Separation of Chloride and Sulfate Ions from Desulfurization Wastewater Using Monovalent Anions Selective Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
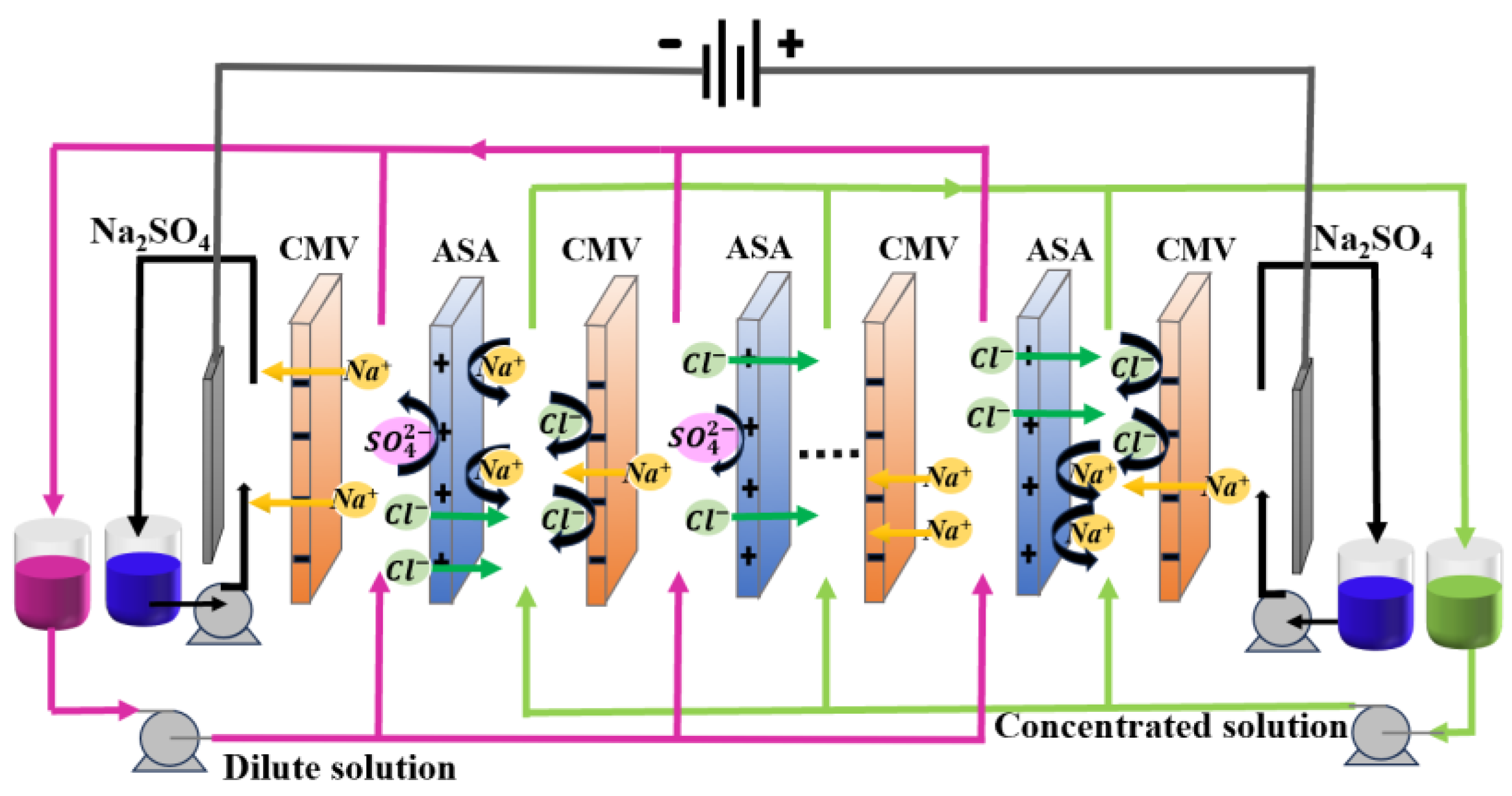
2.2. Experimemtal Setup and Procedure
2.3. Analytical Methods
2.4. Data Analysis
2.4.1. Monovalent Selectivity Coefficient
2.4.2. Removal Rate of Cl−
2.4.3. Ion Flux
2.4.4. Energy Consumption Per Unit of NaCl
2.4.5. Transport Number
3. Results and Discussion
3.1. LCD for Different Concentration of NaCl and Na2SO4
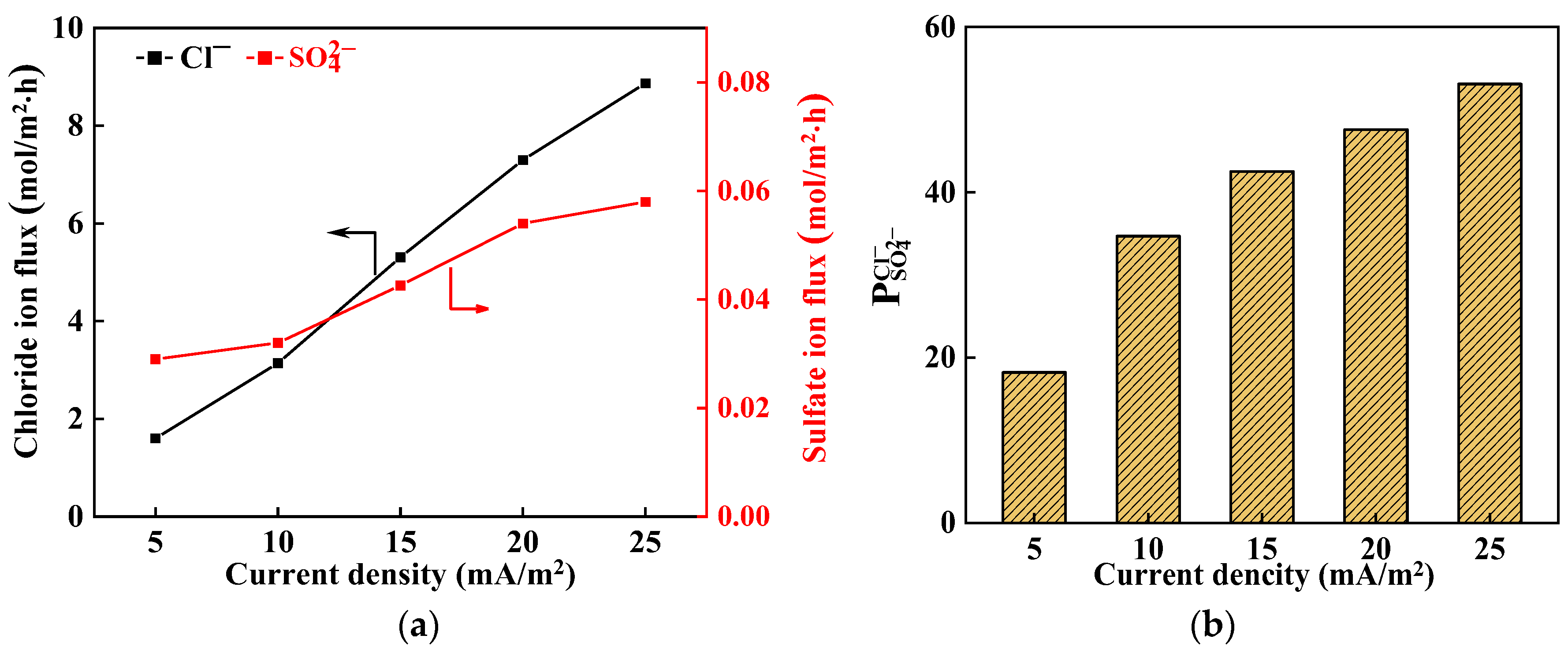
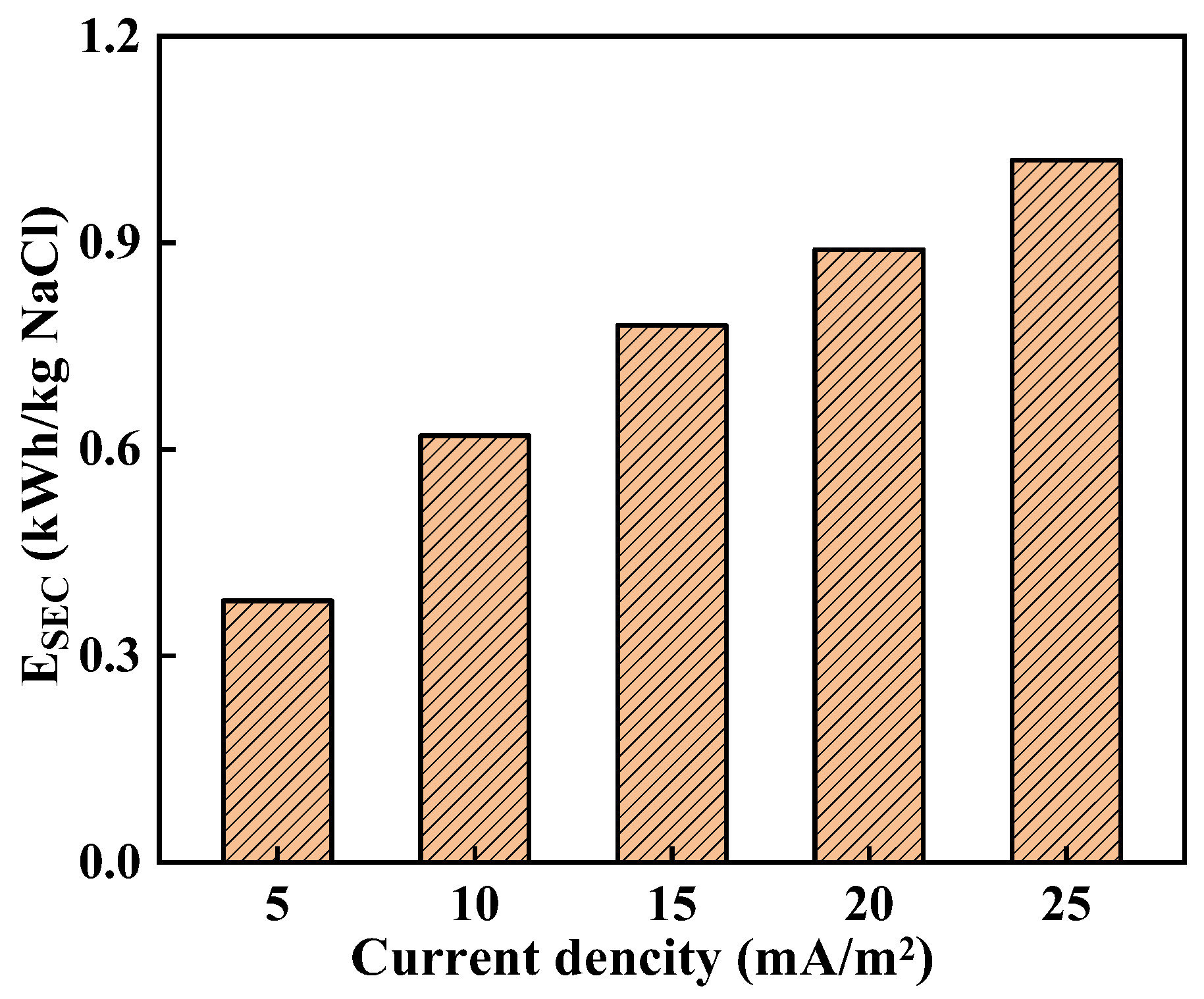
3.2. Effect of Current Density
3.3. Effect of NaCl Concentration
3.4. Effect of Na2SO4 Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Zou, D.; Huang, X.; Lu, W. Coal-Fired Boiler Flue Gas Desulfurization System Based on Slurry Waste Heat Recovery in Severe Cold Areas. Membranes 2021, 12, 47. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Córdoba, P. Status of Flue Gas Desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Lyu, J.; Zhang, H.; Yue, G.; Ni, W. An overview of the development history and technical progress of China’s coal-fired power industry. Front. Energy 2019, 13, 417–426. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, N.; Feng, Y.; Liu, F.; Cai, C.; Che, G.; Zhang, Y.; Wu, H.; Yang, L. Experimental study on the treatment of desulfurization wastewater from coal-fired power plant by spray evaporation. Environ. Sci. Pollut. Res. 2022, 29, 90791–90802. [Google Scholar] [CrossRef]
- Fang, P.; Tang, Z.J.; Chen, X.B.; Huang, J.H.; Tang, Z.X.; Cen, C.P. Chloride Ion Removal from the Wet Flue Gas Desulfurization and Denitrification Wastewater Using Friedel’s Salt Precipitation Method. J. Chem. 2018, 2018, 5461060. [Google Scholar] [CrossRef]
- Brogren, C.; Karlsson, H.T. The impact of the electrical potential gradient on limestone dissolution under wet flue gas desulfurization condition. Chem. Eng. Sci. 1997, 52, 3101–3106. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, W.; Liu, J.; Ye, W.; Lin, J.; Xie, J.; Huang, X.; Gao, S.; Xie, J.; Liu, S.; et al. An integrated process of chemical precipitation and sulfate reduction for treatment of flue gas desulphurization wastewater from coal-fired power plant. J. Clean. Prod. 2019, 228, 63–72. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wei, Y.; Peng, L.; Jiang, B.; Li, G.; Yu, G.; Du, C. Wet flue gas desulfurization wastewater treatment with reclaimed water treatment plant sludge: A case study. Water Sci. Technol. 2018, 78, 2392–2403. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J. Treatment of flue gas desulfurization wastewater with near-zero liquid discharge by nanofiltration-membrane distillation process. Sep. Sci. Technol. 2018, 53, 146–153. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, L.; Yang, Z.; Cheng, X.; Pu, G.; Ran, J. The effect of solid particles on the evaporation and crystallization processes of the desulfurization wastewater droplet. Appl. Therm. Eng. 2018, 134, 141–151. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Zhao, N.; Feng, Y.; Liu, F.; Cai, C.; Che, G.; Yang, L. Experimental research and engineering application on the treatment of desulfurization wastewater from coal-fired power plants by spray evaporation—ScienceDirect. J. Water Process Eng. 2021, 40, 101960. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, C.; Wang, S.; Yang, D.; Guo, B.; An, X.; Yu, A. Simulation of desulphurization wastewater evaporation through flue gas. Powder Technol. 2020, 361, 462–472. [Google Scholar] [CrossRef]
- Wales, M.D.; Gebremichael, E.; Wang, X.; Perea, E.; Jayaweera, P.; Jayaweera, I. Flue Gas Desulfurization (FGD) Wastewater Treatment Using Polybenzimidazole (PBI) Hollow Fiber (HF). Membranes 2021, 11, 430. [Google Scholar] [CrossRef]
- Conidi, C.; Macedonio, F.; Ali, A.; Cassano, A.; Criscuoli, A.; Argurio, P.; Drioli, E. Treatment of Flue Gas Desulfurization Wastewater by an Integrated Membrane-Based Process for Approaching Zero Liquid Discharg. Membranes 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.; Hong, S. Treatment of industrial wastewater produced by desulfurization process in a coal-fired power plant via FO-MD hybrid process. Chemosphere 2018, 210, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; He, W.; Wei, L.; Ma, J.; Li, C. The performance and microbial communities of biodegradation-electron transfer with sulfur metabolism integrated process for flue gas desulfurization wastewater treatment. Bioprocess Biosyst. Eng. 2017, 40, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.B.; Grol, E.; Mauter, M.S. Fundamental challenges and engineering opportunities in flue gas desulfurization wastewater treatment at coal fired power plants. Environ. Sci. Water Res. Technol. 2018, 4, 909–925. [Google Scholar] [CrossRef]
- You, L.; Han, Q.; Yin, J.; Zhu, Y. Discussion on the technical route of water pollution prevention and comprehensive utilization in thermal power plant. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Lee, J.; Kang, M. Heterogeneous Anion-Exchange Membranes with Enhanced Ion Conductivity for Continuous Electrodeionization. Separations 2023, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Cui, L.; Li, G.; Li, Y.; Yang, B.; Zhang, L.; Dong, Y.; Ma, C. Electrolysis-electrodialysis process removing chloride ion in wet flue gas desulfurization wastewater(DW): Influencing factors and energy consumption analysis. Chem. Eng. Res. Des. 2017, 123, 240–247. [Google Scholar] [CrossRef]
- Yao, J.; Wen, D.; Shen, J.; Wang, J. Zero discharge process for dyeing wastewater treatment. J. Water Process Eng. 2016, 11, 98–103. [Google Scholar] [CrossRef]
- An, W.; Zhao, J.; Lu, J.; Han, Y.; Li, D. Zero-liquid discharge technologies for desulfurization wastewater: A review. J. Environ. Manag. 2022, 321, 115953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Meng, F.; Wang, C.; Ren, P.; Zou, Q.; Luan, J. Near-zero liquid discharge of desulfurization wastewater by electrodialysis-reverse osmosis hybrid system. J. Water Process Eng. 2021, 40, 101962. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T. Comparative study on the regeneration of flue-gas desulfurizing agents by using conventional electrodialysis (ED) and bipolar membrane electrodialysis (BMED). Environ. Sci. Technol. 2006, 40, 5527–5531. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.; Kang, M. Surface-Modified Pore-Filled Anion-Exchange Membranes for Efficient Energy Harvesting via Reverse Electrodialysis. Membranes 2023, 13, 894. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566. [Google Scholar] [CrossRef]
- Casas, S.; Bonet, N.; Aladjem, C.; Cortina, J.L.; Larrotcha, E.; Cremades, L.V. Modelling sodium chloride concentration from seawater reverse osmosis brine by electrodialysis: Preliminary results. Solvent Extr. Ion Exch. 2011, 29, 488–508. [Google Scholar] [CrossRef]
- Zhou, H.; Ju, P.; Hu, S.; Shi, L.; Yuan, W.; Chen, D.; Wang, Y.; Shi, S. Separation of Hydrochloric Acid and Oxalic Acid from Rare Earth Oxalic Acid Precipitation Mother Liquor by Electrodialysis. Membranes 2023, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, S.; Guo, Z. Selective electrodialysis process for the separation of potassium: Transmembrane transport of ions in multicomponent solution systems. Separataion Purif. Technol. 2022, 300, 121926. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- Sharma, P.P.; Mohammed, S.; Aburabie, J.; Hashaikeh, R. Valorization of Seawater Reverse Osmosis Brine by Monovalent Ion-Selective Membranes through Electrodialysis. Membranes 2023, 13, 562. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Song, X.; Yu, J. Separation of mono- and di-valent ions from seawater reverse osmosis brine using selective electrodialysis. Environ. Sci. Pollut. Res. 2021, 28, 18754–18767. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, D.; Zhu, D.; Xu, J.; Jiang, H.; Geng, W.; Wei, W.; Lian, Z. Separation of fluoride and chloride ions from ammonia-based flue gas desulfurization slurry using a two-stage electrodialysis. Chem. Eng. Res. Des. 2019, 147, 73–82. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, C.; Li, X.; Xu, S.; Liu, S.; Zhang, Y.; Weng, W.; Gao, X. Evaporation and concentration of desulfurization wastewater with waste heat from coal-fired power plants. Environ. Sci. Pollut. Res. 2019, 26, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, L.; Chen, H.; Mao, J.; Chen, H.; Ma, X.; Yang, L. Study on the evaporation performance of concentrated desulfurization wastewater and its products analysis. J. Water Process Eng. 2024, 58, 104862. [Google Scholar] [CrossRef]
- Nie, X.; Sun, S.; Song, X.; Yu, J. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J Membr Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, Z.; Liu, J.; Zhao, Y.; Wang, S.; Yuan, J. Development of recovering lithium from brines by selective-electrodialysis: Effect of coexisting cations on the migration of lithium. J. Membr. Sci. 2018, 548, 408–420. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Lee, H.; Sarfert, F.; Strathmann, H.; Moon, S. Designing of an electrodialysis desalination plant. Desalination 2002, 142, 267–286. [Google Scholar] [CrossRef]
- Fan, H.; Yip, N. Elucidating conductivity-permselectivity tradeoffs in electrodialysis and reverse electrodialysis by structure-property analysis of ion-exchange membranes. J. Membr. Sci. 2019, 573, 668–681. [Google Scholar] [CrossRef]
- Golubenko, D.; Yaroslavtsev, A. Effect of current density, concentration of ternary electrolyte and type of cations on the monovalent ion selectivity of surface-sulfonated graft anion-exchange membranes: Modelling and experiment. J. Membr. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical modeling of monovalent permselectivity of a bilayer ion-exchange membrane as a function of current density. Int. J. Mol. Sci. 2022, 23, 4711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Bruggen, B. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]




| Characteristic | CMV | ASA | |
|---|---|---|---|
| Thickness (μm) | 120 | 120 | |
| Counter ion | Na+ | Cl− | |
| Burst strength (MPa) | 0.16 | 0.14 | |
| Exchange capacity (meq g−1 dry) | 2.01 | 2.0–2.1 | |
| Resistance (Ω cm2) | 0.5 M NaCl | 3 | 3.7 |
| 0.5 M Na2SO4 | 13 | ||
| Current Density (mA/cm2) | 5 | 10 | 15 | 20 | 25 |
|---|---|---|---|---|---|
| t (Cl−) | 0.9651 | 0.9800 | 0.9842 | 0.9854 | 0.9871 |
| t (SO42−) | 0.0349 | 0.0200 | 0.0158 | 0.0146 | 0.0129 |
| NaCl Concentration (g/L) | 15 | 25 | 40 | 66 |
|---|---|---|---|---|
| t(Cl−) | 0.9861 | 0.9916 | 0.9941 | 0.9956 |
| t(SO42−) | 0.0139 | 0.0084 | 0.0059 | 0.0044 |
| Na2SO4 Concentration (g/L) | 10 | 20 | 30 | 40 |
|---|---|---|---|---|
| t(Cl−) | 0.9909 | 0.9835 | 0.9754 | 0.9721 |
| t(SO42−) | 0.0091 | 0.0165 | 0.0246 | 0.0279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Yue, D.; Hou, T.; Xiao, F.; Wang, Z.; Cai, W. Separation of Chloride and Sulfate Ions from Desulfurization Wastewater Using Monovalent Anions Selective Electrodialysis. Membranes 2024, 14, 73. https://doi.org/10.3390/membranes14040073
Tian X, Yue D, Hou T, Xiao F, Wang Z, Cai W. Separation of Chloride and Sulfate Ions from Desulfurization Wastewater Using Monovalent Anions Selective Electrodialysis. Membranes. 2024; 14(4):73. https://doi.org/10.3390/membranes14040073
Chicago/Turabian StyleTian, Xufeng, Dongbei Yue, Tao Hou, Fuyuan Xiao, Zhiping Wang, and Weibin Cai. 2024. "Separation of Chloride and Sulfate Ions from Desulfurization Wastewater Using Monovalent Anions Selective Electrodialysis" Membranes 14, no. 4: 73. https://doi.org/10.3390/membranes14040073
APA StyleTian, X., Yue, D., Hou, T., Xiao, F., Wang, Z., & Cai, W. (2024). Separation of Chloride and Sulfate Ions from Desulfurization Wastewater Using Monovalent Anions Selective Electrodialysis. Membranes, 14(4), 73. https://doi.org/10.3390/membranes14040073






