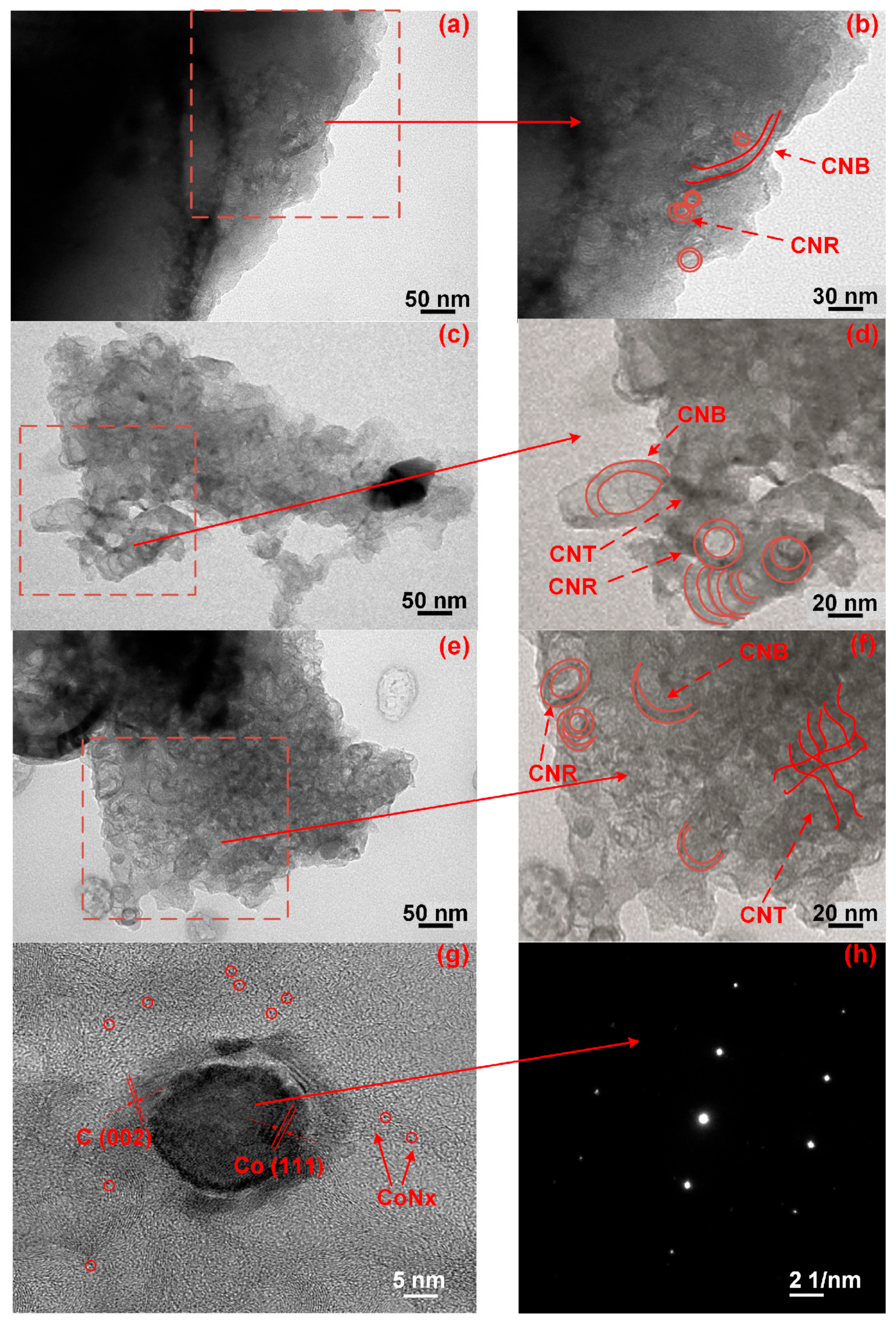
3.4. TEM
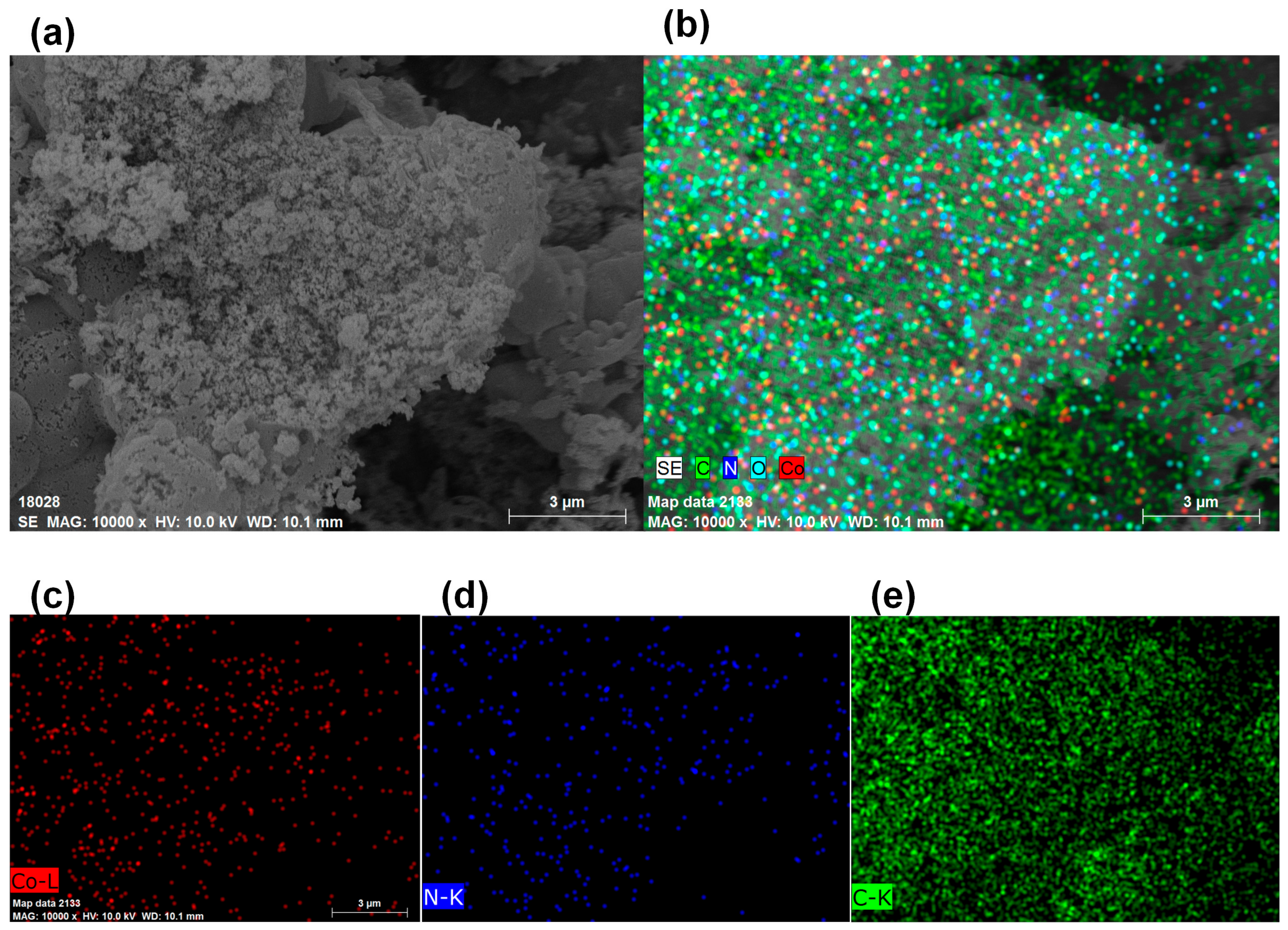
3.5. ED Mappings
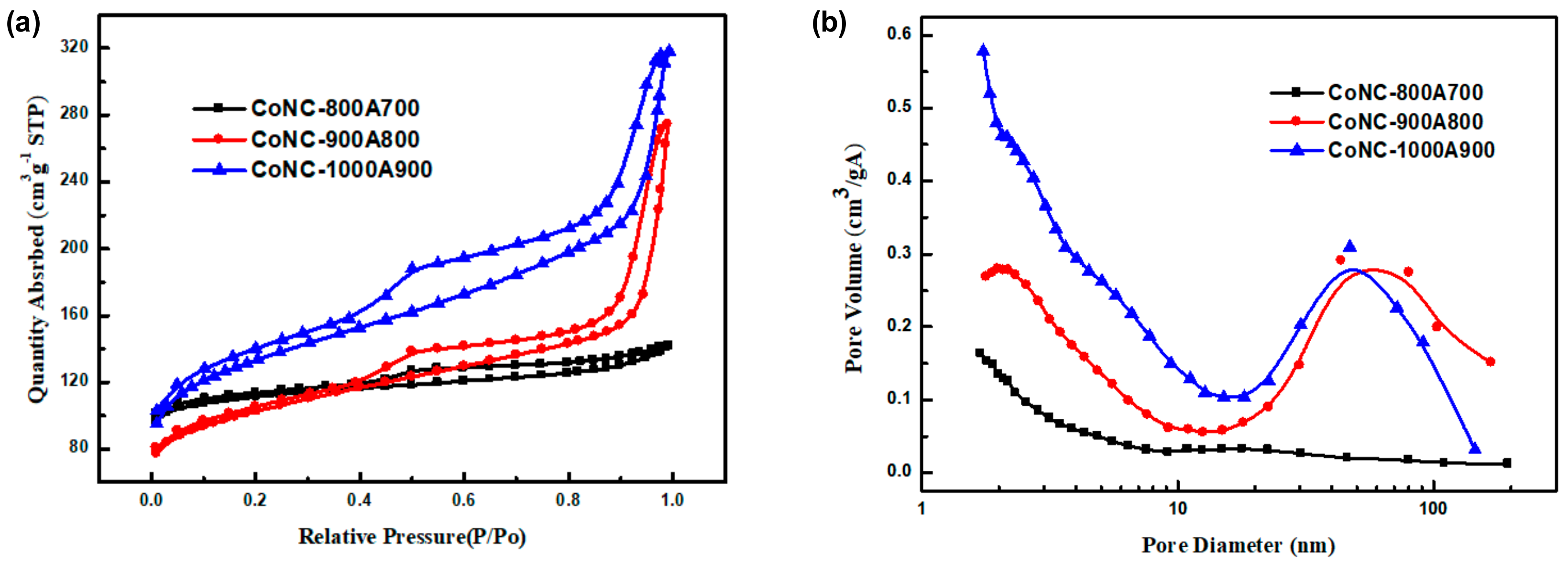
3.6. BET Surface Area and Pore Size
3.7. Electrochemical Testing
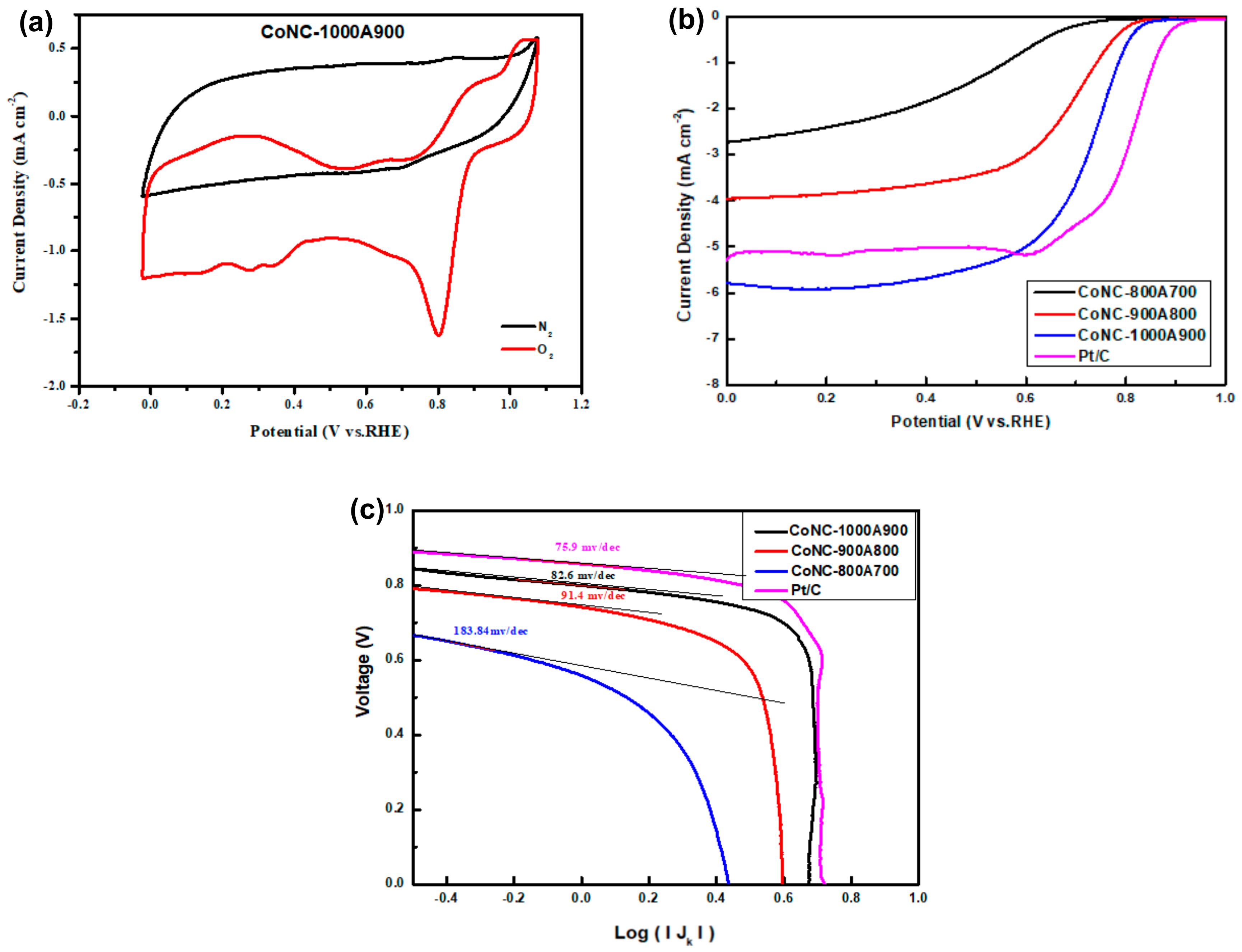
3.7.1. C–V and LSV Curves
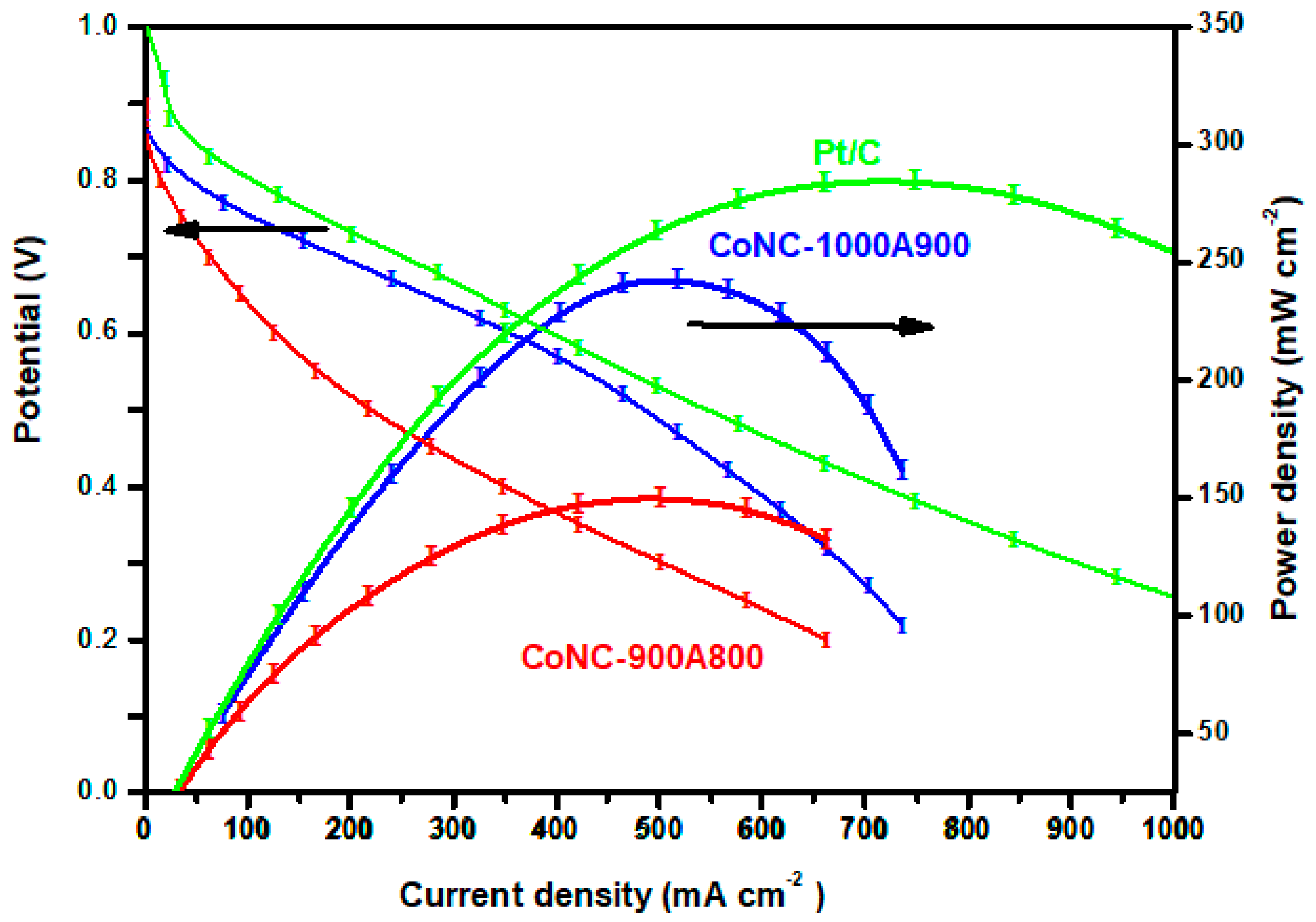
3.7.2. Polarization Curve and Power Density Curve Measurements
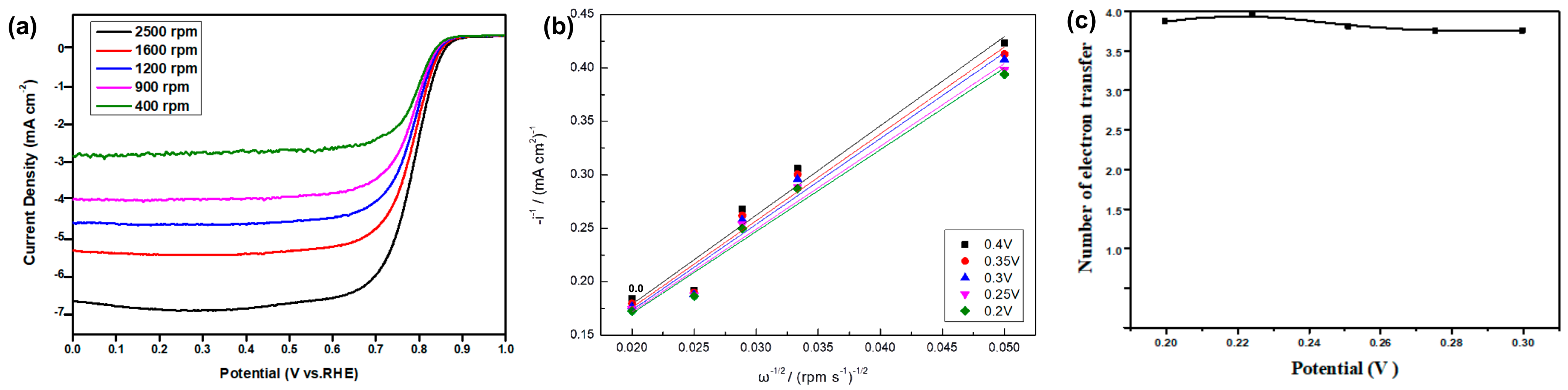
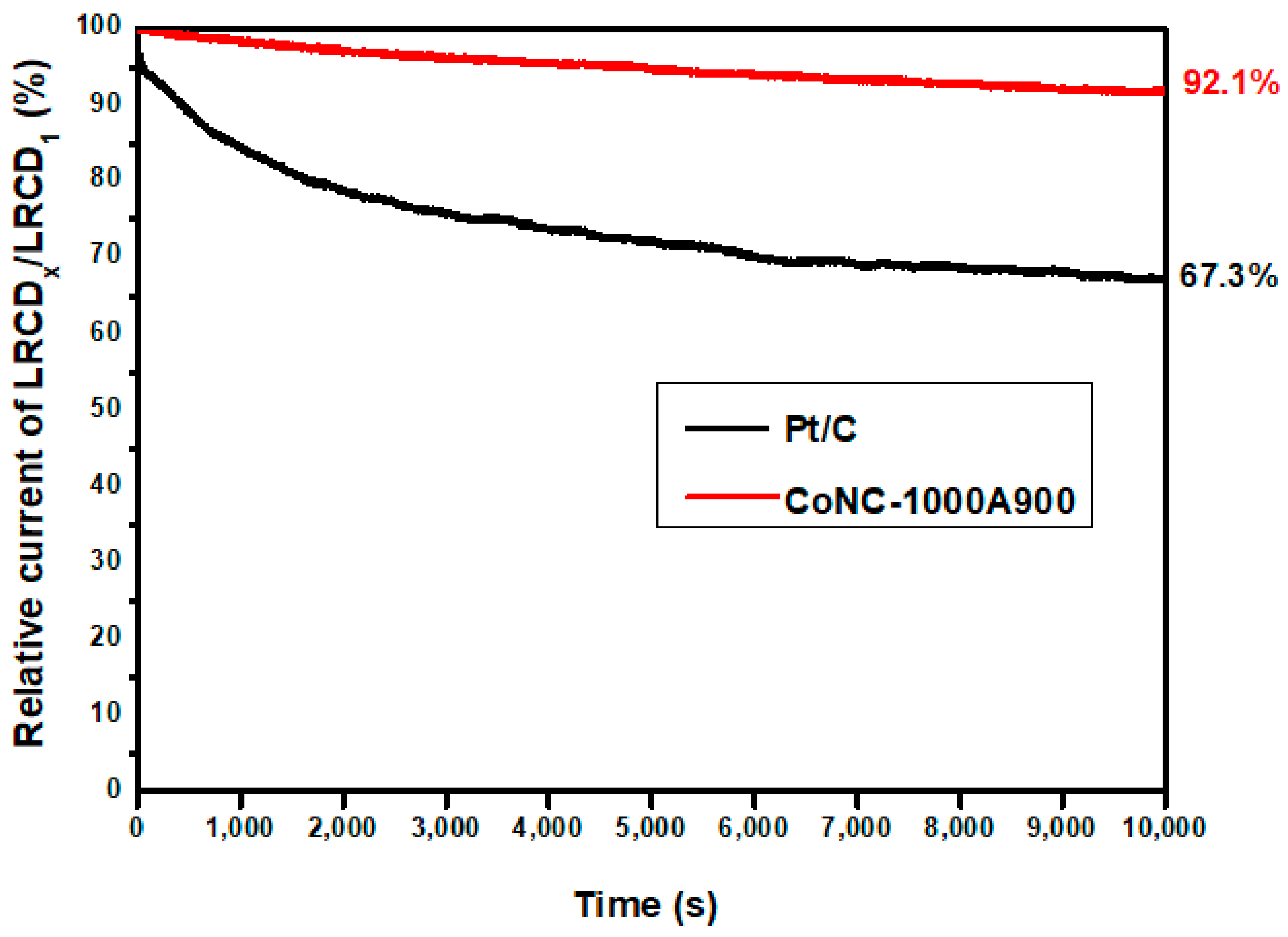
3.7.3. Electrochemical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janošević, A.; Pašti, I.A.; Gavrilov, N.; Mentus, S.; Krstić, J.; Mitrić, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G. Microporous conducting carbonized polyaniline nanorods: Synthesis, characterization and electrocatalytic properties. Microporous Mesoporous Mater. 2012, 152, 50–57. [Google Scholar] [CrossRef]
- Michel, M.; Ettingshausen, F.; Scheiba, F.; Wolz, A.; Roth, C. Using layer-by-layer assembly of polyaniline fibers in the fast preparation of high performance fuel cell nanostructured membrane electrodes. Phys. Chem. Chem. Phys. 2008, 10, 3796–3801. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Chang, M.-Y.; Wang, Y.-Z.; Huang, Y.-C.; Ho, K.-S.; Hsieh, T.-H.; Kuo, Y.-C. Polyaniline Based Pt-Electrocatalyst for a Proton Exchanged Membrane Fuel Cell. Polymers 2020, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Ko, T.-H.; Huang, W.-Y.; Hsieh, T.-H.; Ho, K.-S.; Chen, Y.-Y.; Hsieh, S.-J. Preparation of Pt-Catalyst by Poly(p-phenylenediamine) Nanocomposites Assisted by Microwave Radiation for Proton Exchange Membrane Fuel Cell. Polymers 2018, 10, 1388. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Hsieh, T.-H.; Wang, Y.-Z.; Ho, K.-S.; Chang, C.-Y. Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, L.M.; Sievers, G.; Brüser, V.; Dyck, A.; Wittstock, G. Characterization of different plasma-treated cobalt oxide catalysts for oxygen reduction reaction in alkaline media. Sci. Bull. 2016, 61, 612–618. [Google Scholar]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar]
- Yang, Y.; Zeng, R.; Xiong, Y.; DiSalvo, F.J.; Abruña, H.D. Cobalt-Based Nitride-Core Oxide-Shell Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2019, 141, 19241–19245. [Google Scholar]
- Zhang, G.; Huang, C.; Wang, X. Dispersing Molecular Cobalt in Graphitic Carbon Nitride Frameworks for Photocatalytic Water Oxidation. Small 2015, 11, 1215–1221. [Google Scholar]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar]
- Venegas, R.; Recio, F.J.; Zuñiga, C.; Viera, M.; Oyarzún, M.-P.; Silva, N.; Neira, K.; Marco, J.F.; Zagal, J.H.; Tasca, F. Comparison of the catalytic activity for O2 reduction of Fe and Co MN4 adsorbed on graphite electrodes and on carbon nanotubes. Phys. Chem. Chem. Phys. 2017, 19, 20441–20450. [Google Scholar]
- Bai, L.; Hsu, C.-S.; Alexander, D.T.L.; Chen, H.M.; Hu, X. A Cobalt–Iron Double-Atom Catalyst for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 14190–14199. [Google Scholar] [PubMed]
- Gong, L.; Zhang, H.; Wang, Y.; Luo, E.; Li, K.; Gao, L.; Wang, Y.; Wu, Z.; Jin, Z.; Ge, J.; et al. Bridge Bonded Oxygen Ligands between Approximated FeN(4) Sites Confer Catalysts with High ORR Performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 13923–13928. [Google Scholar]
- Liang, X.; Li, Z.; Xiao, H.; Zhang, T.; Xu, P.; Zhang, H.; Gao, Q.; Zheng, L. Two Types of Single-Atom FeN4 and FeN5 Electrocatalytic Active Centers on N-Doped Carbon Driving High Performance of the SA-Fe-NC Oxygen Reduction Reaction Catalyst. Chem. Mater. 2021, 33, 5542–5554. [Google Scholar]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar]
- Gonen, S.; Lori, O.; Cohen-Taguri, G.; Elbaz, L. Metal organic frameworks as a catalyst for oxygen reduction: An unexpected outcome of a highly active Mn-MOF-based catalyst incorporated in activated carbon. Nanoscale 2018, 10, 9634–9641. [Google Scholar] [PubMed]
- Chen, X.; Wang, N.; Shen, K.; Xie, Y.; Tan, Y.; Li, Y. MOF-Derived Isolated Fe Atoms Implanted in N-Doped 3D Hierarchical Carbon as an Efficient ORR Electrocatalyst in Both Alkaline and Acidic Media. ACS Appl. Mater. Interfaces 2019, 11, 25976–25985. [Google Scholar]
- Anwar, R.; Iqbal, N.; Hanif, S.; Noor, T.; Shi, X.; Zaman, N.; Haider, D.; Rizvi, S.A.M.; Kannan, A.M. MOF-Derived CuPt/NC Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2020, 10, 799. [Google Scholar]
- Zhang, J. Preparation and Properties of MOF-derived Porous Carbon Nanosheets as Electrocatalyst for Oxygen Reduction Reaction. Int. J. Electrochem. Sci. 2022, 17, 220922. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Panneerselvam, I.R.; Ramakrishnan, S.; Kumar, R.S.; Kim, A.R.; Wang, Y.; Yoo, D.J. Quasihexagonal Platinum Nanodendrites Decorated over CoS2-N-Doped Reduced Graphene Oxide for Electro-Oxidation of C1-, C2-, and C3-Type Alcohols. Adv. Sci. 2022, 9, 2105344. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Hsieh, T.H.; Huang, Y.C.; Tseng, P.H.; Wang, Y.Z.; Ho, K.S.; Huang, Y.J. Calcined Co(II)-Chelated Polyazomethine as Cathode Catalyst of Anion Exchange Membrane Fuel Cells. Polymers 2022, 14, 1784. [Google Scholar]
- Hsieh, T.H.; Wang, Y.Z.; Ho, K.S. Cobalt-Based Cathode Catalysts for Oxygen-Reduction Reaction in an Anion Exchange Membrane Fuel Cell. Membranes 2022, 12, 699. [Google Scholar]
- Nasalska, A.; Skompska, M. Removal of toxic chromate ions by the films of poly(1,8-diaminonaphthalene). J. Appl. Electrochem. 2003, 33, 113–119. [Google Scholar]
- Hernadi, K.; Fonseca, A.; Nagy, J.B.; Bernaerts, D.; Lucas, A.A. Fe-catalyzed carbon nanotube formation. Carbon 1996, 34, 1249–1257. [Google Scholar]
- Sano, M.; Kamino, A.; Okamura, J.; Shinkai, S. Ring closure of carbon nanotubes. Science 2001, 293, 1299–1301. [Google Scholar] [PubMed]
- Huh, Y.; Green, M.L.H.; Kim, Y.H.; Lee, J.Y.; Lee, C.J. Control of carbon nanotube growth using cobalt nanoparticles as catalyst. Appl. Surf. Sci. 2005, 249, 145–150. [Google Scholar]
- Lin, M.; Tan, J.P.Y.; Boothroyd, C.; Loh, K.P.; Tok, E.S.; Foo, Y.-L. Dynamical Observation of Bamboo-like Carbon Nanotube Growth. Nano Lett. 2007, 7, 2234–2238. [Google Scholar]
- Chen, Z.; Higgins, D.; Tao, H.; Hsu, R.S.; Chen, Z. Highly Active Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction in Fuel Cell Applications. J. Phys. Chem. C 2009, 113, 21008–21013. [Google Scholar]
- Fan, W.; Li, Z.; You, C.; Zong, X.; Tian, X.; Miao, S.; Shu, T.; Li, C.; Liao, S. Binary Fe, Cu-doped bamboo-like carbon nanotubes as efficient catalyst for the oxygen reduction reaction. Nano Energy 2017, 37, 187–194. [Google Scholar]
- Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Synthesis of a carbon nanobelt. Science 2017, 356, 172. [Google Scholar]
- Chen, H.; Gui, S.; Zhang, Y.; Liu, Z.; Miao, Q. Synthesis of a Hydrogenated Zigzag Carbon Nanobelt. CCS Chem. 2021, 3, 613–619. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Chen, Y.; Liu, C.; Li, Q. Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials. Metals 2023, 13, 703. [Google Scholar] [CrossRef]
- Kruusenberg, I.; Ramani, D.; Ratso, S.; Joost, U.; Saar, R.; Rauwel, P.; Kannan, A.M.; Tammeveski, K. Cobalt–Nitrogen Co-doped Carbon Nanotube Cathode Catalyst for Alkaline Membrane Fuel Cells. ChemElectroChem 2016, 3, 1455–1465. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Bhange, S.N.; Sivasankaran, V.P.; Kurungot, S. Single Cell Fabrication Towards the Realistic Evaluation of a CNT-Strung ZIF-Derived Electrocatalyst as a Cathode Material in Alkaline Fuel Cells and Metal-Air Batteries. ChemElectroChem 2017, 4, 2928–2933. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Käärik, M.; Kook, M.; Puust, L.; Saar, R.; Leis, J.; Tammeveski, K. Highly efficient transition metal and nitrogen co-doped carbide-derived carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 2018, 375, 233–243. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.; Pei, Y.; Liu, Y.; Qin, Y.; Zhang, X.; Wang, Q.; Yin, Y.; Guiver, M.D. Hierarchically Porous Co-N-C Cathode Catalyst Layers for Anion Exchange Membrane Fuel Cells. ChemSusChem 2019, 12, 4165–4169. [Google Scholar] [CrossRef]

| CoNC- | ID/IG | ρ (Ω cm) |
|---|---|---|
| 800A700 | 1.072 | 221.6 |
| 900A800 | 0.941 | 74.7 |
| 1000A900 | 0.907 | 29.7 |
| 1000 | 0.866 | 9.3 |
| CoNC- | BET Surface Area (m2 g−1) | Pore Diameter (nm) |
|---|---|---|
| 800A700 | 342.07 | 4.09 |
| 900A800 | 335.13 | 7.4 |
| 1000A900 | 437.80 | 5.3 |
| Catalyst | Half-Wave Potential (V) a | LRCD (mA cm−2) a | Tafel Slope (mV dec−1) | Eonset (V) a |
|---|---|---|---|---|
| CoNC-800A700 | 0.62 | −2.72 | 183.8 | - |
| CoNC-900A800 | 0.72 | −3.92 | 91.4 | 0.870 |
| CoNC-1000A900 | 0.75 | −5.80 | 82.6 | 0.897 |
| Pt/C | 0.81 | −5.26 | 75.9 | 1.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Wang, Y.-Z.; Hsieh, T.-H.; Ho, K.-S. Co(II)-Chelated Polyimines as Oxygen Reduction Reaction Catalysts in Anion Exchange Membrane Fuel Cells. Membranes 2023, 13, 769. https://doi.org/10.3390/membranes13090769
Huang Y-C, Wang Y-Z, Hsieh T-H, Ho K-S. Co(II)-Chelated Polyimines as Oxygen Reduction Reaction Catalysts in Anion Exchange Membrane Fuel Cells. Membranes. 2023; 13(9):769. https://doi.org/10.3390/membranes13090769
Chicago/Turabian StyleHuang, Yu-Chang, Yen-Zen Wang, Tar-Hwa Hsieh, and Ko-Shan Ho. 2023. "Co(II)-Chelated Polyimines as Oxygen Reduction Reaction Catalysts in Anion Exchange Membrane Fuel Cells" Membranes 13, no. 9: 769. https://doi.org/10.3390/membranes13090769
APA StyleHuang, Y.-C., Wang, Y.-Z., Hsieh, T.-H., & Ho, K.-S. (2023). Co(II)-Chelated Polyimines as Oxygen Reduction Reaction Catalysts in Anion Exchange Membrane Fuel Cells. Membranes, 13(9), 769. https://doi.org/10.3390/membranes13090769








