Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Enzyme Preparation
2.3. Multi-Enzyme Immobilization
2.4. Microfiltration Tests
2.5. Characterization
3. Results
3.1. Enzymes Preparation
3.2. Multi-Enzyme Immobilization
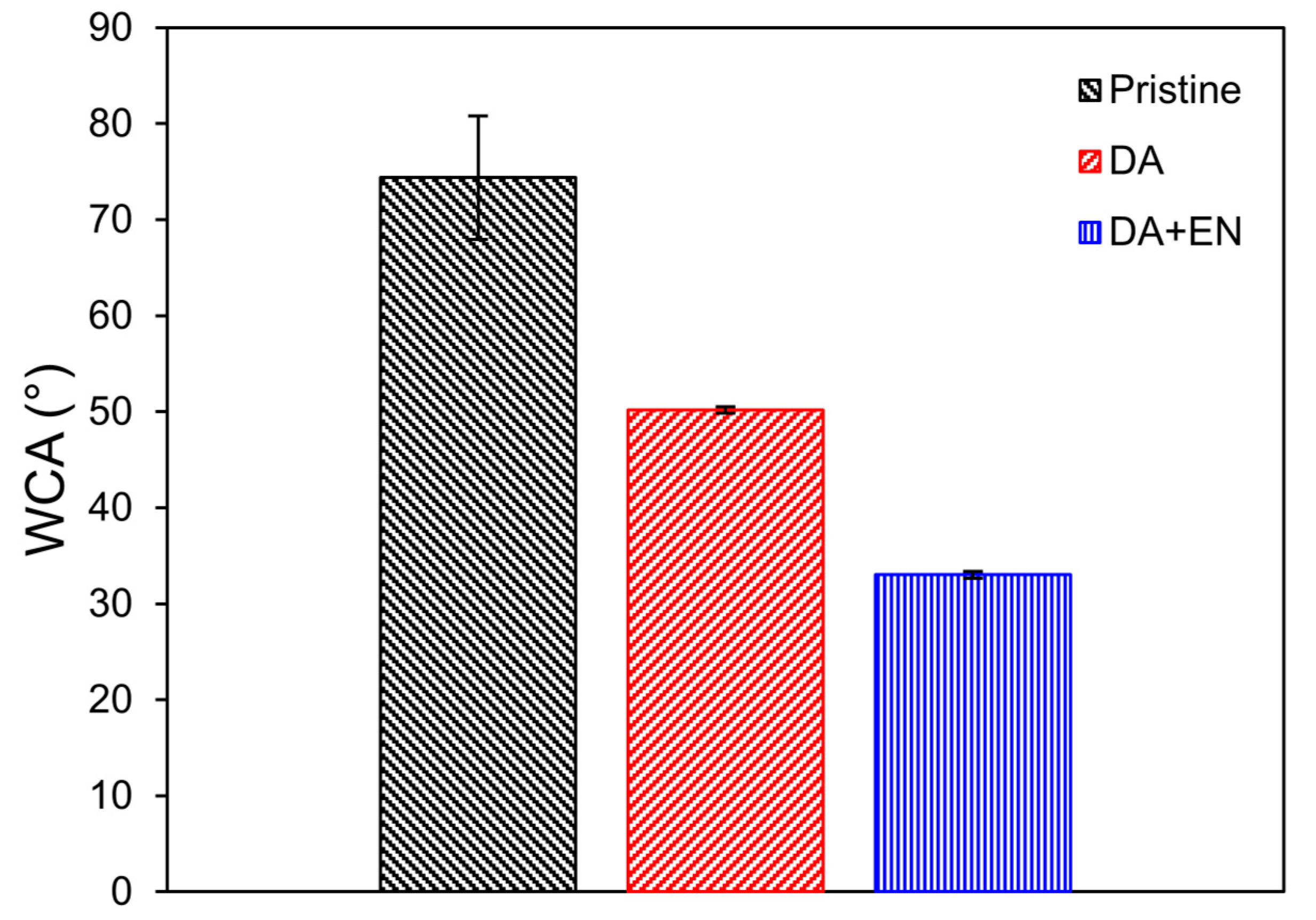
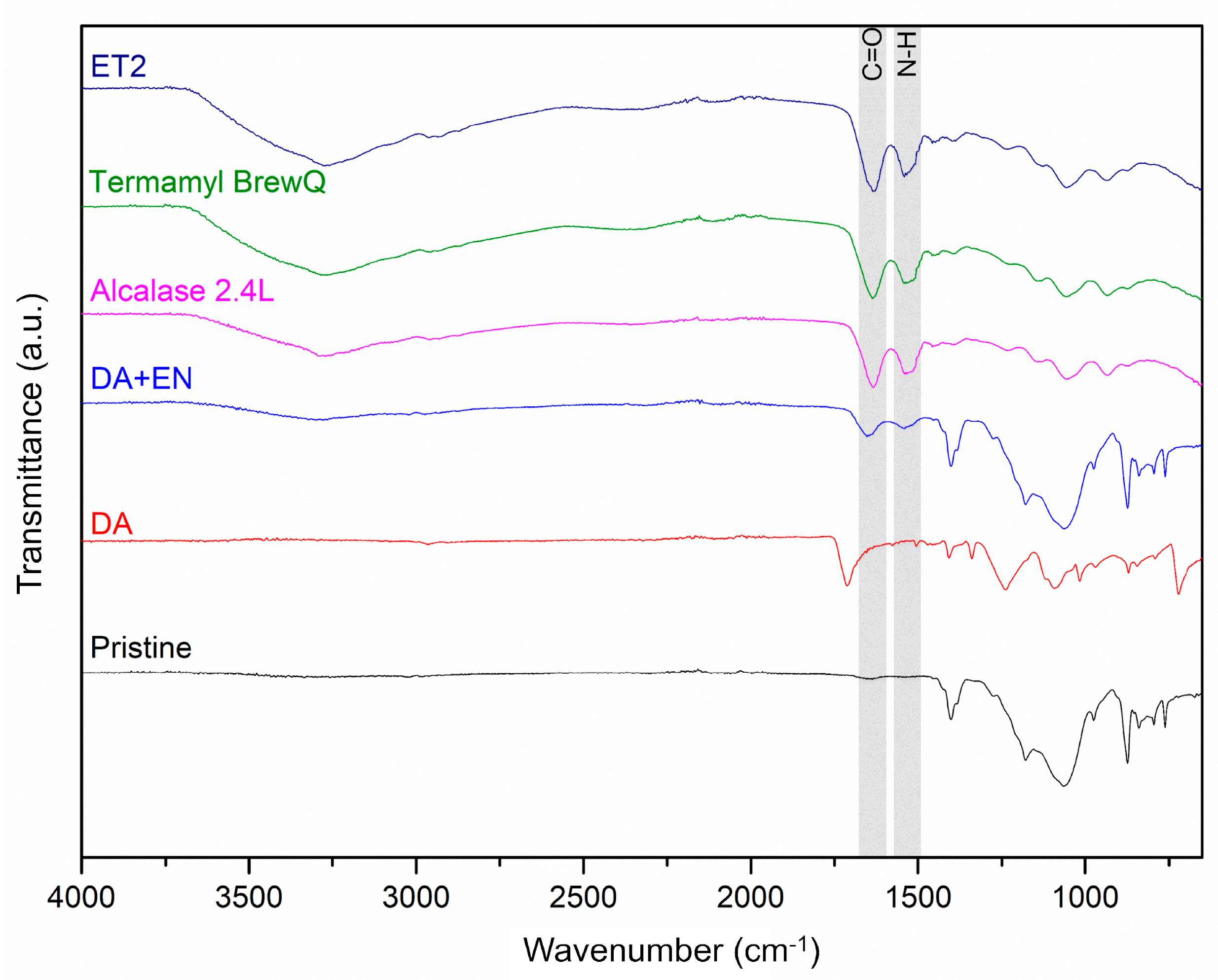
3.3. Membrane Characterization
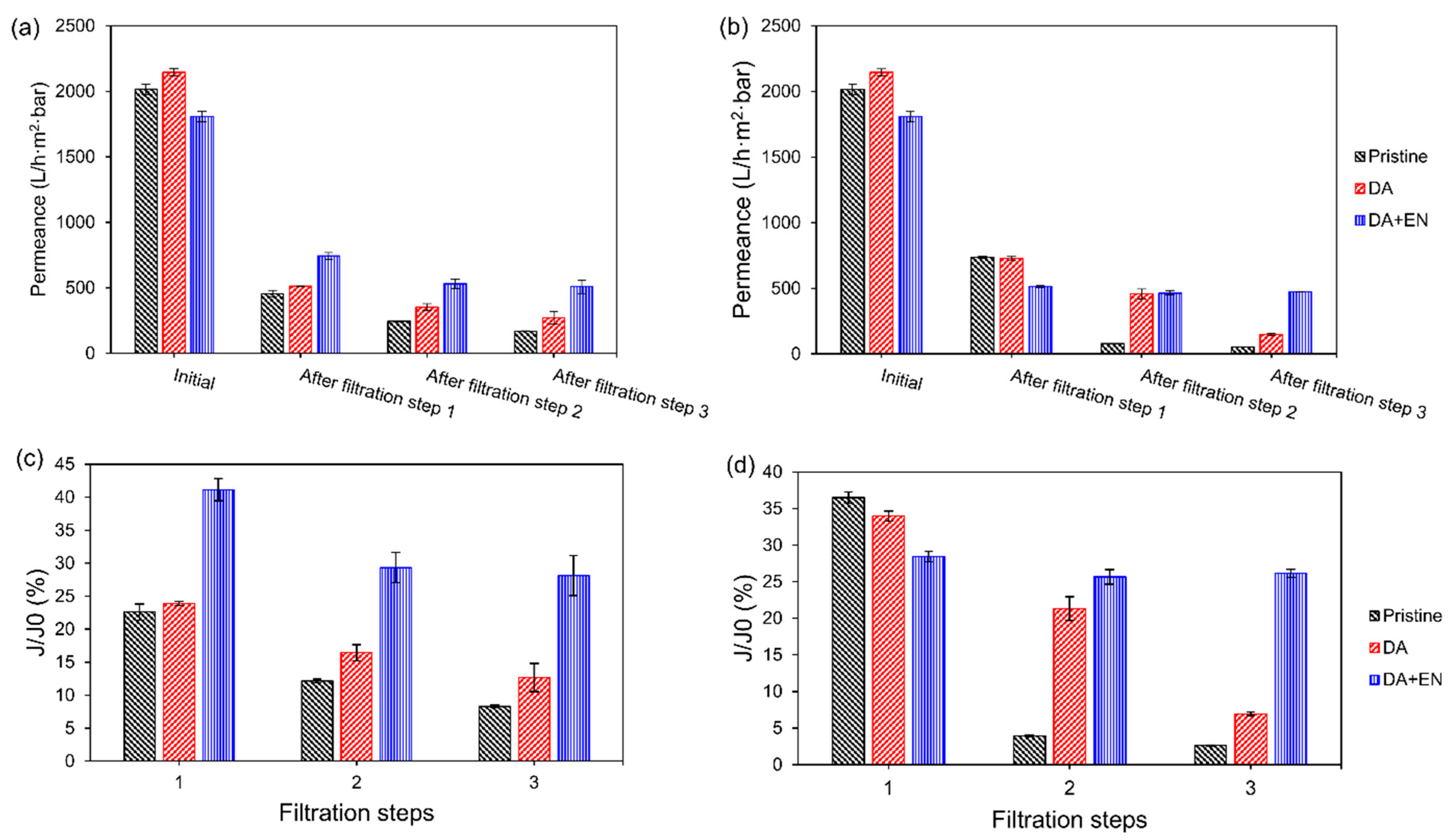
3.4. Filtration Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, D.; Lee, Y.M. Design Strategy of Poly(Vinylidene Fluoride) Membranes for Water Treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-Based Separation for Oily Wastewater: A Practical Perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Schmidt, M.; Breite, D.; Thomas, I.; Went, M.; Prager, A.; Schulze, A. Polymer Membranes for Active Degradation of Complex Fouling Mixtures. J. Membr. Sci. 2018, 563, 481–491. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Konovalova, V.; Kharchenko, K.; Burban, A.; Knozowska, K.; Kujawski, W.; Kujawa, J. Improved Antifouling Properties of Polyethersulfone Membranes Modified with α-Amylase Entrapped in Tetronic® Micelles. J. Membr. Sci. 2019, 570–571, 436–444. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Dizge, N.; Koyuncu, I. Enzymatic Activation of Cellulose Acetate Membrane for Reducing of Protein Fouling. Colloids Surf. B Biointerfaces 2012, 92, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Hiebner, D.W.; Casey, E. Self-Assembly and Regeneration Strategy for Mitigation of Membrane Biofouling by the Exploitation of Enzymatic Nanoparticles. Chem. Eng. J. 2021, 412, 128666. [Google Scholar] [CrossRef]
- Mulinari, J.; Feng, Y.; Huang, X.; Shen, H.; Ambrosi, A.; Di Luccio, M.; Li, Q.; Hotza, D.; Oliveira, J.V. In-Situ Immobilization of Lipase on α-Alumina Membrane for Oil Fouling Control and Cleaning. Sep. Purif. Technol. 2024, 330, 125493. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in Membrane Bioreactors Used in Wastewater Treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Burzio, L.A.; Waite, J.H. Cross-Linking in Adhesive Quinoproteins: Studies with Model Decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, Y.; Yang, H.; Fan, G.; Ding, A.; Liang, H.; Li, G.; Ren, N.; Van der Bruggen, B. Mussel-Inspired Polydopamine Modification of Polymeric Membranes for the Application of Water and Wastewater Treatment: A Review. Chem. Eng. Res. Des. 2020, 157, 195–214. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.-H.; Lee, B.P.; Messersmith, P.B. Mussel Adhesive Protein Mimetic Polymers for the Preparation of Nonfouling Surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef]
- Gao, N.; Xu, Z.-K. Ceramic Membranes with Mussel-Inspired and Nanostructured Coatings for Water-in-Oil Emulsions Separation. Sep. Purif. Technol. 2019, 212, 737–746. [Google Scholar] [CrossRef]
- Kasemset, S.; He, Z.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of Polydopamine Deposition Conditions on Polysulfone Ultrafiltration Membrane Properties and Threshold Flux during Oil/Water Emulsion Filtration. Polymer 2016, 97, 247–257. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, F.; Xue, L. Under Seawater Superoleophobic PVDF Membrane Inspired by Polydopamine for Efficient Oil/Seawater Separation. J. Membr. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Mulinari, J.; Ambrosi, A.; Innocentini, M.D.d.M.; Feng, Y.; Li, Q.; Di Luccio, M.; Hotza, D.; Oliveira, J.V. Lipase Immobilization on Alumina Membranes Using a Traditional and a Nature-Inspired Method for Active Degradation of Oil Fouling. Sep. Purif. Technol. 2022, 287, 120527. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M. Preparation, Characterization and Fouling Analysis of in-Air Hydrophilic/Underwater Oleophobic Bio-Inspired Polydopamine Coated PES Membranes for Oily Wastewater Treatment. J. Membr. Sci. 2019, 582, 402–413. [Google Scholar] [CrossRef]
- Zin, G.; Wu, J.; Rezzadori, K.; Petrus, J.C.C.; Di Luccio, M.; Li, Q. Modification of Hydrophobic Commercial PVDF Microfiltration Membranes into Superhydrophilic Membranes by the Mussel-Inspired Method with Dopamine and Polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, M.; Zhai, Q.; Li, S.; Jiang, Y. Polydopamine Tethered CPO/HRP-TiO2 Nano-Composites with High Bio-Catalytic Activity, Stability and Reusability: Enzyme-Photo Bifunctional Synergistic Catalysis in Water Treatment. Chem. Eng. J. 2018, 347, 703–710. [Google Scholar] [CrossRef]
- Cheng, W.; Li, Y.; Li, X.; Bai, W.; Liang, Y. Preparation and Characterization of PDA/SiO2 Nanofilm Constructed Macroporous Monolith and Its Application in Lipase Immobilization. J. Taiwan Inst. Chem. Eng. 2019, 104, 351–359. [Google Scholar] [CrossRef]
- Luo, J.; Meyer, A.S.; Mateiu, R.V.; Kalyani, D.; Pinelo, M. Functionalization of a Membrane Sublayer Using Reverse Filtration of Enzymes and Dopamine Coating. ACS Appl. Mater. Interfaces 2014, 6, 22894–22904. [Google Scholar] [CrossRef]
- Morthensen, S.T.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. Significance of Membrane Bioreactor Design on the Biocatalytic Performance of Glucose Oxidase and Catalase: Free vs. Immobilized Enzyme Systems. Biochem. Eng. J. 2017, 117, 41–47. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef]
- Mulinari, J.; Ambrosi, A.; Feng, Y.; He, Z.; Huang, X.; Li, Q.; Di Luccio, M.; Hotza, D.; Oliveira, J.V. Polydopamine-Assisted One-Step Immobilization of Lipase on α-Alumina Membrane for Fouling Control in the Treatment of Oily Wastewater. Chem. Eng. J. 2023, 459, 141516. [Google Scholar] [CrossRef]
- Daverey, A.; Pakshirajan, K. Pretreatment of Synthetic Dairy Wastewater Using the Sophorolipid-Producing Yeast Candida Bombicola. Appl. Biochem. Biotechnol. 2011, 163, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Gopinatha Kurup, G.; Adhikari, B.; Zisu, B. Recovery of Proteins and Lipids from Dairy Wastewater Using Food Grade Sodium Lignosulphonate. Water Resour. Ind. 2019, 22, 100114. [Google Scholar] [CrossRef]
- Fuwa, H. A New Method for Microdetermination of Amylase Activity by the Use of Amylose as the Substrate. J. Biochem. 1954, 41, 583–603. [Google Scholar] [CrossRef]
- De Yan, H.; Zhang, Y.J.; Liu, H.C.; Zheng, J.Y.; Wang, Z. Influence of Ammonium Salts on the Lipase/Esterase Activity Assay Using p -nitrophenyl Esters as Substrates. Biotech. App. Biochem. 2013, 60, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M. Crystalline Soybean Trypsin Inhibitor: II. General Properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]
- Walter, H.E. Proteinases: Methods with Haemoglobin, Casein and Azocoll as Substrates. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Editora Verlag Chemie: Weinheim, Germany, 1984; Volume 5, pp. 270–277. [Google Scholar]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef]
- Bresolin, D.; Hawerroth, B.; de Oliveira Romera, C.; Sayer, C.; de Araújo, P.H.H.; de Oliveira, D. Immobilization of Lipase Eversa Transform 2.0 on Poly(Urea–Urethane) Nanoparticles Obtained Using a Biopolyol from Enzymatic Glycerolysis. Bioprocess Biosyst. Eng. 2020, 43, 1279–1286. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Et Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Proner, M.C.; Ramalho Marques, I.; Ambrosi, A.; Rezzadori, K.; da Costa, C.; Zin, G.; Tres, M.V.; Di Luccio, M. Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes. Membranes 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | Protein Content (mg/g) | Specific Hydrolytic Activity | Unit |
|---|---|---|---|
| Amylase—crude extract | 41.65 ± 0.16 | 401.76 ± 15.80 | gstarch/min·g |
| Amylase—lyophilized | 657.76 ± 0.83 | 402.34 ± 17.13 | gstarch/min·g |
| Lipase—crude extract | 28.62 ± 2.65 | 6.43 ± 0.83 | mmolp-nitrophenol/min·g |
| Lipase—lyophilized | 536.02 ± 1.44 | 6.63 ± 0.24 | mmolp-nitrophenol/min·g |
| Protease—crude extract | 82.37 ± 1.23 | 9.29 ± 1.34 | mmoltyrosine/min·g |
| Protease—lyophilized | 599.10 ± 6.27 | 12.33 ± 1.02 | mmoltyrosine/min·g |
| Assay | DA (mg/mL) | EN (mg/mL) | Amylase Activity (mgstarch/min·cm2) | Lipase Activity (µmoln-nitrophenol/min·cm2) | Protease Activity (nmoltyrosine/min·cm2) |
|---|---|---|---|---|---|
| 1 | 0.3 | 3.0 | 0.80 | 18.52 | 5.24 |
| 2 | 0.7 | 3.0 | 0.49 | 13.25 | 4.56 |
| 3 | 0.3 | 5.0 | 0.62 | 18.11 | 10.16 |
| 4 | 0.7 | 5.0 | 0.80 | 13.35 | 8.00 |
| 5 | 0.2 | 4.0 | 0.82 | 20.48 | 7.82 |
| 6 | 0.8 | 4.0 | 0.69 | 12.30 | 5.60 |
| 7 | 0.5 | 2.6 | 0.68 | 15.20 | 3.27 |
| 8 | 0.5 | 5.4 | 0.83 | 16.11 | 8.22 |
| 9 | 0.5 | 4.0 | 0.85 | 15.15 | 6.05 |
| 10 | 0.5 | 4.0 | 0.90 | 15.19 | 7.01 |
| 11 | 0.5 | 4.0 | 0.86 | 14.64 | 6.63 |
| DA (mg/mL) | EN (mg/mL) | Amylase Activity (mgstarch/min·cm2) | Lipase Activity (µmolp-nitrophenol/min·cm2) | Protease Activity (nmoltyrosine/min·cm2) |
|---|---|---|---|---|
| 0.3 | 5.0 | 0.62 ± 0.03 a | 18.11 ± 0.31 a | 10.16 ± 0.48 a |
| 0.2 | 4.0 | 0.82 ± 0.03 b | 20.48 ± 0.37 b | 7.82 ± 0.33 b |
| 0.5 | 4.0 | 0.87 ± 0.02 b | 14.99 ± 0.29 c | 6.56 ± 0.41 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulinari, J.; Rigo, D.; Demaman Oro, C.E.; Meneses, A.C.d.; Zin, G.; Eleutério, R.V.; Tres, M.V.; Dallago, R.M. Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency. Membranes 2024, 14, 208. https://doi.org/10.3390/membranes14100208
Mulinari J, Rigo D, Demaman Oro CE, Meneses ACd, Zin G, Eleutério RV, Tres MV, Dallago RM. Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency. Membranes. 2024; 14(10):208. https://doi.org/10.3390/membranes14100208
Chicago/Turabian StyleMulinari, Jéssica, Diane Rigo, Carolina Elisa Demaman Oro, Alessandra Cristina de Meneses, Guilherme Zin, Rafael Vidal Eleutério, Marcus Vinícius Tres, and Rogério Marcos Dallago. 2024. "Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency" Membranes 14, no. 10: 208. https://doi.org/10.3390/membranes14100208
APA StyleMulinari, J., Rigo, D., Demaman Oro, C. E., Meneses, A. C. d., Zin, G., Eleutério, R. V., Tres, M. V., & Dallago, R. M. (2024). Multienzyme Immobilization on PVDF Membrane via One-Step Mussel-Inspired Method: Enhancing Fouling Resistance and Self-Cleaning Efficiency. Membranes, 14(10), 208. https://doi.org/10.3390/membranes14100208







