Hydrophilic Modification of Dialysis Membranes Sustains Middle Molecule Removal and Filtration Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigated Dialyzers
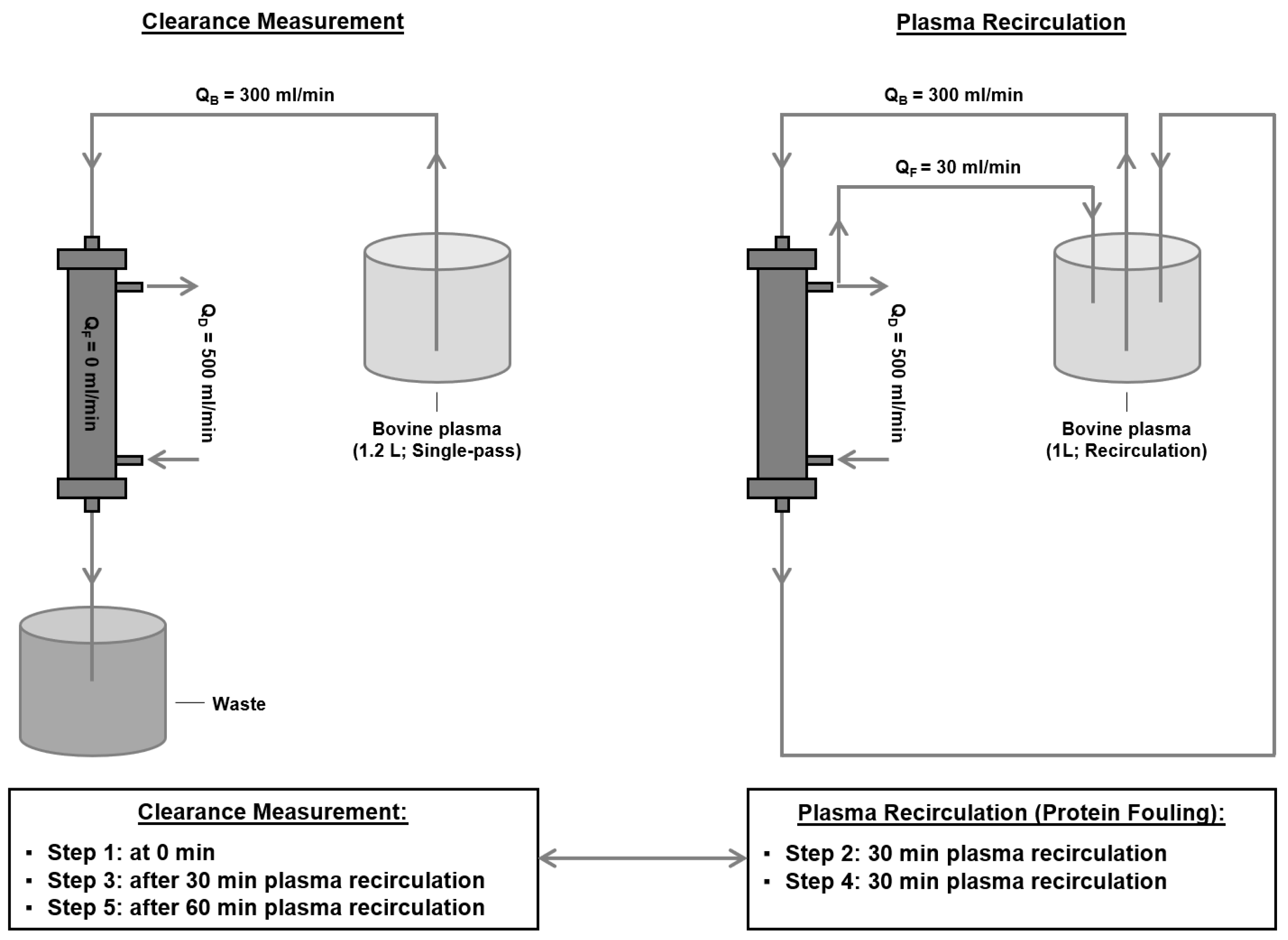
2.2. Determination of Middle Molecule Clearance after Protein Adsorption
- (1)
- Determination of β2-microglobulin clearance:
- (2)
- Induction of protein adsorption to the membrane:
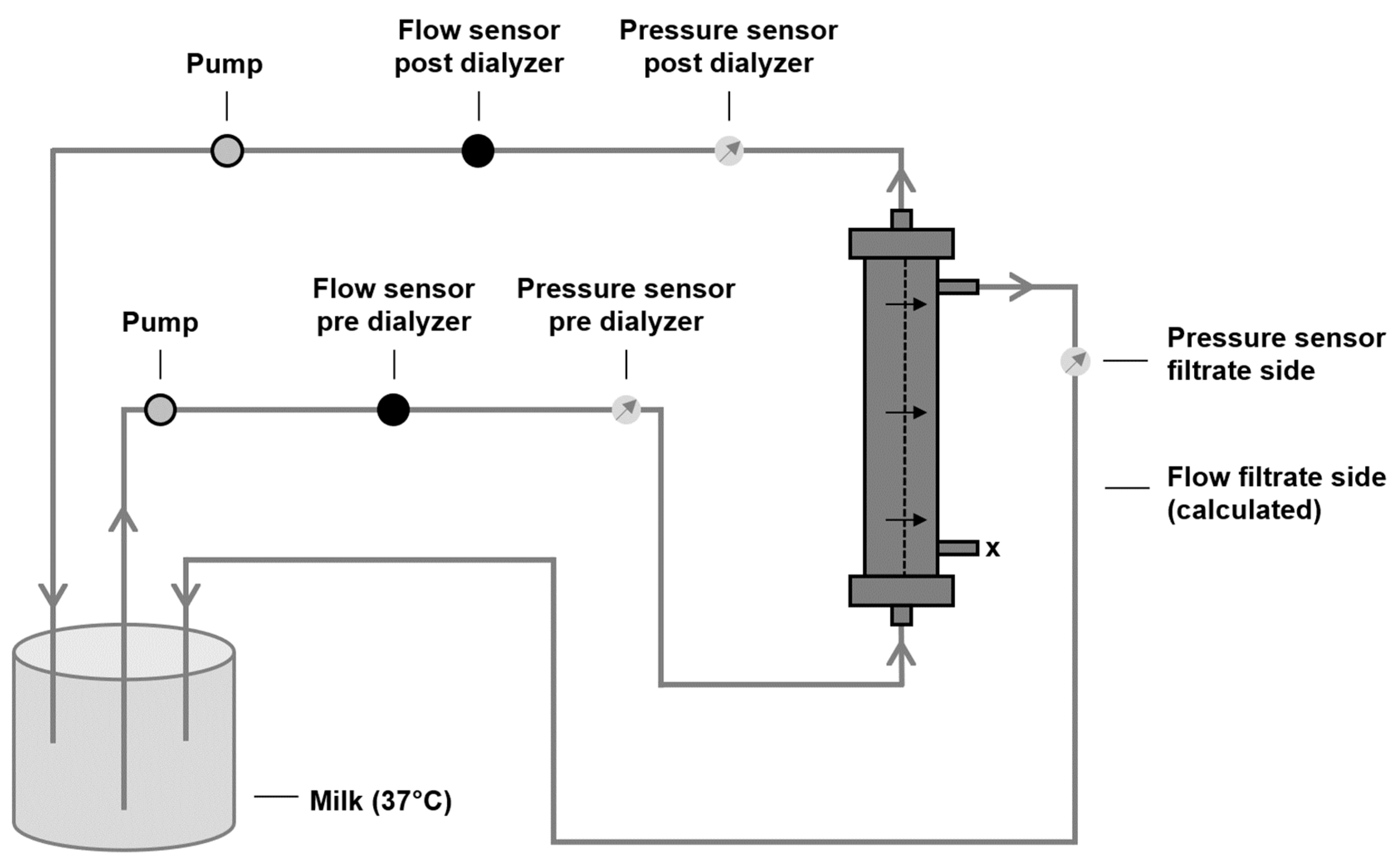
2.3. Characterization of Filtration Performance after Protein Adsorption
2.4. Statistics
3. Results
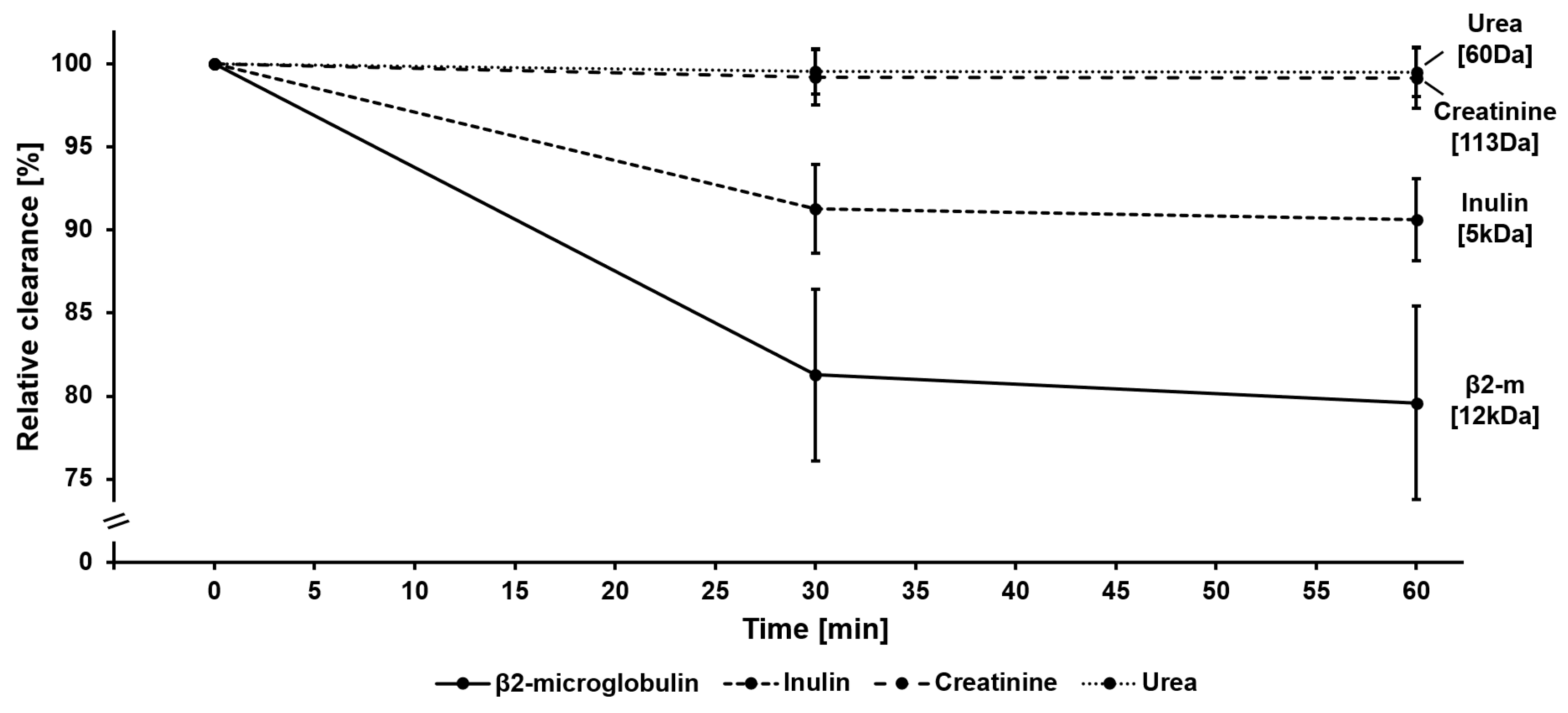
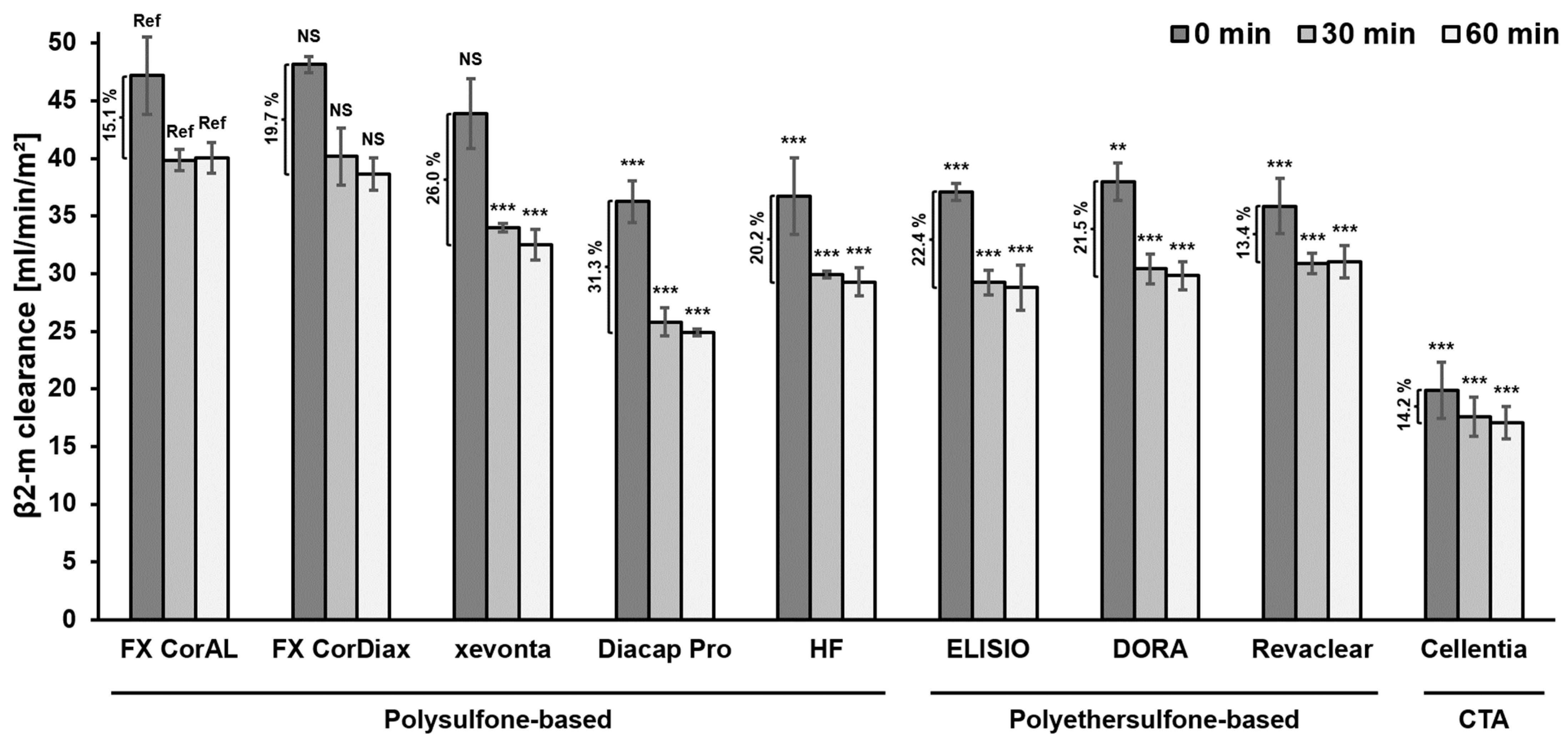
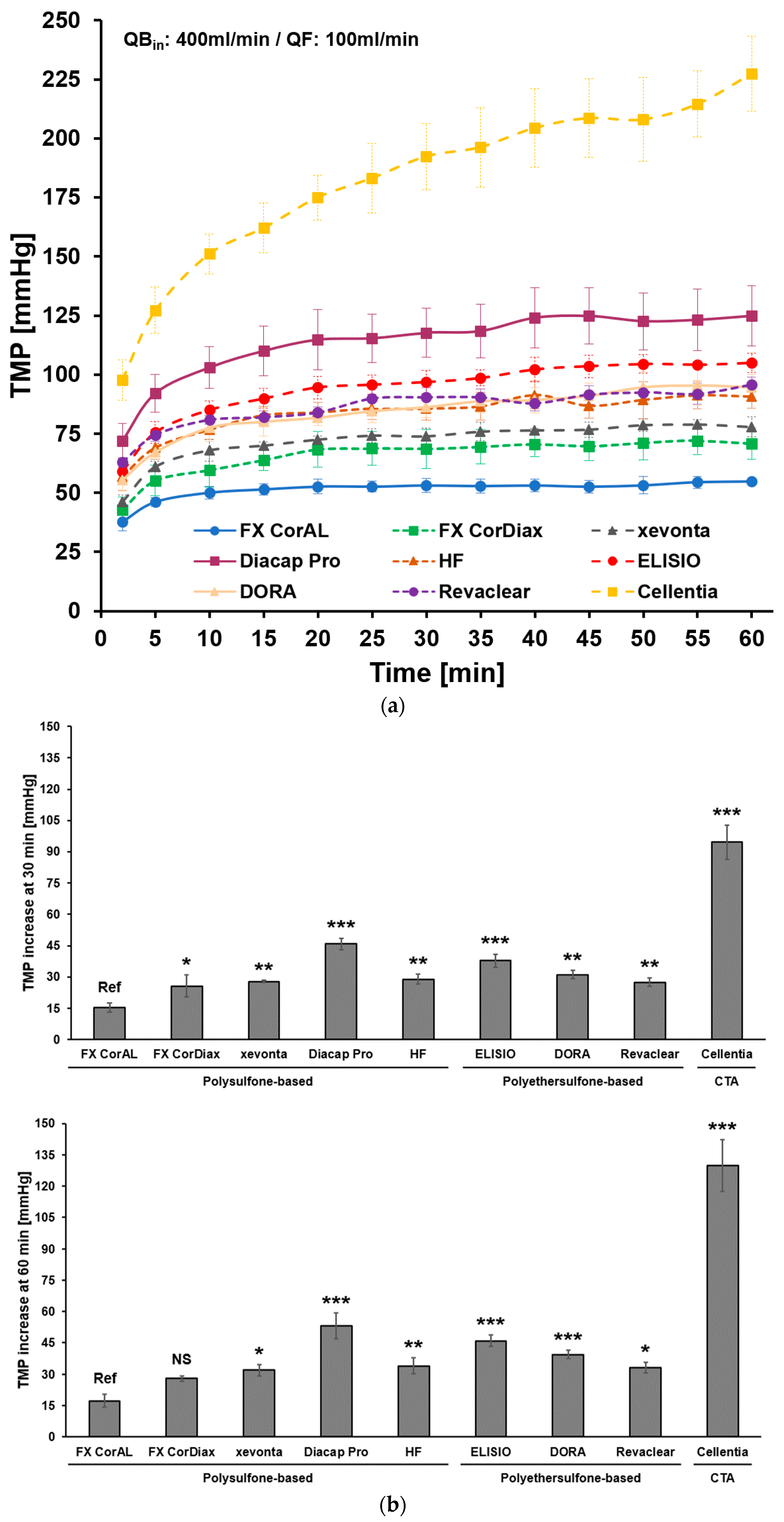
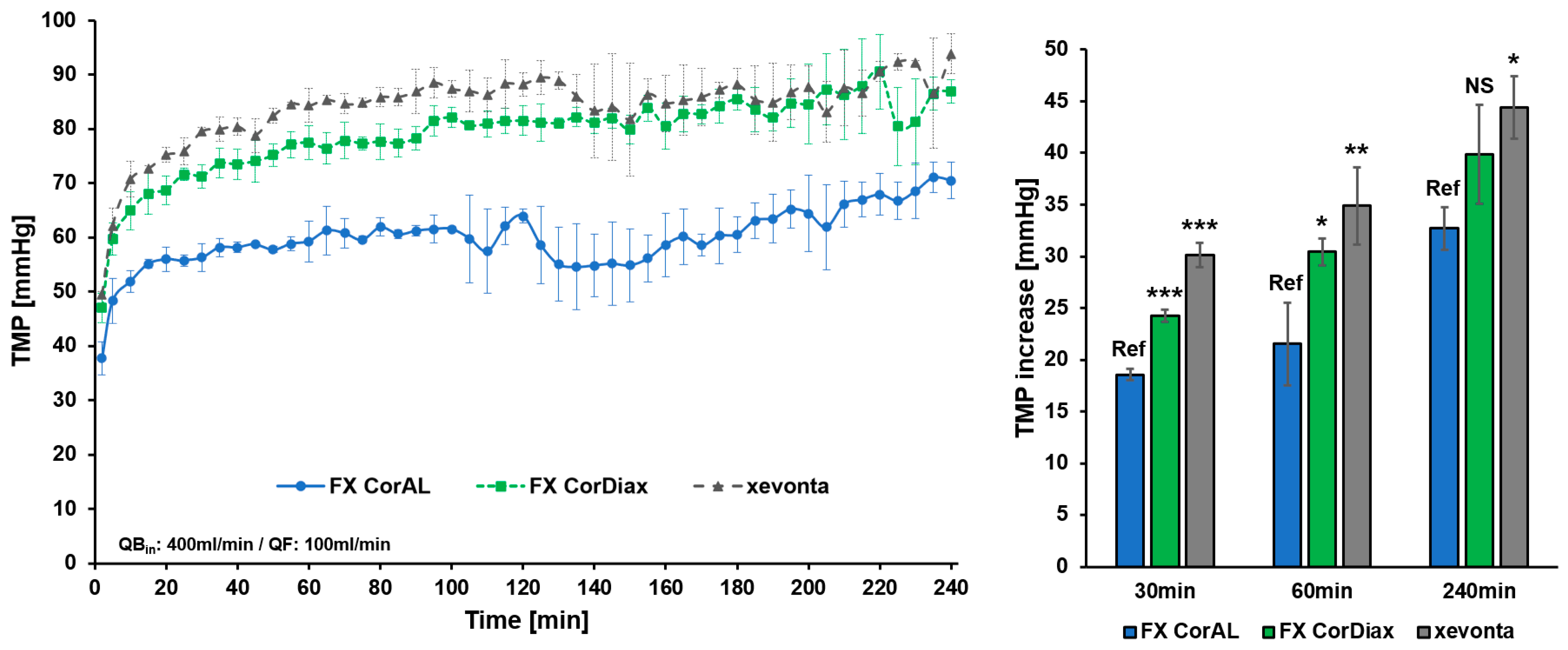
3.1. Impact of Protein Adsorption on Middle Molecule Clearance
3.2. Impact of Protein Adsorption on Filtration Performance
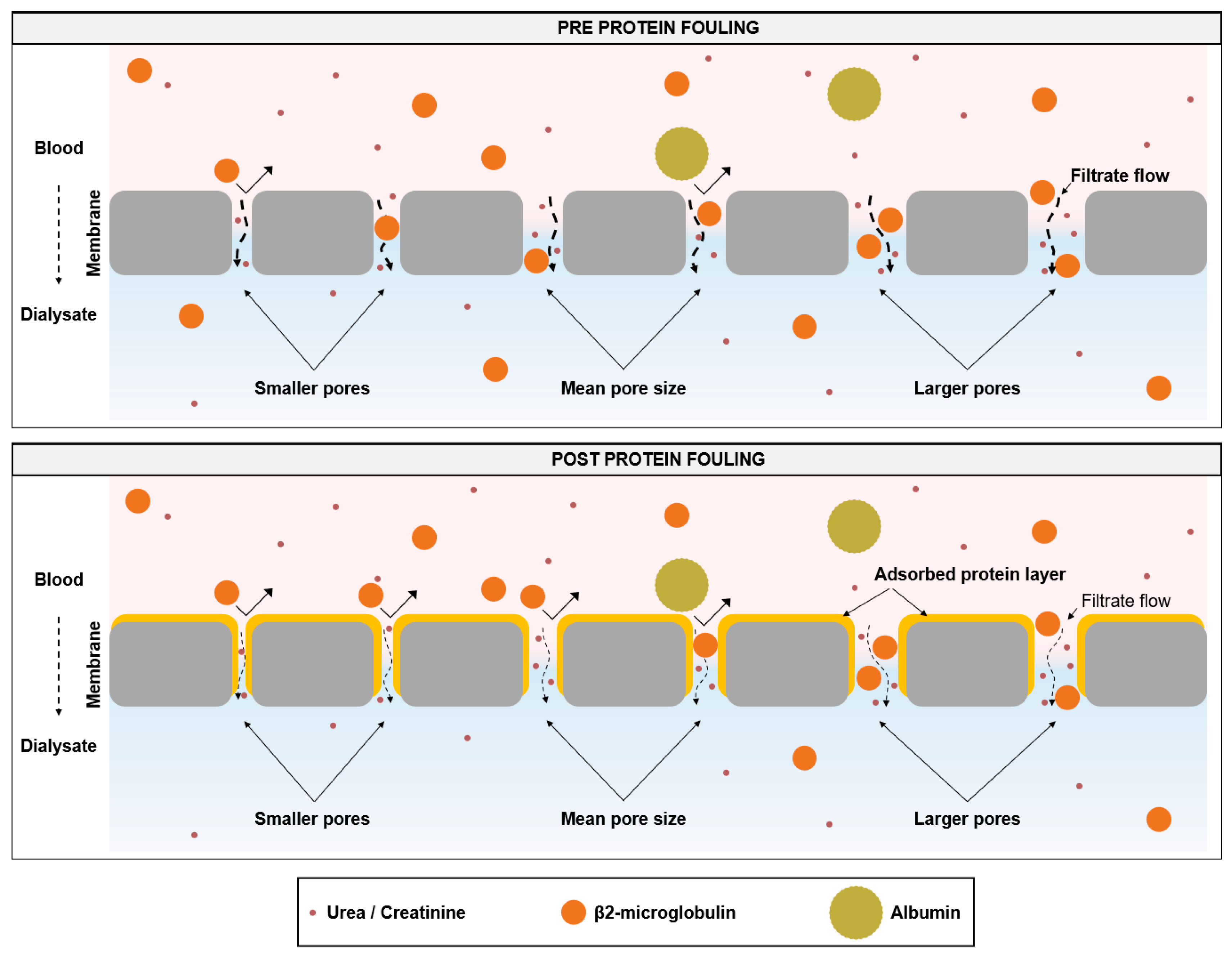
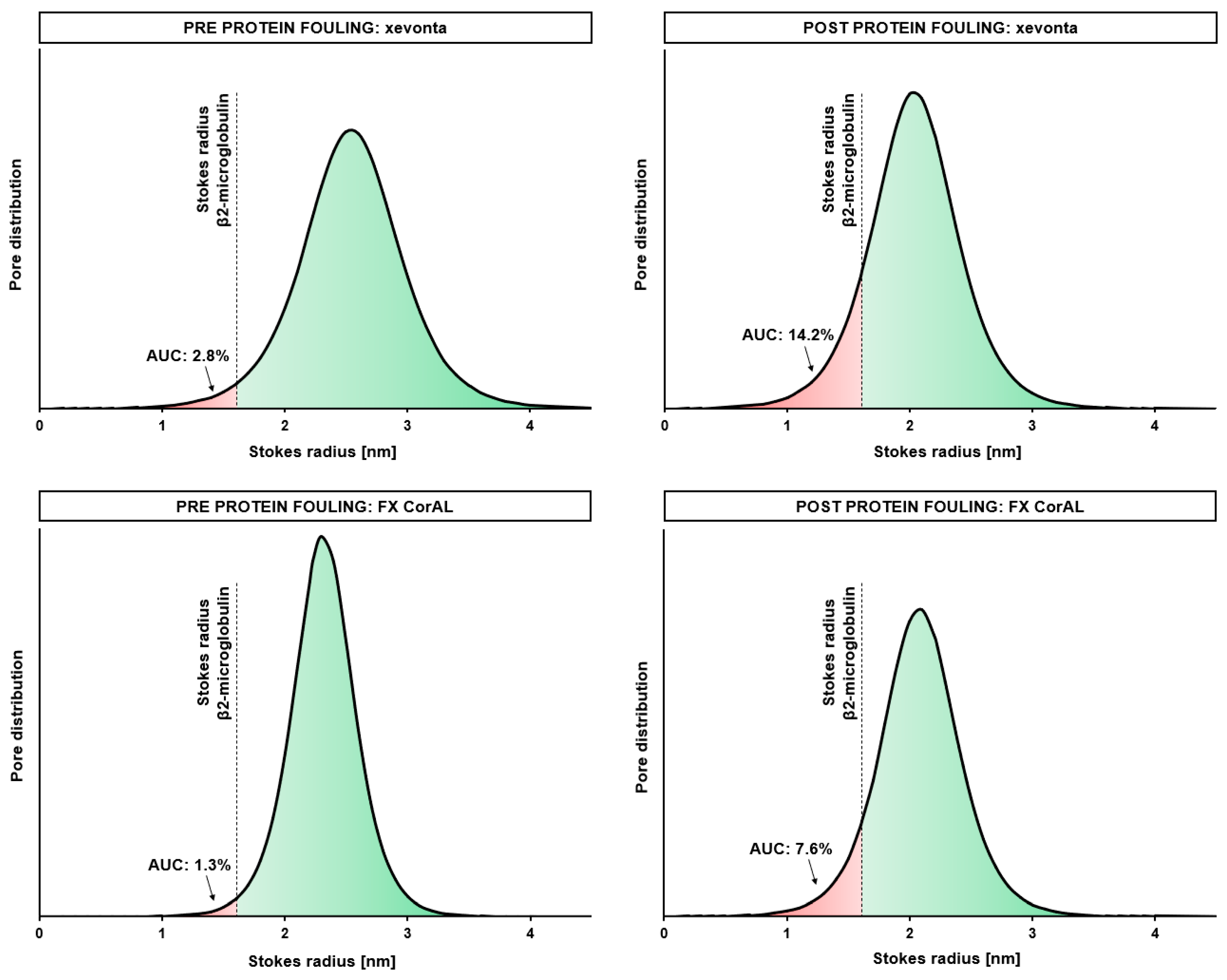
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canaud, B.; Blankestijn, P.J.; Grooteman, M.P.C.; Davenport, A. Why and How High Volume Hemodiafiltration May Reduce Cardiovascular Mortality in Stage 5 Chronic Kidney Disease Dialysis Patients? A Comprehensive Literature Review on Mechanisms Involved. Semin. Dial. 2022, 35, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An Update on Uremic Toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Kaizu, Y.; Ohkawa, S.; Odamaki, M.; Ikegaya, N.; Hibi, I.; Miyaji, K.; Kumagai, H. Association between Inflammatory Mediators and Muscle Mass in Long-Term Hemodialysis Patients. Am. J. Kidney Dis. 2003, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T.; Kida, A.; Furuta, M.; Nanami, M.; Otaki, Y.; Hasuike, Y.; Nonoguchi, H.; Nakanishi, T. The Impact of Β2-Microglobulin Clearance on the Risk Factors of Cardiovascular Disease in Hemodialysis Patients. ASAIO J. 2010, 56, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Okuno, S.; Ishimura, E.; Kohno, K.; Fujino-Katoh, Y.; Maeno, Y.; Yamakawa, T.; Inaba, M.; Nishizawa, Y. Serum 2-Microglobulin Level Is a Significant Predictor of Mortality in Maintenance Haemodialysis Patients. Nephrol. Dial. Transpl. 2008, 24, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum β-2 Microglobulin Levels Predict Mortality in Dialysis Patients: Results of the HEMO Study. J. Am. Soc. Nephrol. 2006, 17, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Lornoy, W.; Becaus, I.; Billiouw, J.-M.; Sierens, L.; Van Malderen, P. Remarkable Removal of Beta-2-Microglobulin by On-Line Hemodiafiltration. Am. J. Nephrol. 1998, 18, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Wizemann, V.; Külz, M.; Techert, F.; Nederlof, B. Efficacy of Haemodiafiltration. Nephrol. Dial. Transpl. 2001, 16, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Zawada, A.M.; Theis, L.; Braun, J.; Ottillinger, B.; Kopperschmidt, P.; Gagel, A.; Kotanko, P.; Stauss-Grabo, M.; Kennedy, J.P.; et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering 2023, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Lang, T.; Ottillinger, B.; Kircelli, F.; Stauss-Grabo, M.; Kennedy, J.P. Impact of Hydrophilic Modification of Synthetic Dialysis Membranes on Hemocompatibility and Performance. Membranes 2022, 12, 932. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.C.; Sakurai, K. Dialysis Membranes—Physicochemical Structures and Features. In Updates in Hemodialysis; Suzuki, H., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Bowry, S.K. Dialysis Membranes Today. Int. J. Artif. Organs 2002, 25, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Bowry, S.K.; Chazot, C. The Scientific Principles and Technological Determinants of Haemodialysis Membranes. Clin. Kidney J. 2021, 14, i5–i16. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Clark, W.R. Haemodialysis Membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B. Recent Advances in Dialysis Membranes. Curr. Opin. Nephrol. Hypertens 2021, 30, 613–622. [Google Scholar] [CrossRef]
- Cancilla, N.; Gurreri, L.; Marotta, G.; Ciofalo, M.; Cipollina, A.; Tamburini, A.; Micale, G. Performance Comparison of Alternative Hollow-Fiber Modules for Hemodialysis by Means of a CFD-Based Model. Membranes 2022, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Melchior, P.; Schall, C.; Erlenkötter, A.; Lang, T.; Keller, T.; Stauss-Grabo, M.; Kennedy, J.P. Time-resolving Characterization of Molecular Weight Retention Changes among Three Synthetic High-flux Dialyzers. Artif. Organs 2022, 46, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Raharjo, Y.; Zainol Abidin, M.N.; Ismail, A.F.; Fahmi, M.Z.; Saiful; Elma, M.; Santoso, D.; Haula’, H.; Habibi, A.R. Dialysis Membranes for Acute Kidney Injury. Membranes 2022, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Kohlová, M.; Amorim, C.G.; Araújo, A.; Santos-Silva, A.; Solich, P.; Montenegro, M.C.B.S.M. The Biocompatibility and Bioactivity of Hemodialysis Membranes: Their Impact in End-Stage Renal Disease. J. Artif. Organs 2019, 22, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Vienken, J. Polymers in Nephrology Characteristics and Needs. Int. J. Artif. Organs 2002, 25, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Goh, K.; Lai, G.S.; Zhao, Y.; Torres, J.; Wang, R. Unraveling the Role of Support Membrane Chemistry and Pore Properties on the Formation of Thin-Film Composite Polyamide Membranes. J. Membr. Sci. 2021, 640, 119805. [Google Scholar] [CrossRef]
- Röckel, A.; Hertel, J.; Fiegel, P.; Abdelhamid, S.; Panitz, N.; Walb, D. Permeability and Secondary Membrane Formation of a High Flux Polysulfone Hemofilter. Kidney Int. 1986, 30, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Hadidi, M.; Motevalian, S.P.; Sunohara, T.; Zydney, A.L. Effects of Plasma Proteins on the Transport and Surface Characteristics of Polysulfone/Polyethersulfone and Asymmetric Cellulose Triacetate High Flux Dialyzers. Artif. Organs 2018, 42, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Langsdorf, L.J.; Zydney, A.L. Effect of Blood Contact on the Transport Properties of Hemodialysis Membranes: A Two-Layer Membrane Model. Blood Purif. 1994, 12, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Morti, S.M.; Zydney, A.L. Protein-Membrane Interactions during Hemodialysis: Effects on Solute Transport. ASAIO J. 1998, 44, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Boschetti-de-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO Membranes: Enhanced Selectivity in High-Flux Class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, T.; Ito, H.; Yamashita, A.C. Effect of Membrane Surface Area on Solute Removal Performance of Dialyzers with Fouling. Membranes 2022, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Hayama, M.; Yamamoto, K.; Kohori, F.; Uesaka, T.; Ueno, Y.; Sugaya, H.; Itagaki, I.; Sakai, K. Nanoscopic Behavior of Polyvinylpyrrolidone Particles on Polysulfone/Polyvinylpyrrolidone Film. Biomaterials 2004, 25, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Song, H.; Wang, J.; Xue, L. Polysulfone Hemodiafiltration Membranes with Enhanced Anti-Fouling and Hemocompatibility Modified by Poly(Vinyl Pyrrolidone) via in Situ Cross-Linked Polymerization. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly(N-Vinyl Pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Zhao, W.; Li, J.; Su, B.; Sun, S.; Zhao, C. Biocompatibility of Modified Polyethersulfone Membranes by Blending an Amphiphilic Triblock Co-Polymer of Poly(Vinyl Pyrrolidone)-b-Poly(Methyl Methacrylate)-b-Poly(Vinyl Pyrrolidone). Acta Biomater. 2011, 7, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Hayama, M.; Yamamoto, K.; Kohori, F.; Sakai, K. How Polysulfone Dialysis Membranes Containing Polyvinylpyrrolidone Achieve Excellent Biocompatibility? J. Membr. Sci. 2004, 234, 41–49. [Google Scholar] [CrossRef]
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of Hydrophilicity and Blood Compatibility on Polyethersulfone Membrane by Adding Polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Zawada, A.M.; Melchior, P.; Erlenkötter, A.; Delinski, D.; Stauss-Grabo, M.; Kennedy, J.P. Polyvinylpyrrolidone in Hemodialysis Membranes: Impact on Platelet Loss during Hemodialysis. Hemodial. Int. 2021, 25, 498–506. [Google Scholar] [CrossRef]
- Ehlerding, G.; Ries, W.; Kempkes-Koch, M.; Ziegler, E.; Erlenkoetter, A.; Zawada, A.M.; Kennedy, J.; Ottillinger, B.; Stauss-Grabo, M.; Lang, T. Randomized Comparison of Three High-Flux Dialyzers during High Volume Online Hemodiafiltration—The comPERFORM Study. Clin. Kidney J. 2022, 15, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement Activation by Dialysis Membranes and Its Association with Secondary Membrane Formation and Surface Charge. Artif. Organs 2021, 45, 770–778. [Google Scholar] [CrossRef]
- Ehlerding, G.; Erlenkötter, A.; Gauly, A.; Griesshaber, B.; Kennedy, J.; Rauber, L.; Ries, W.; Schmidt-Gürtler, H.; Stauss-Grabo, M.; Wagner, S.; et al. Performance and Hemocompatibility of a Novel Polysulfone Dialyzer: A Randomized Controlled Trial. Kidney360 2021, 2, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Ehlerding, G.; Ronova, P.; Krizsán, M.; Griesshaber, B.; Erlenkötter, A.; Nitschel, R.; Zawada, A.; Ottillinger, B.; Braun, J.; Stauss-Grabo, M. #3944 Comparison of clinical performance and hemocompatibility of dialyzers applied during post-dilution online hemodiafiltration (HDF)—eMPORA III study. Nephrol. Dial. Transpl. 2023, 38, gfad063c_3944. [Google Scholar] [CrossRef]
- Kempkes-Koch, M.; Stauss-Grabo, M.; Erlenkötter, A.; Rauber, L.; Kennedy, J.; Gauly, A.; Schmidt-Gürtler, H. MO387 Clinical performance, hemocompatibility and safety of a new dialyzer with a modified polysulfone membrane. Nephrol. Dial. Transpl. 2021, 36, gfab082.0041. [Google Scholar] [CrossRef]
- Ficheux, A.; Kerr, P.G.; Brunet, P.; Argiles, A. The Ultrafiltration Coefficient of a Dialyser (KUF) Is Not a Fixed Value, and It Follows a Parabolic Function: The New Concept of KUF Max. Nephrol. Dial. Transpl. 2011, 26, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of Haemodialysis Outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, D.A.; Bragg-Gresham, J.L.; Koenig, K.G.; Wolfe, R.A.; Akiba, T.; Andreucci, V.E.; Saito, A.; Rayner, H.C.; Kurokawa, K.; Port, F.K.; et al. Association of Comorbid Conditions and Mortality in Hemodialysis Patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J. Am. Soc. Nephrol. 2003, 14, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.M.; Akizawa, T.; Jager, K.J.; Kerr, P.G.; Saran, R.; Pisoni, R.L. Factors Affecting Outcomes in Patients Reaching End-Stage Kidney Disease Worldwide: Differences in Access to Renal Replacement Therapy, Modality Use, and Haemodialysis Practices. Lancet 2016, 388, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wachterman, M.W.; O’Hare, A.M.; Rahman, O.-K.; Lorenz, K.A.; Marcantonio, E.R.; Alicante, G.K.; Kelley, A.S. One-Year Mortality after Dialysis Initiation among Older Adults. JAMA Intern. Med. 2019, 179, 987. [Google Scholar] [CrossRef] [PubMed]
- de Jager, D.J. Cardiovascular and Noncardiovascular Mortality among Patients Starting Dialysis. JAMA 2009, 302, 1782. [Google Scholar] [CrossRef]
- Villar, E.; Remontet, L.; Labeeuw, M.; Ecochard, R. Effect of Age, Gender, and Diabetes on Excess Death in End-Stage Renal Failure. J. Am. Soc. Nephrol. 2007, 18, 2125–2134. [Google Scholar] [CrossRef]
- Bloembergen, W.E.; Port, F.K.; Mauger, E.A.; Wolfe, R.A. Causes of Death in Dialysis Patients: Racial and Gender Differences. J. Am. Soc. Nephrol. 1994, 5, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Grooteman, M.P.C.; Van Den Dorpel, M.A.; Bots, M.L.; Penne, E.L.; Van Der Weerd, N.C.; Mazairac, A.H.A.; Den Hoedt, C.H.; Van Der Tweel, I.; Lévesque, R.; Nubé, M.J.; et al. Effect of Online Hemodiafiltration on All-Cause Mortality and Cardiovascular Outcomes. J. Am. Soc. Nephrol. 2012, 23, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and Cardiovascular Events in Online Haemodiafiltration (OL-HDF) Compared with High-Flux Dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transpl. 2013, 28, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-S.; Tsai, Y.-C.; Chen, T.-W.; Li, S.-Y. Artificial Kidney Engineering: The Development of Dialysis Membranes for Blood Purification. Membranes 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Golper, T.A. Improving Dialysis Techniques for Patients? N. Engl. J. Med. 2023, 389, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, D.; Letteri, J.J.; Clark, W.R. Blood-Membrane Interactions during Dialysis. Semin. Dial. 2009, 22, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Gao, D. Low-Molecular Weight Proteins in End-Stage Renal Disease: Potential Toxicity and Dialytic Removal Mechanisms. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S1), S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.; Schmidt, B.; Samtleben, W.; Gurland, H.J. Effect of Protein Adsorption on Diffusive and Convective Transport through Polysulfone Membranes. Contrib. Nephrol. 1985, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gayrard, N.; Ficheux, A.; Duranton, F.; Guzman, C.; Szwarc, I.; Vetromile, F.; Cazevieille, C.; Brunet, P.; Servel, M.-F.; Argilés, À.; et al. Consequences of Increasing Convection onto Patient Care and Protein Removal in Hemodialysis. PLoS ONE 2017, 12, e0171179. [Google Scholar] [CrossRef] [PubMed]
- Haq, Z.; Wang, X.; Cheng, Q.; Dias, G.F.; Moore, C.; Piecha, D.; Kotanko, P.; Ho, C.-H.; Grobe, N. Bisphenol A and Bisphenol S in Hemodialyzers. Toxins 2023, 15, 465. [Google Scholar] [CrossRef]

| Dialyzer | Manufacturer | Membrane Name | Membrane Material | Sterilization | Surface [m2] |
|---|---|---|---|---|---|
| FX CorAL 80 | Fresenius Medical Care | Helixone hydro | Polysulfone, polyvinylpyrrolidone | INLINE steam | 1.8 |
| FX CorDiax 80 | Fresenius Medical Care | Helixone plus | Polysulfone, polyvinylpyrrolidone | INLINE steam | 1.8 |
| xevonta® Hi 18 | B. Braun | amembris polysulfone | Polysulfone, polyvinylpyrrolidone | Gamma | 1.8 |
| Diacap® Pro 19H | B. Braun | α polysulfone pro | Polysulfone, polyvinylpyrrolidone | Gamma | 1.9 |
| HF18 | Wego | N/A | Polysulfone-based * | Radiation * | 1.8 |
| ELISIOTM-17H | Nipro | PolynephronTM | Polyethersulfone, polyvinylpyrrolidone | Gamma | 1.7 |
| DORA® B-18HF | Bain Medical Equipment | N/A | Polyethersulfone-based * | Radiation * | 1.8 |
| Revaclear 400 | Baxter | Poracton | Polyarylethersulfone, polyvinylpyrrolidone | Steam | 1.8 |
| CellentiaTM 17H | Nipro | N/A | Cellulose triacetate | Gamma | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawada, A.M.; Emal, K.; Förster, E.; Saremi, S.; Delinski, D.; Theis, L.; Küng, F.; Xie, W.; Werner, J.; Stauss-Grabo, M.; et al. Hydrophilic Modification of Dialysis Membranes Sustains Middle Molecule Removal and Filtration Characteristics. Membranes 2024, 14, 83. https://doi.org/10.3390/membranes14040083
Zawada AM, Emal K, Förster E, Saremi S, Delinski D, Theis L, Küng F, Xie W, Werner J, Stauss-Grabo M, et al. Hydrophilic Modification of Dialysis Membranes Sustains Middle Molecule Removal and Filtration Characteristics. Membranes. 2024; 14(4):83. https://doi.org/10.3390/membranes14040083
Chicago/Turabian StyleZawada, Adam M., Karlee Emal, Eva Förster, Saeedeh Saremi, Dirk Delinski, Lukas Theis, Florian Küng, Wenhao Xie, Joanie Werner, Manuela Stauss-Grabo, and et al. 2024. "Hydrophilic Modification of Dialysis Membranes Sustains Middle Molecule Removal and Filtration Characteristics" Membranes 14, no. 4: 83. https://doi.org/10.3390/membranes14040083
APA StyleZawada, A. M., Emal, K., Förster, E., Saremi, S., Delinski, D., Theis, L., Küng, F., Xie, W., Werner, J., Stauss-Grabo, M., Faust, M., Boyington, S., & Kennedy, J. P. (2024). Hydrophilic Modification of Dialysis Membranes Sustains Middle Molecule Removal and Filtration Characteristics. Membranes, 14(4), 83. https://doi.org/10.3390/membranes14040083






