Controlled Growth of ZIF-8 Membranes on GO-Coated α-Alumina Supports via ZnO Atomic Layer Deposition for Improved Gas Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. α-Alumina Hollow Fiber Preparation
2.2.2. Rapid Thermal Deposition Technique
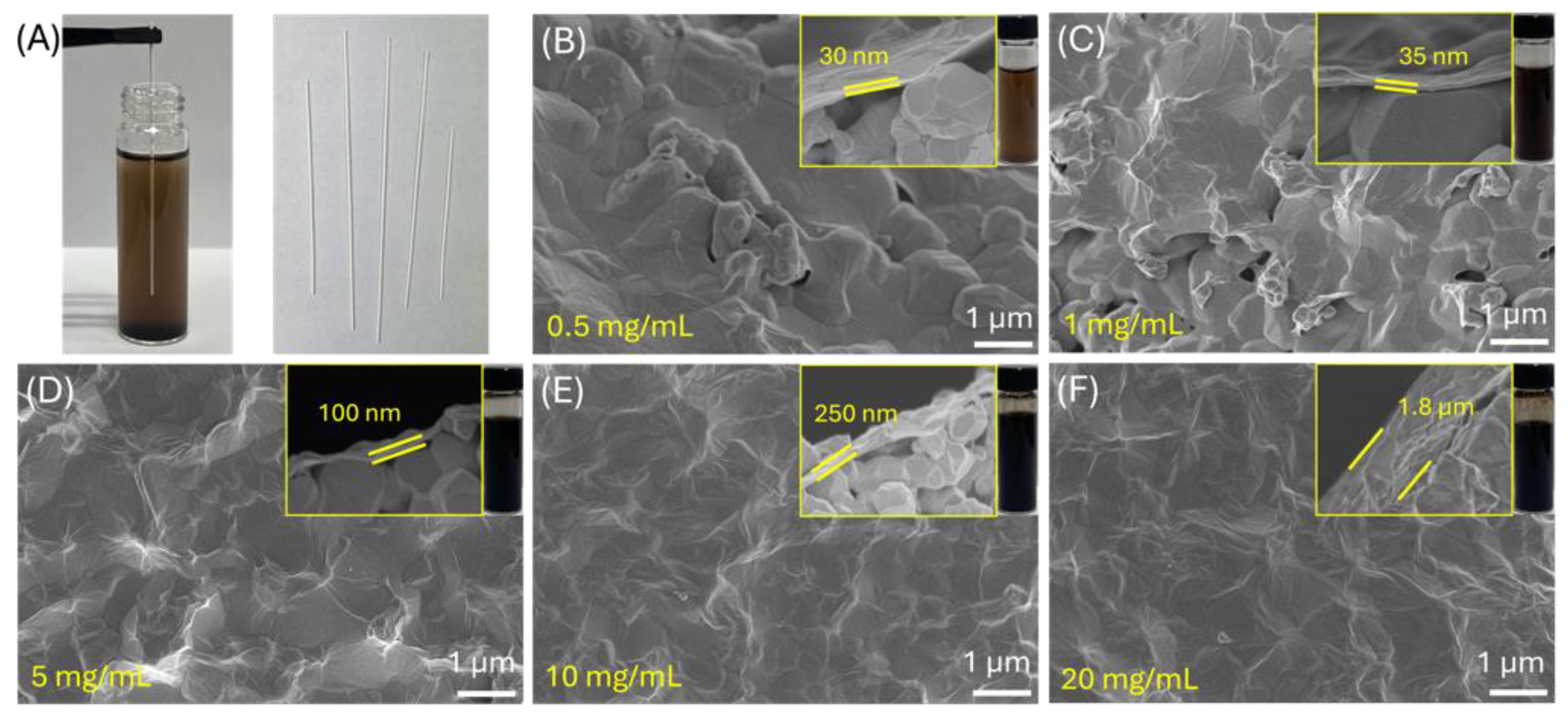
2.2.3. Graphene Oxide (GO) Synthesis
2.2.4. Deposition of GO on α-Alumina Hollow Fiber
2.3. Characterization
2.4. Permeation Test
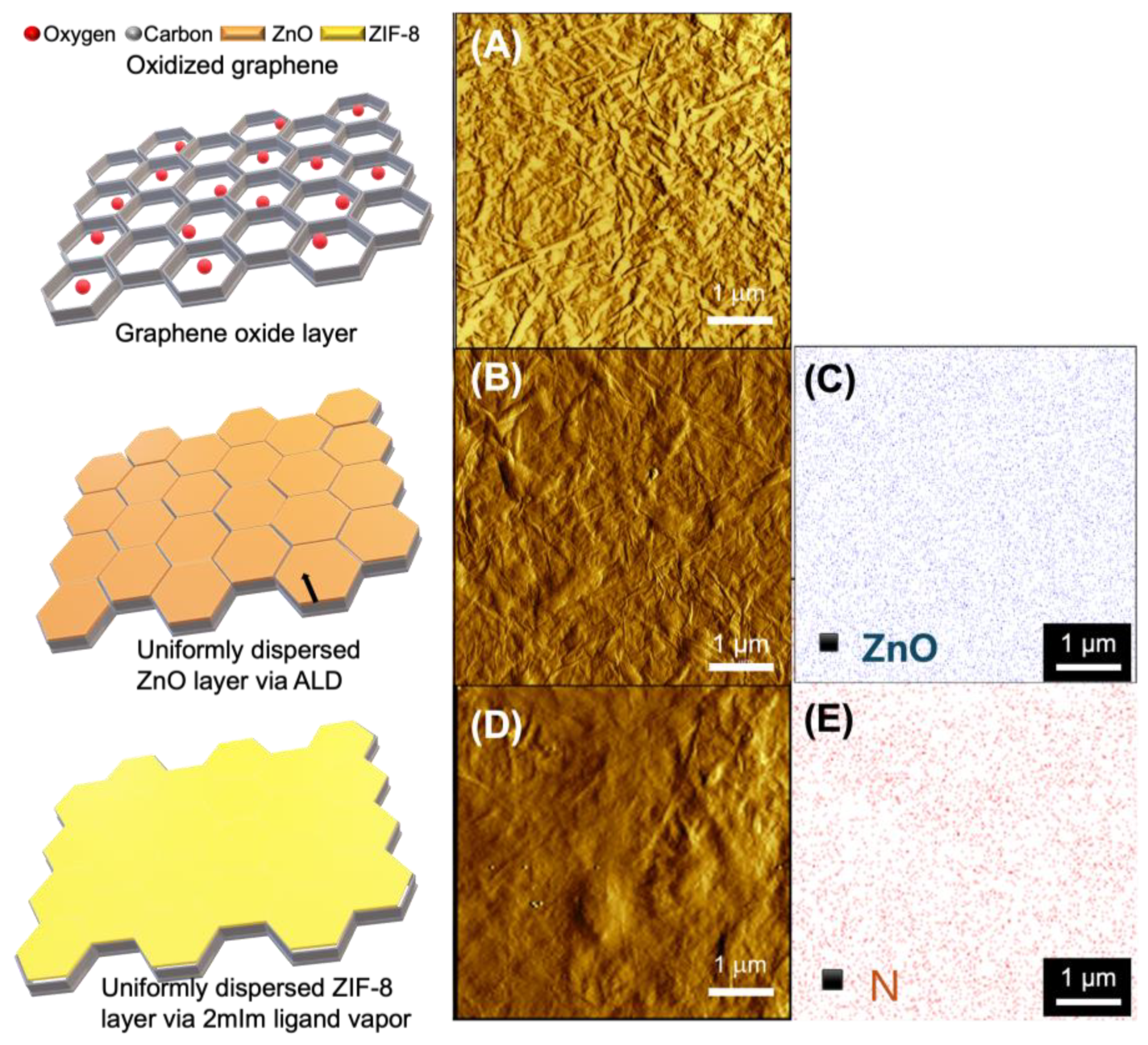
2.5. Zinc Oxide Atomic Layer Deposition
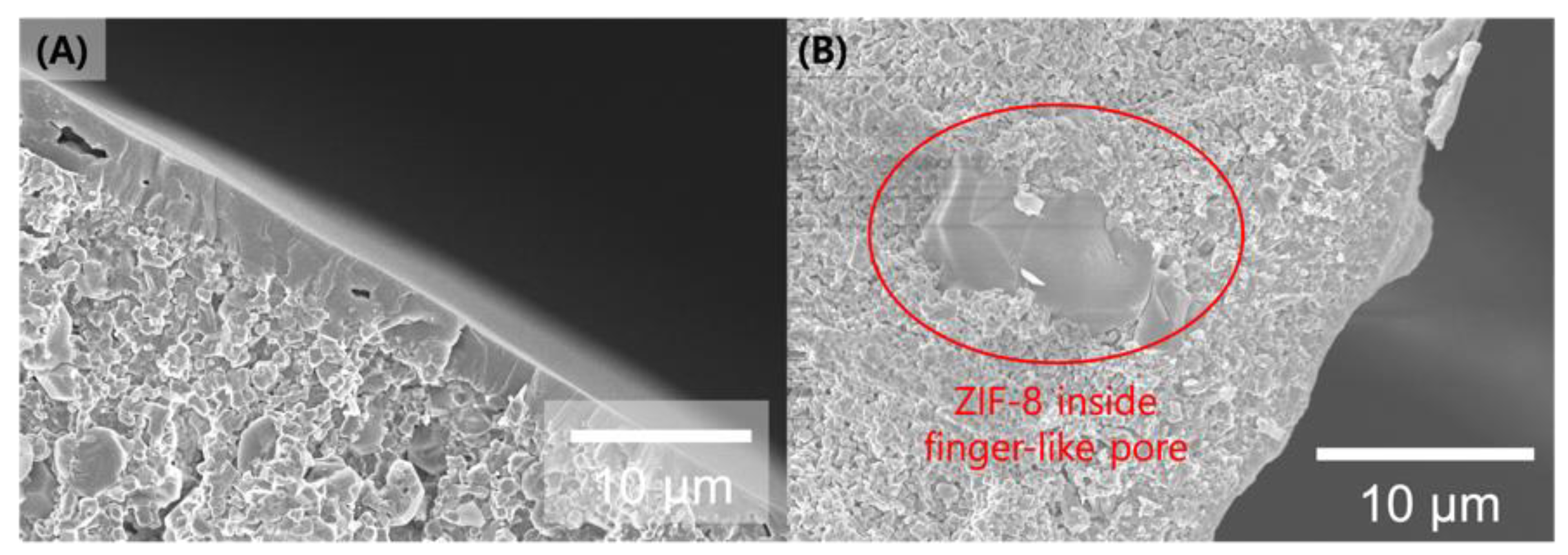
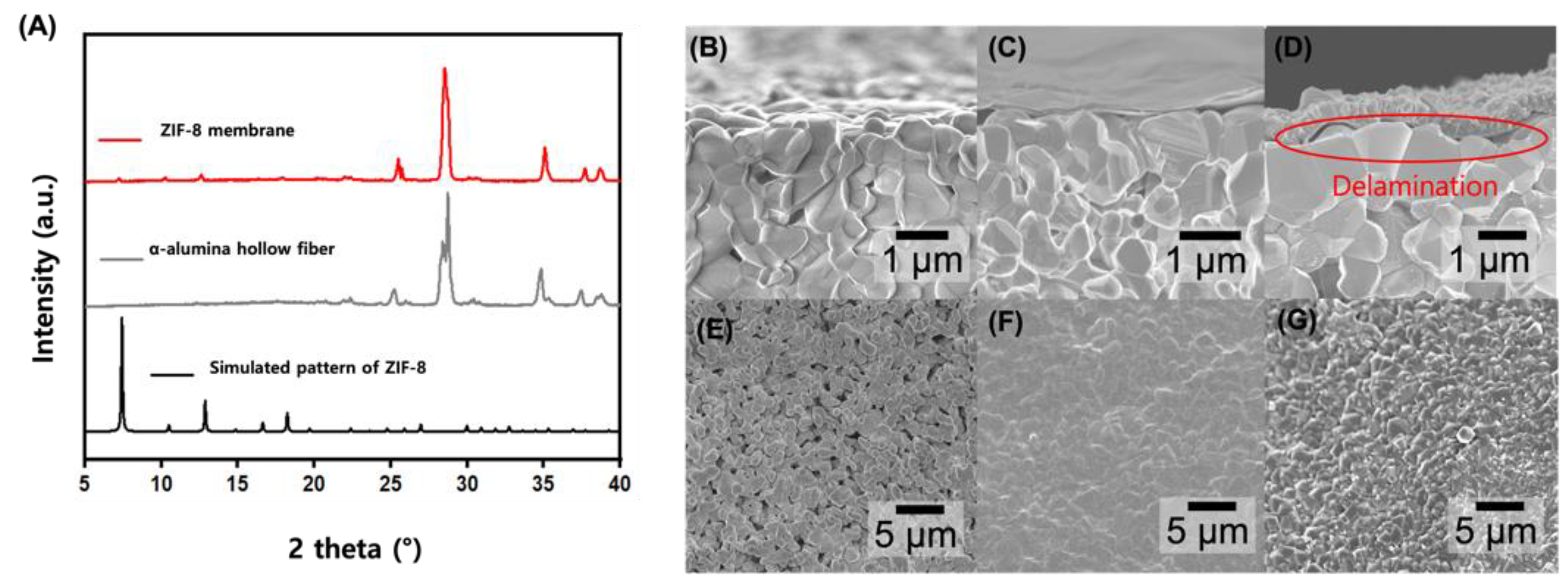
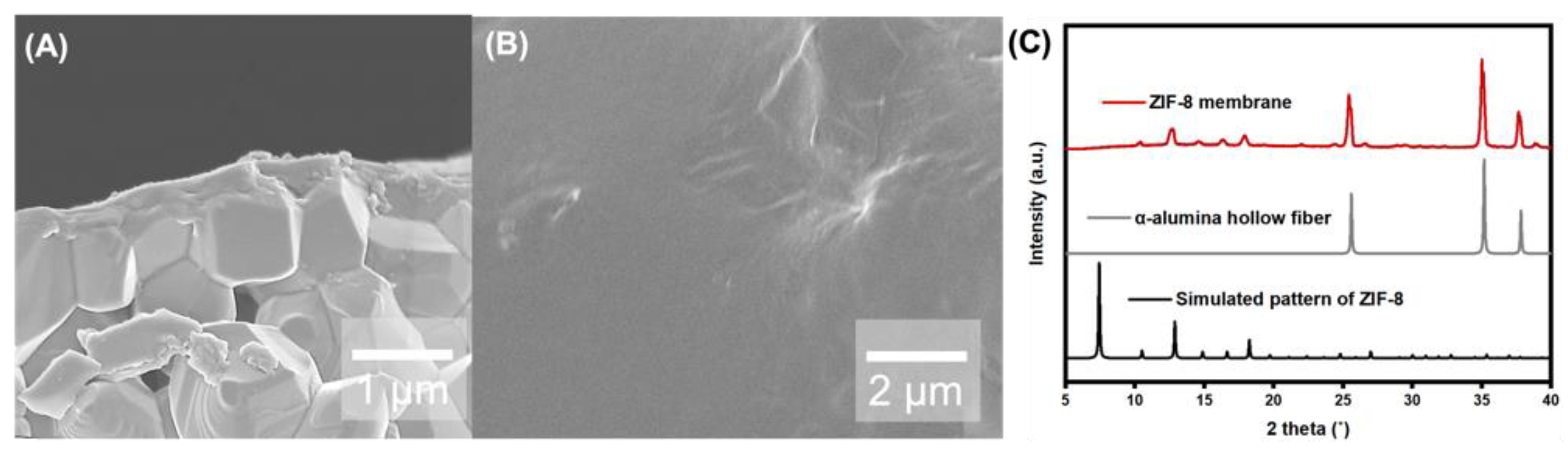
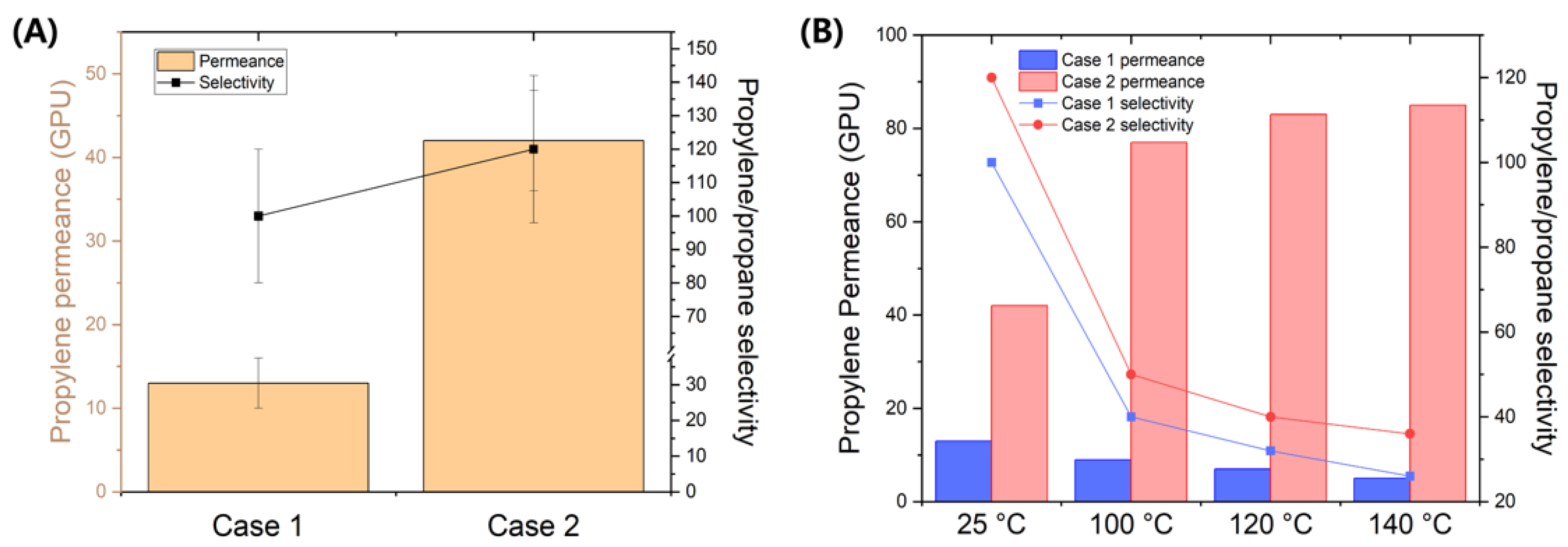
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, T.; Hu, W.; Sun, Y.; He, Y.; Yan, J.; He, G.; Liu, Y. Epitaxial supercritical fluid processing of ZIF-8 membranes towards efficient C3H6/C3H8 separation. J. Membr. Sci. 2023, 669, 121300. [Google Scholar] [CrossRef]
- Hu, W.; Liu, L.; Ji, T.; Meng, S.; Yan, J.; He, Y.; Wu, M.; Liu, Y. Supercritical Processing of a Highly C3H6-Permselective ZIF-8 Membrane on a Tubular Porous α-Al2O3 Substrate. Ind. Eng. Chem. Res. 2023, 62, 21297–21303. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. In situ synthesis of thin zeolitic-imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kim, Y.J.; Kim, J.; Eum, K. ZIF-L to ZIF-8 Transformation: Morphology and Structure Controls. Nanomaterials 2022, 12, 4224. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Xia, Y.; Xiang, L. Highly selective methane sensing based on enhanced dual sieving effects by ZnO/Pd@Co node-replaced ZIF-8 nanorods. J. Environ. Chem. Eng. 2024, 12, 112497. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, X.; Shi, D.; Wang, Z. Zeolitic imidazolate framework-8 (ZIF-8) for drug delivery: A critical review. Front. Chem. Sci. Eng. 2021, 15, 221–237. [Google Scholar] [CrossRef]
- Zhu, C.; Peng, Y.; Yang, W. Modification strategies for metal-organic frameworks targeting at membrane-based gas separations. Green Chem. Eng. 2021, 2, 17–26. [Google Scholar] [CrossRef]
- Awang Chee, D.N.; Aziz, F.; Mohamed Amin, M.A.; Ismail, A.F. ZIF-8 membrane: The synthesis technique and nanofiltration application. Emergent Mater. 2022, 5, 1289–1310. [Google Scholar] [CrossRef]
- Yu, C.; Kim, Y.J.; Kim, J.; Hayashi, M.; Kim, D.W.; Kwon, H.T.; Eum, K. A sacrificial ZIF-L seed layer for sub-100 nm thin propylene-selective ZIF-8 membranes. J. Mater. Chem. A 2022, 10, 15390–15394. [Google Scholar] [CrossRef]
- Shah, M.N.; Gonzalez, M.A.; McCarthy, M.C.; Jeong, H.K. An unconventional rapid synthesis of high performance metal-organic framework membranes. Langmuir 2013, 29, 7896–7902. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Mo, K.; Gao, S.; Xie, Y.; Wang, J.; Jin, H.; Feldhoff, A.; Xu, S.; Lin, J.Y.S.; Li, Y. Ultrafast Semi-Solid Processing of Highly Durable ZIF-8 Membranes for Propylene/Propane Separation. Angew. Chem.-Int. Ed. 2020, 59, 21909–21914. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, Y.J.; Yu, C.; Kim, J.; Eum, K. Facile Fabrication of α-Alumina Hollow Fiber-Supported ZIF-8 Membrane Module and Impurity Effects on Propylene Separation Performance. Membranes 2022, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Hughes, R. Preparation of porous aluminium oxide (Al2O3) hollow fibre membranes by a combined phase-inversion and sintering method. Ceram. Int. 2003, 29, 875–881. [Google Scholar] [CrossRef]
- Kingsbury, B.F.K.; Li, K. A morphological study of ceramic hollow fibre membranes. J. Membr. Sci. 2009, 328, 134–140. [Google Scholar] [CrossRef]
- Choi, S.E.; Kim, S.S.; Choi, E.; Kim, J.H.; Choi, Y.; Kang, J.; Kwon, O.; Kim, D.W. Diamine vapor treatment of viscoelastic graphene oxide liquid crystal for gas barrier coating. Sci. Rep. 2021, 11, 9518. [Google Scholar] [CrossRef]
- Ji, T.; Liu, L.; Wu, M.; Yu, K.; He, X.; Liu, Y. Subfreezing conversion of ALD-derived ZnO layer to ultra-thin ZIF-8 membrane for high-flux C3H6 production. Chem. Eng. Sci. 2023, 282, 119293. [Google Scholar] [CrossRef]
- Young, C.; Salunkhe, R.R.; Tang, J.; Hu, C.C.; Shahabuddin, M.; Yanmaz, E.; Hossain, M.S.A.; Kim, J.H.; Yamauchi, Y. Ze-olitic im-idazolate framework (ZIF-8) derived nanoporous carbon: The effect of carbonization temperature on the supercapacitor performance in an aqueous electrolyte. Phys. Chem. Chem. Phys. 2016, 18, 29308–29315. [Google Scholar] [CrossRef]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane binary mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390–391, 93–98. [Google Scholar] [CrossRef]
- Jain, A.; Ahmad, M.Z.; Linkès, A.; Martin-gil, V.; Castro-muñoz, R.; Izak, P.; Sofer, Z.; Hintz, W.; Fila, V. 6fda-dam:Daba co-polyimide mixed matrix membranes with go and zif-8 mixtures for effective co2/ch4 separation. Nanomaterials 2021, 11, 668. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Moutloali, R.M. The Effect of Zeolitic Imidazole Framework-8@Graphene Oxide on the Performance of Polymeric Membranes Used for Wasterwater Treatment. In Functional Polymer Nanocomposites for Wastewater Treatment; Hato, M.J., Sinha Ray, S., Eds.; Springer Series in Materials Science; Springer: Cham, Switzerland, 2022; Volume 323. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Xi, H.; Lin, Y.S. Gas transport properties and propylene/propane separation characteristics of ZIF-8 membranes. J. Membr. Sci. 2014, 451, 85–93. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. ZIF-8 membranes prepared at miscible and immiscible liquid–liquid interfaces. Microporous Mesoporous Mater. 2015, 206, 75–80. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. Highly propylene-selective supported zeolite-imidazolate framework (ZIF-8) membranes synthesized by rapid microwave-assisted seeding and secondary growth. Chem. Commun. 2013, 49, 3854–3856. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Rownaghi, A.; Choi, D.; Bhave, R.R.; Jones, C.W.; Nair, S. Fluidic Processing of High-Performance ZIF-8 Membranes on Polymeric Hollow Fibers: Mechanistic Insights and Microstructure Control. Adv. Funct. Mater. 2016, 26, 5011–5018. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially Grown Zeolitic Imidazolate Framework Membranes with Unprecedented Propylene/Propane Separation Performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef]
- Bai, S.; Sridhar, S.; Khan, A. Metal-ion mediated separation of propylene from propane using PPO membranes. J. Membr. Sci. 1998, 147, 131–139. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Chung, T.-S. Flexible thermally treated 3D PIM-CD molecular sieve membranes exceeding the upper bound line for propylene/propane separation. J. Mater. Chem. A 2017, 5, 4583–4595. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Okamoto, K.; Noborio, K.; Hao, J.; Tanaka, K.; Kita, H. Permeation and separation properties of polyimide membranes to 1,3-butadiene and n-. J. Membr. Sci. 1997, 134, 171–179. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.-G.; Kim, S.; Kim, S.S. PDMS–silica composite membranes with silane coupling for propylene separation. J. Membr. Sci. 2009, 344, 211–218. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; Park, J.; Lee, A.S.; Hwang, S.S.; Kim, S.-J.; Park, S.; Cho, K.Y.; Won, W.; Lee, J.S. Fluorine-containing polyimide/polysilsesquioxane carbon molecular sieve membranes and techno-economic evaluation thereof for C3H6/C3H8 separation. J. Membr. Sci. 2019, 598, 117660. [Google Scholar] [CrossRef]
- Chng, M.L.; Xiao, Y.; Chung, T.-S.; Toriida, M.; Tamai, S. Enhanced propylene/propane separation by carbonaceous membrane derived from poly (aryl ether ketone)/2,6-bis(4-azidobenzylidene)-4-methyl-cyclohexanone interpenetrating network. Carbon 2009, 47, 1857–1866. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/Paraffin Separation through Carbonized Membranes Derived from an Asymmetric Polyimide Hollow Fiber Membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective Ag-Exchanged X-Type Zeolite Membrane for Propylene/Propane and Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, H.; He, F.; Liu, Q.; Xu, N.; Fan, L.; Wang, C.; Zhang, L.; Zhou, R. High-Performance FAU Zeolite Membranes Derived from Nano-Seeds for Gas Separation. Membranes 2023, 13, 858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Ahn, Y.-H.; Kim, J.; Eum, K. Controlled Growth of ZIF-8 Membranes on GO-Coated α-Alumina Supports via ZnO Atomic Layer Deposition for Improved Gas Separation. Membranes 2024, 14, 216. https://doi.org/10.3390/membranes14100216
Lee N, Ahn Y-H, Kim J, Eum K. Controlled Growth of ZIF-8 Membranes on GO-Coated α-Alumina Supports via ZnO Atomic Layer Deposition for Improved Gas Separation. Membranes. 2024; 14(10):216. https://doi.org/10.3390/membranes14100216
Chicago/Turabian StyleLee, Nahyeon, Yun-Ho Ahn, Jaheon Kim, and Kiwon Eum. 2024. "Controlled Growth of ZIF-8 Membranes on GO-Coated α-Alumina Supports via ZnO Atomic Layer Deposition for Improved Gas Separation" Membranes 14, no. 10: 216. https://doi.org/10.3390/membranes14100216
APA StyleLee, N., Ahn, Y.-H., Kim, J., & Eum, K. (2024). Controlled Growth of ZIF-8 Membranes on GO-Coated α-Alumina Supports via ZnO Atomic Layer Deposition for Improved Gas Separation. Membranes, 14(10), 216. https://doi.org/10.3390/membranes14100216





