Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
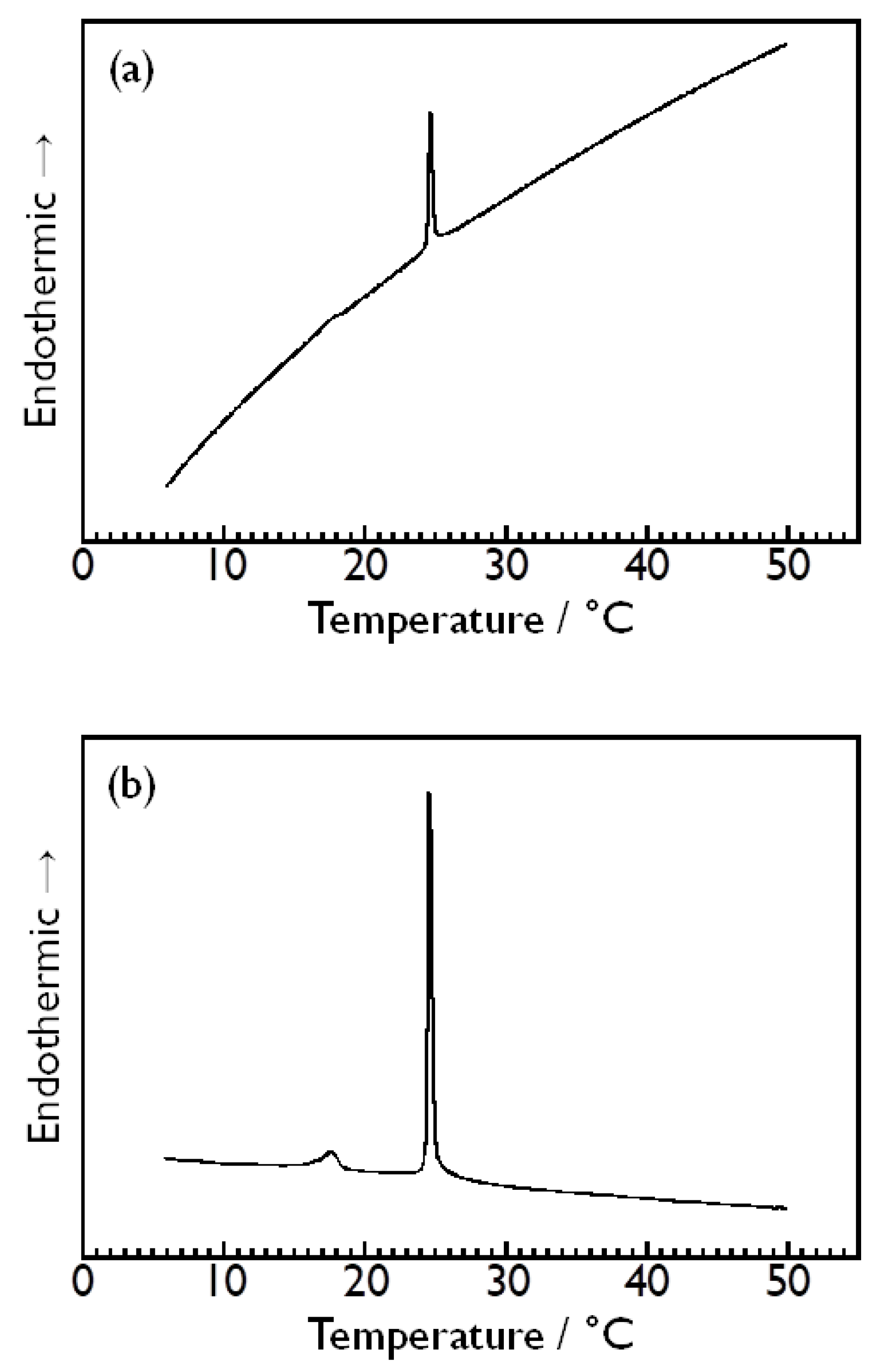
3.1. Choice of Reference Solution and Stability of Baseline
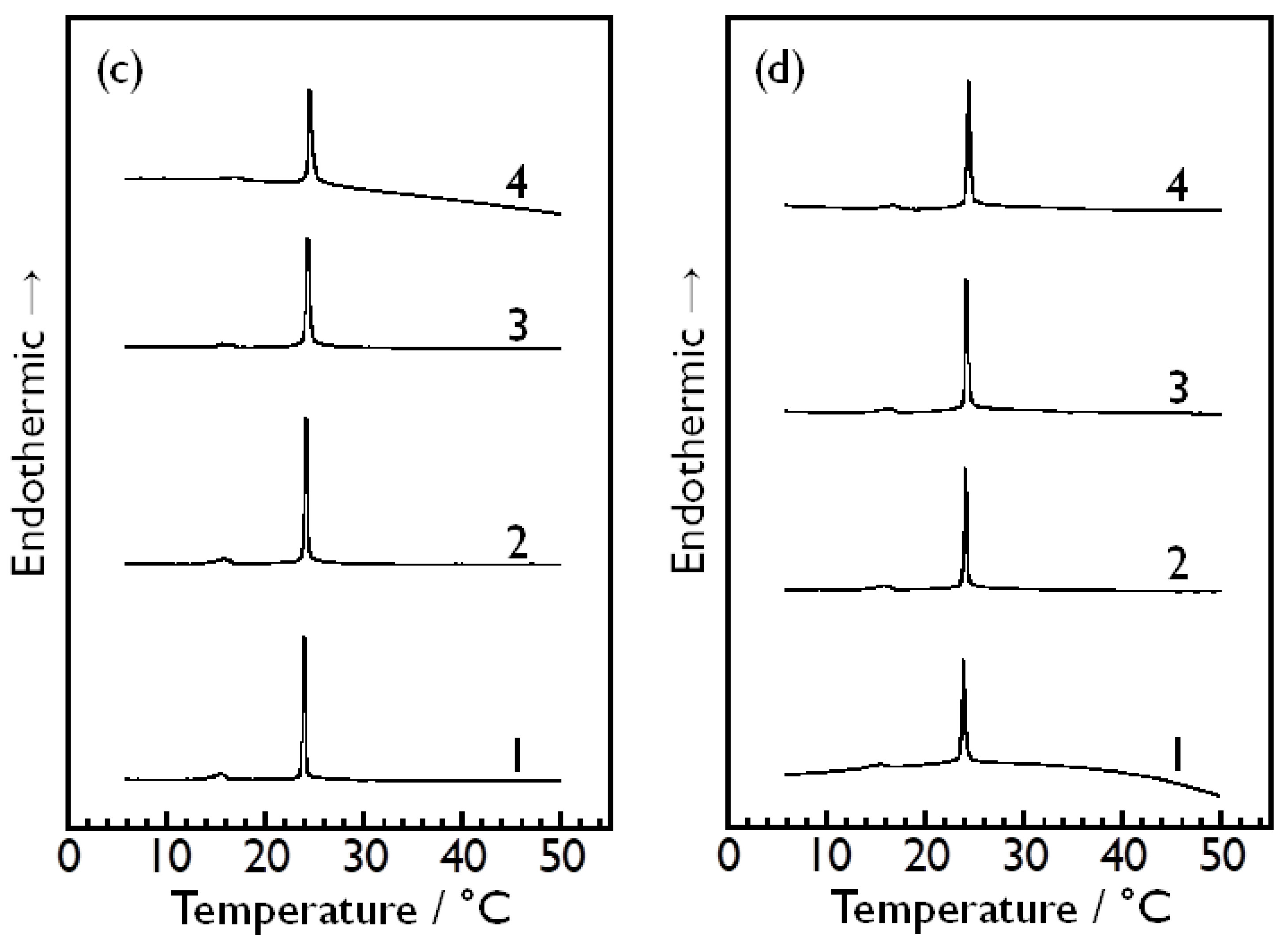
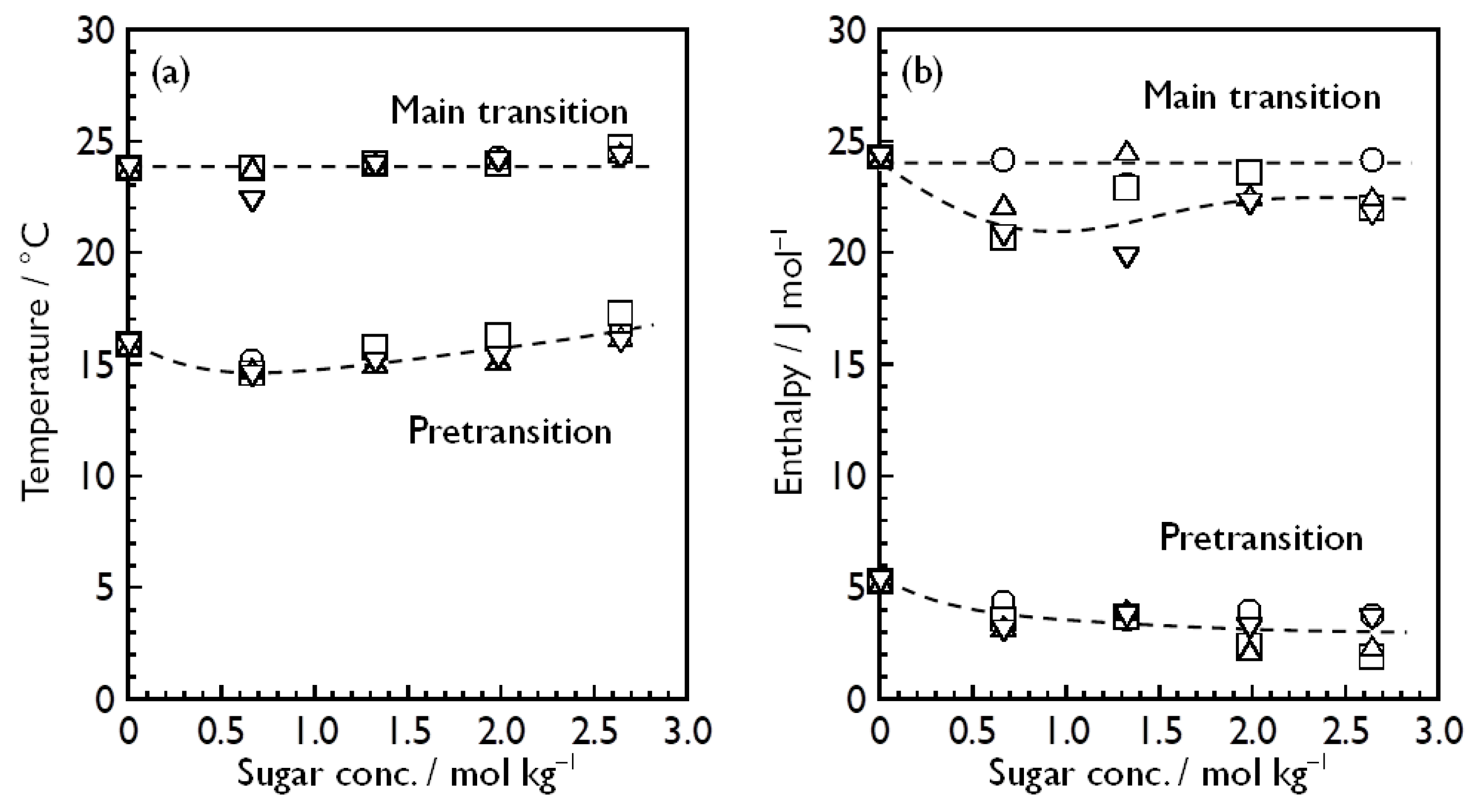
3.2. Bilayer Phase Transitions of DMPC in Aqueous Sugar Solutions
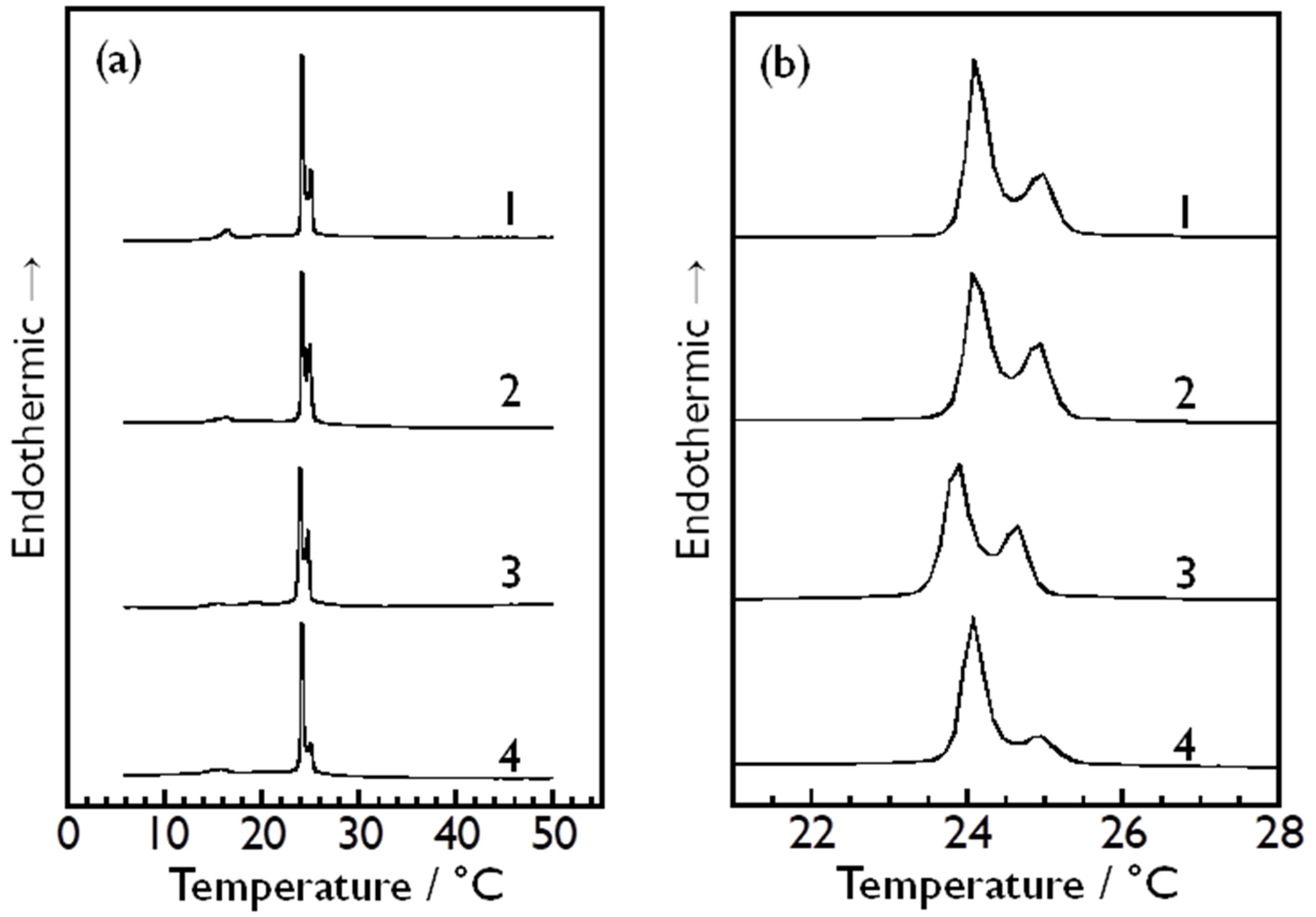
3.3. Effect of Asymmetry in Sugar Concentration Between Inside and Outside of Vesicle Particles on Bilayer Phase Transitions of DMPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, R.N.A.H.; Mak, N.; McElhaney, R.N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry 1987, 26, 6118–6126. [Google Scholar] [CrossRef]
- Ichimori, H.; Hata, T.; Matsuki, H.; Kaneshina, S. Barotropic phase transitions and pressure-induced interdigitation on bilayer membranes of phospholipids with varying acyl chain-lengths. Biochim. Biophys. Acta 1998, 1414, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.J.; Shipley, G.G. Characterization of the sub-transition of hydrated dipalmitoylphosphatidylcholine bilayers: X-ray diffraction study. Biochim. Biophys. Acta 1982, 684, 59–66. [Google Scholar] [CrossRef]
- Nagle, J.F.; Zahng, R.; Tristram-Nagle, S.; Sun, W.; Petrache, H.I.; Suter, R.M. X-ray structure determination of fully hydrated L alpha phase dipalmitoylphosphatidylcholine bilayers. Biophys. J. 1996, 70, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Kawato, S.; Kinosita, K., Jr.; Ikegami, A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry 1977, 16, 2319–2324. [Google Scholar] [CrossRef]
- Zeng, J.; Chong, P.L.G. Interactions between pressure and ethanol on the formation of interdigitated DPPC liposomes: A study with Prodan fluorescence. Biochemistry 1991, 30, 9485–9491. [Google Scholar] [CrossRef]
- Tamai, N.; Matsuki, H.; Goto, M. Phase imaging of phosphatidylcholine bilayer membranes by Prodan fluorescence. Membranes 2022, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Seelig, J. Deuterium magnetic resonance: Theory and application to lipid membranes. Q. Rev. Biophys. 1977, 10, 353–418. [Google Scholar] [CrossRef]
- Watts, A.; Spooner, P.J.R. Phospholipid phase transitions as revealed by NMR. Chem. Phys. Lipid 1991, 57, 195–211. [Google Scholar] [CrossRef]
- Drabik, D.; Chodaczek, G.; Kraszewski, S.; Langner, M. Mechanical Properties Determination of DMPC, DPPC, DSPC, and HSPC Solid-Ordered Bilayers. Langmuir 2020, 36, 3826–3835. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef] [PubMed]
- Braganza, L.F.; Worcester, D.L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry 1986, 25, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Pilgrim, W.C. A SANS study of high pressure phase transitions in model biomembranes. Ber. Bunsenges Phys. Chem. 1989, 93, 708–717. [Google Scholar] [CrossRef]
- Seddon, J.M. Structure of the inverted hexagonal (HII) phase, non-lamellar phase transitions of lipids. Biophys. Biochim. Acta 1990, 1031, 1–69. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Schmidt, M.L.; Bashe, B.Y.M.; Pruim, J.R.; Link, M.L.; Cullis, P.R.; Harper, P.E.; Thewalt, J.L.; Tieleman, D.P. Structural properties of inverted hexagonal phase: A hybrid computational and experimental approach. Langmuir 2020, 36, 6668–6680. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Wu, N.M. Effects of small molecules on the dipalmitoyl lecithin liposomal bilayer: III. Phase transition in lipid bilayer. J. Membrane Biol. 1977, 34, 157–201. [Google Scholar] [CrossRef]
- Nishimoto, M.; Hata, T.; Goto, M.; Tamai, N.; Kaneshina, S.; Matsuki, H.; Ueda, I. Interaction modes of long-chain fatty acids in dipalmitoylphosphatidylcholine bilayer membrane: Contrast to mode of inhalation anesthetics. Chem. Phys. Lipids 2009, 158, 71–80. [Google Scholar] [CrossRef]
- Rowe, E.S.; Cutrera, T.A. Differential calorimetric study of ethanol interaction with distearoylphosphatidylcholine: Transition to the interdigitated phase. Biochemistry 1990, 29, 10398–10404. [Google Scholar] [CrossRef]
- Schullery, S.E.; Seder, T.A.; Weinstein, D.A.; Bryant, D.A. Differential thermal analysis of dipalmitoylphosphatidylcholine-fatty acid mixtures. Biochemistry 1981, 20, 6818–6824. [Google Scholar] [CrossRef]
- Ipsen, J.H.; Karlström, G.; Mouritsen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase equilibria in the phosphatidylcholine–cholesterol system. Biochim. Biophys. Acta 1987, 905, 162–172. [Google Scholar] [CrossRef]
- Vist, M.R.; Davis, J.H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry 1990, 29, 451–464. [Google Scholar] [CrossRef] [PubMed]
- McMullen, T.P.W.; Lewis, R.N.A.H.; McElhaney, R.N. Differential calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholine. Biochemistry 1993, 32, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Izumikawa, T.; Fukui, S.; Uemura, M.; Goto, M.; Matsuki, H.; Kaneshina, S. How does acyl chain length affect thermotropic phase behavior of saturated diacylphosphatidylcholines–cholesterol binary bilayers? Biochim. Biophys. Acta 2013, 1828, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Lois, M.; Crowe, L.M.; Reid, D.S.; Crowe, J.H. Is Trehalose Special for Preserving Dry Biomaterials? Biophys. J. 1996, 71, 2087–2093. [Google Scholar]
- Koster, K.L.; Leopold, A.C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef]
- Oliver, A.E.; Hincha, D.K.; Crowe, J.H. Looking beyond Sugars: The Role of Amphiphilic Solutes in Preventing Adventitious Reactions in Anhydrobiotes at Low Water Contents. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 515–525. [Google Scholar] [CrossRef]
- Anchordoguy, T.J.; Rudolph, A.S.; Carpenter, J.F.; Crowe, J.H. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 1987, 24, 324–331. [Google Scholar] [CrossRef]
- Crowe, J.H.; Whittam, M.A.; Chapman, D.; Crowe, R.M. Interactions of phospholipid monolayers with carbohydrates. Biochim. Biophys. Acta 1984, 769, 151–159. [Google Scholar] [CrossRef]
- Viera, L.I.; Alonso-Romanowski, S.; Borovyagin, V.; Feliz, M.R.; Disalvo, E.A. Properties of gel-phase lipid-trehalose bilayers upon rehydration. Biochim. Biophys. Acta 1993, 1145, 157–167. [Google Scholar] [CrossRef]
- Nagase, H.; Ueda, H.; Nakagaki, M. Effect of water on lamellar structure of DPPC/sugar systems. Biochim. Biophys. Acta 1997, 1328, 197–206. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, R.; Kundu, N.; Banik, D.; Sarkar, N. A comparative study of the influence of sugars sucrose, trehalose and maltose on the hydration and diffusion of DMPC lipid bilayer at complete hydration: Investigation of structural and spectroscopic aspect of lipid-sugar interaction. Langmuir 2016, 32, 5124–5134. [Google Scholar] [CrossRef] [PubMed]
- Westh, P. Glucose, sucrose and trehalose are partially excluded from the interface of hydrated DMPC bilayer. Phys. Chem. Chem. Phys. 2008, 10, 4110–4112. [Google Scholar] [CrossRef] [PubMed]
- Lenné, T.; Garvey, C.J.; Koster, K.L.; Bryant, G. Effects of sugars on lipid bilayers during dehydration—SAXS/WAXS measurements and quantitative model. J. Phys. Chem. B 2009, 113, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Lenné, T.; Garvey, C.J.; Koster, K.L.; Bryant, G. Kinetics of the lamellar-gel fluid transition in phosphatidylcholine membranes in the presence of sugars. Chem. Phys. Lipids 2010, 163, 236–242. [Google Scholar] [CrossRef]
- Kent, B.; Hunt, T.; Darwish, T.A.; Hauß, T.; Garvey, C.J.; Bryant, G. Localization of trehalose in partially hydrated DOPC bilayers: Insights into cryoprotective mechanisms. J. R. Soc. Interface 2014, 11, 20140069. [Google Scholar] [CrossRef]
- Anderson, H.D.; Wang, C.; Arleth, L.; Peters, G.H.; Westh, P. Reconciliation of opposing views on membrane-sugar interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, B.Z.; Lipka, G.; Sturtevant, J.M. Thermodynamics of phospholipid-sucrose interactions. Biophys. J. 1984, 46, 419–422. [Google Scholar] [CrossRef]
- Strauss, G.; Schurtenberger, P.; Hauser, H. The interaction of saccharides with lipid bilayer vesicles: Stabilization during freeze-thawing and freeze-drying. Biochim. Biophys. Acta 1986, 858, 169–180. [Google Scholar] [CrossRef]
- Fabrie, C.H.J.P.; De Kruijff, B.; De Gier, J. Protection by sugars against phase transition-induced leak in hydrated dimyristoylphosphatidylcholine liposomes. Biochim. Biophys. Acta 1990, 1024, 380–384. [Google Scholar] [CrossRef]
- Crowe, L.M.; Crowe, J.H. Solution effects on the thermotropic phase transition of unilamellar liposomes. Biochim. Biophys. Acta 1991, 1064, 267–274. [Google Scholar] [CrossRef]
- Morandi, M.I.; Sommer, M.; Kluzek, M.; Thalmann, F.; Schroder, A.P.; Marques, C.M. DPPC bilayers in solutions of high sucrose content. Biophys. J. 2018, 114, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, J.; Vaz, W.L.C.; Hallmann, D. An X-ray diffraction and differential scanning calorimetric study on the effect of sucrose on the properties of phosphatidylcholine bilayers. Biochim. Biophys. Acta 1985, 821, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.H.; Itami, Y.; Rokui, Y.; Katayama, T.; Izumori, K. D-Allose production from D-psicose using immobilised L-rhamnose isomerase. J. Ferment. Bioeng. 1998, 85, 539–541. [Google Scholar] [CrossRef]
- Takeshita, K.; Suga, A.; Takada, G.; Izumori, K. Mass production of D-psicose from D-fructose by a continuous bioreactor system using immobilized D-tagatose 3-epimerase. J. Biosci. Bioeng. 2000, 90, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Granström, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Yanai, S.; Kanasaki, A.; Tanaka, M.; Iida, T.; Ozawa, G.; Kunihiro, T.; Endo, S. Long-term D-allose administration favorably alters the intestinal environment in aged male mice. J. Appl. Glycosci. 2022, 69, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nakamura, T.; Toyoshima, T.; Shinomiya, A.; Tamiya, T.; Tokuda, M.; Keep, R.F.; Itano, T. The effects of D-allose on transient ischemic neuronal death and analysis of its mechanism. Brain Res. Bull. 2014, 109, 127–131. [Google Scholar] [CrossRef]
- Kimura, S.; Zhang, G.-X.; Nishiyama, A.; Nagai, Y.; Nakagawa, T.; Miyanaka, H.; Fujisawa, Y.; Miyatake, A.; Nagai, T.; Tokuda, M.; et al. D-allose, an all-cis aldo-hexose, suppresses development of salt-induced hypertension in Dahl rats. J. Hypertens. 2005, 23, 1887–1894. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Takata, M.; Kamitori, K.; Nonaka, M.; Dong, Y.; Sui, L.; Tokuda, M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int. J. Oncol. 2008, 32, 377–385. [Google Scholar] [CrossRef]
- Matsumoto, E.; Postrado, M.; Takahashi, H. Induction of the interdigitated gel phase of hydrated dipalmitoylphosphatidylcholine bilayers by the artificial sweetener sucralose. J. Phys. Chem. B 2024, 128, 9745–9755. [Google Scholar] [CrossRef]
- Kamaya, H.; Kaneshina, S.; Ueda, I. Partition equilibrium of inhalation anesthetics and alcohols between water and membranes of phospholipids with varying acyl chain lengths. Biochim. Biophys. Acta 1981, 646, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Okamura, E.; Nakahara, M. NMR Study Directly Determining Drug Delivery Sites in Phospholipid Bilayer Membranes. J. Phys. Chem. B 1999, 103, 3505–3509. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D. The interaction of small molecules with phospholipid membranes studied by 1H NOESY NMR under magic-angle spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipid 1991, 57, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Hincha, D.K. Specific interactions of tryptophan with phosphatidylcholine and digalactosyldiacylglycerol in pure and mixed bilayers in the dry and hydrated state. Chem. Phys. Lipid 2004, 132, 171–184. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, N.; Kamiya, M.; Kiriyama, N.; Goto, M.; Fukada, K.; Matsuki, H. Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine. Membranes 2024, 14, 258. https://doi.org/10.3390/membranes14120258
Tamai N, Kamiya M, Kiriyama N, Goto M, Fukada K, Matsuki H. Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine. Membranes. 2024; 14(12):258. https://doi.org/10.3390/membranes14120258
Chicago/Turabian StyleTamai, Nobutake, Mei Kamiya, Nono Kiriyama, Masaki Goto, Kazuhiro Fukada, and Hitoshi Matsuki. 2024. "Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine" Membranes 14, no. 12: 258. https://doi.org/10.3390/membranes14120258
APA StyleTamai, N., Kamiya, M., Kiriyama, N., Goto, M., Fukada, K., & Matsuki, H. (2024). Effect of Monosaccharides Including Rare Sugars on the Bilayer Phase Behavior of Dimyristoylphosphatidylcholine. Membranes, 14(12), 258. https://doi.org/10.3390/membranes14120258







