Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
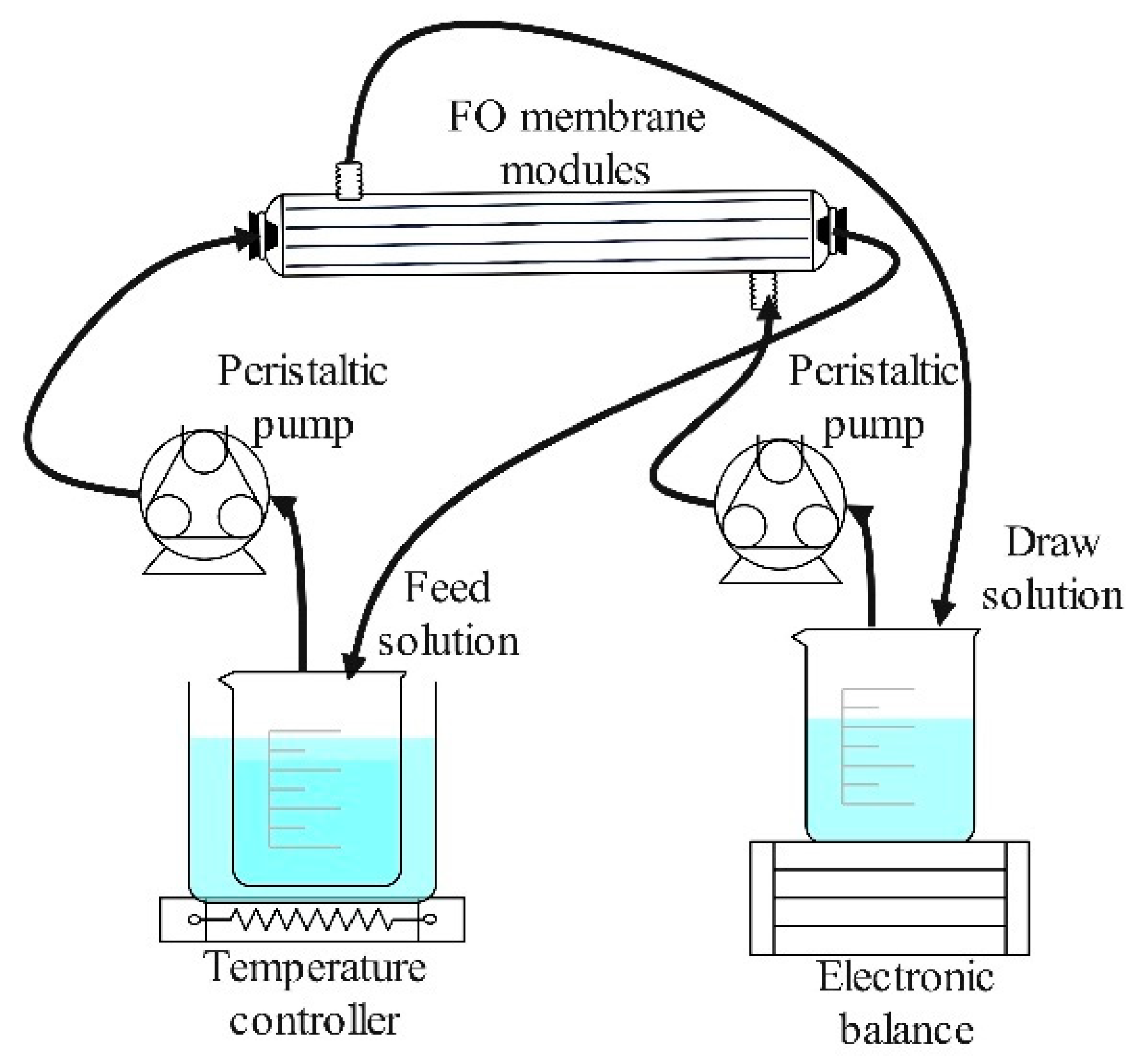
2.2. Optimization of FO Operating Condition
2.3. Concentration of Apple Juice
2.4. Membrane Characterization
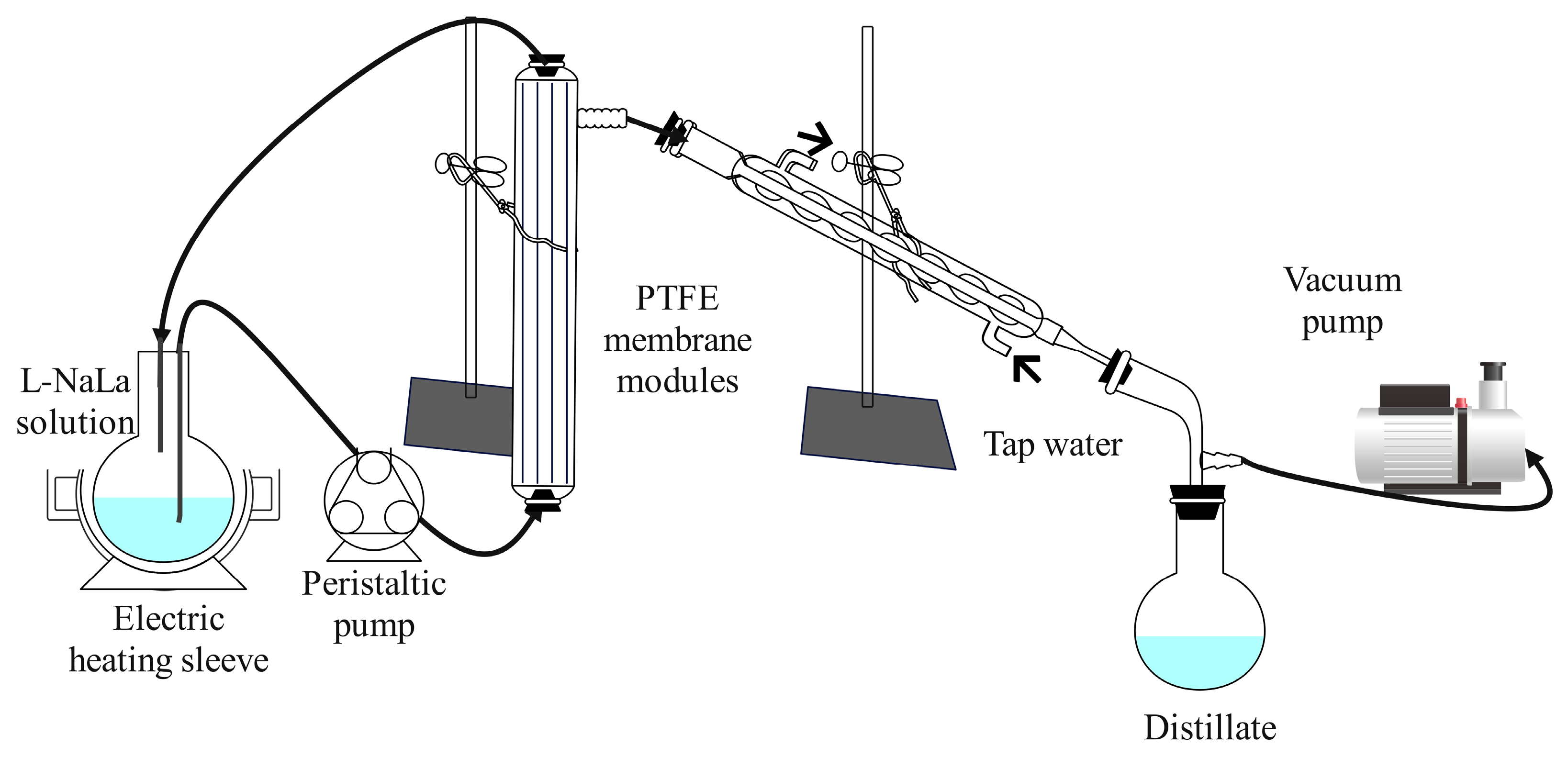
2.5. Recycling of Draw Solution
3. Results and Discussion
3.1. Effect of Operating Conditions on FO Performance
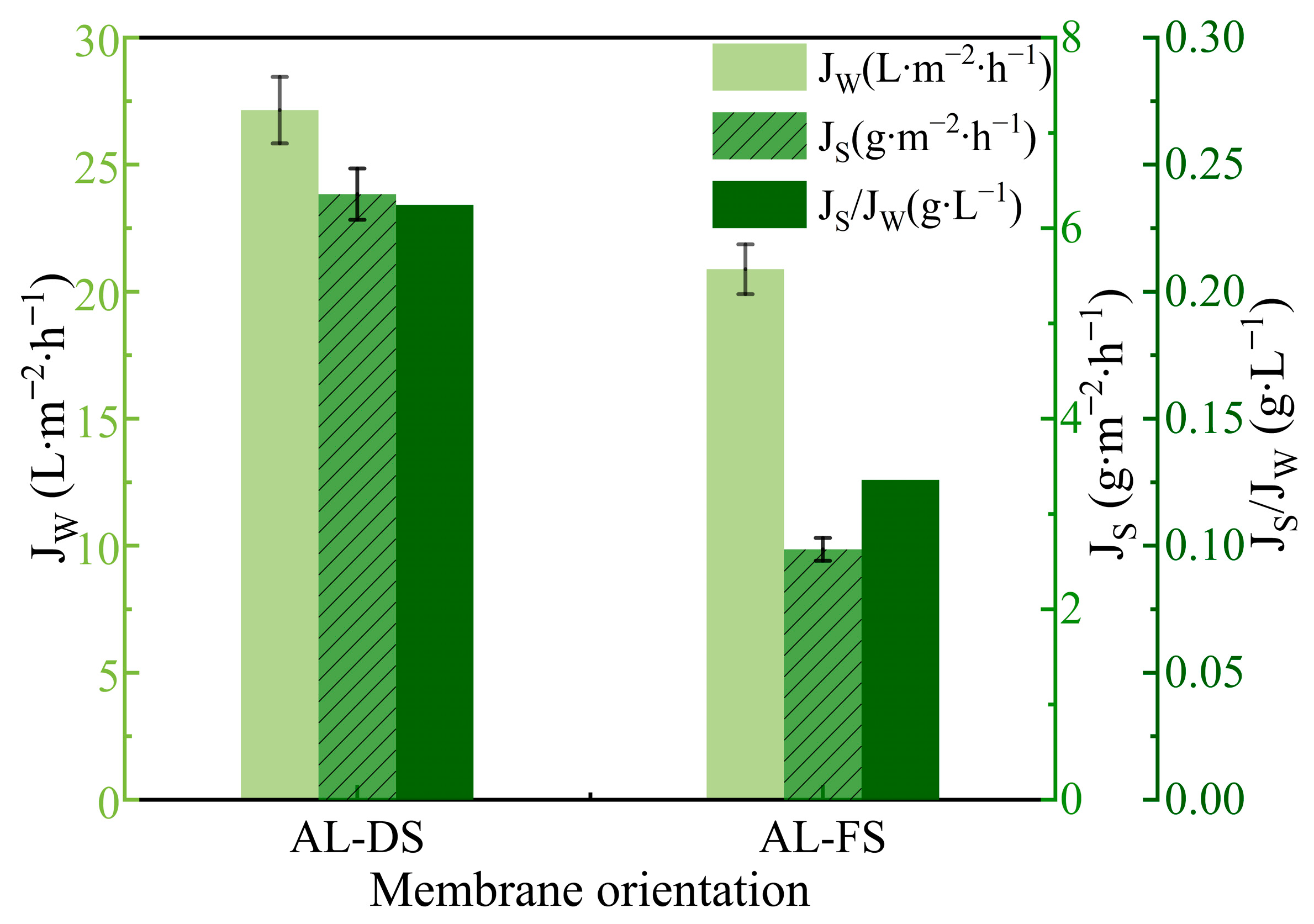
3.1.1. Effect of Membrane Orientation on FO Performance
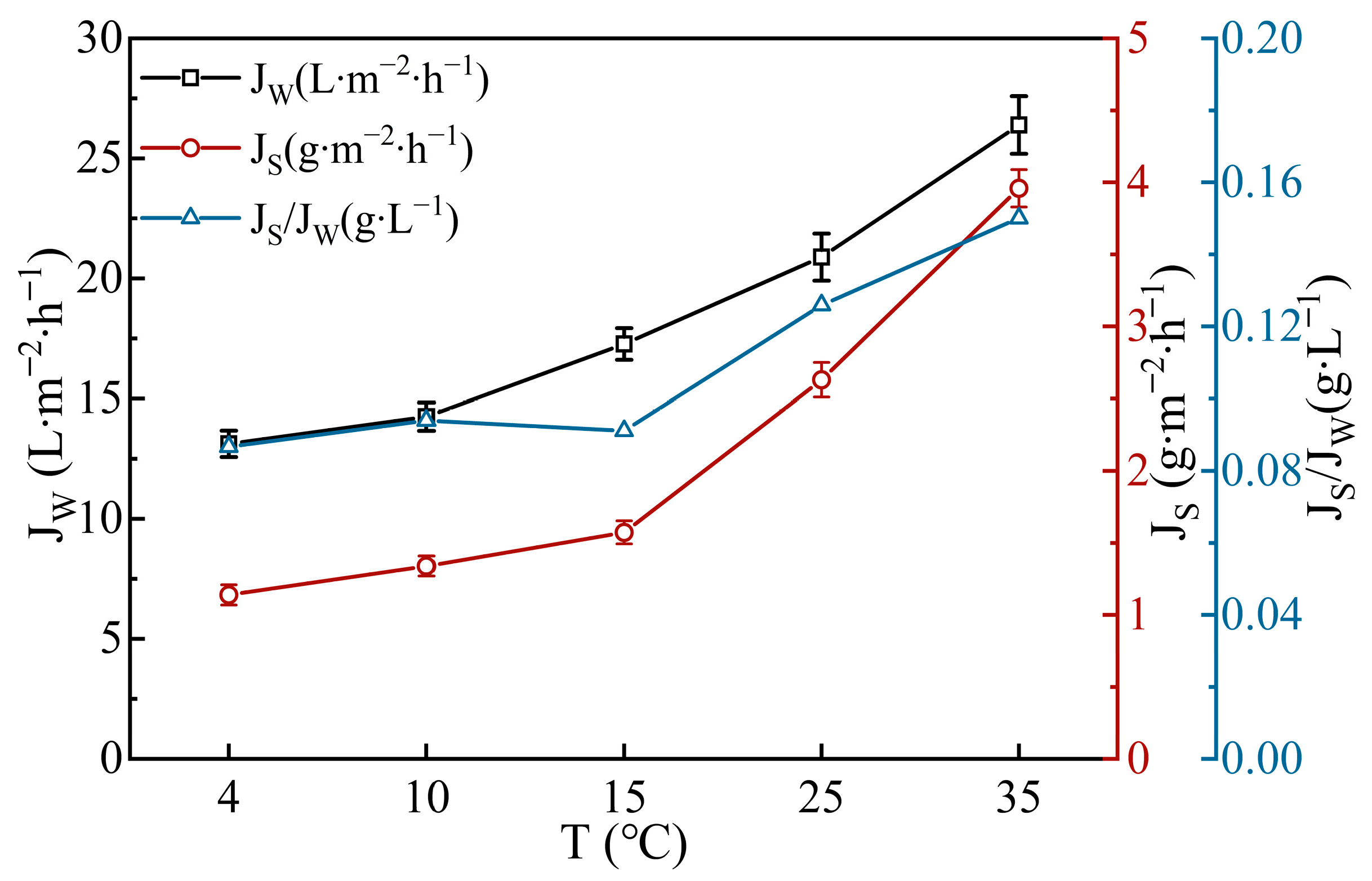
3.1.2. Effect of FS Temperature on FO Performance
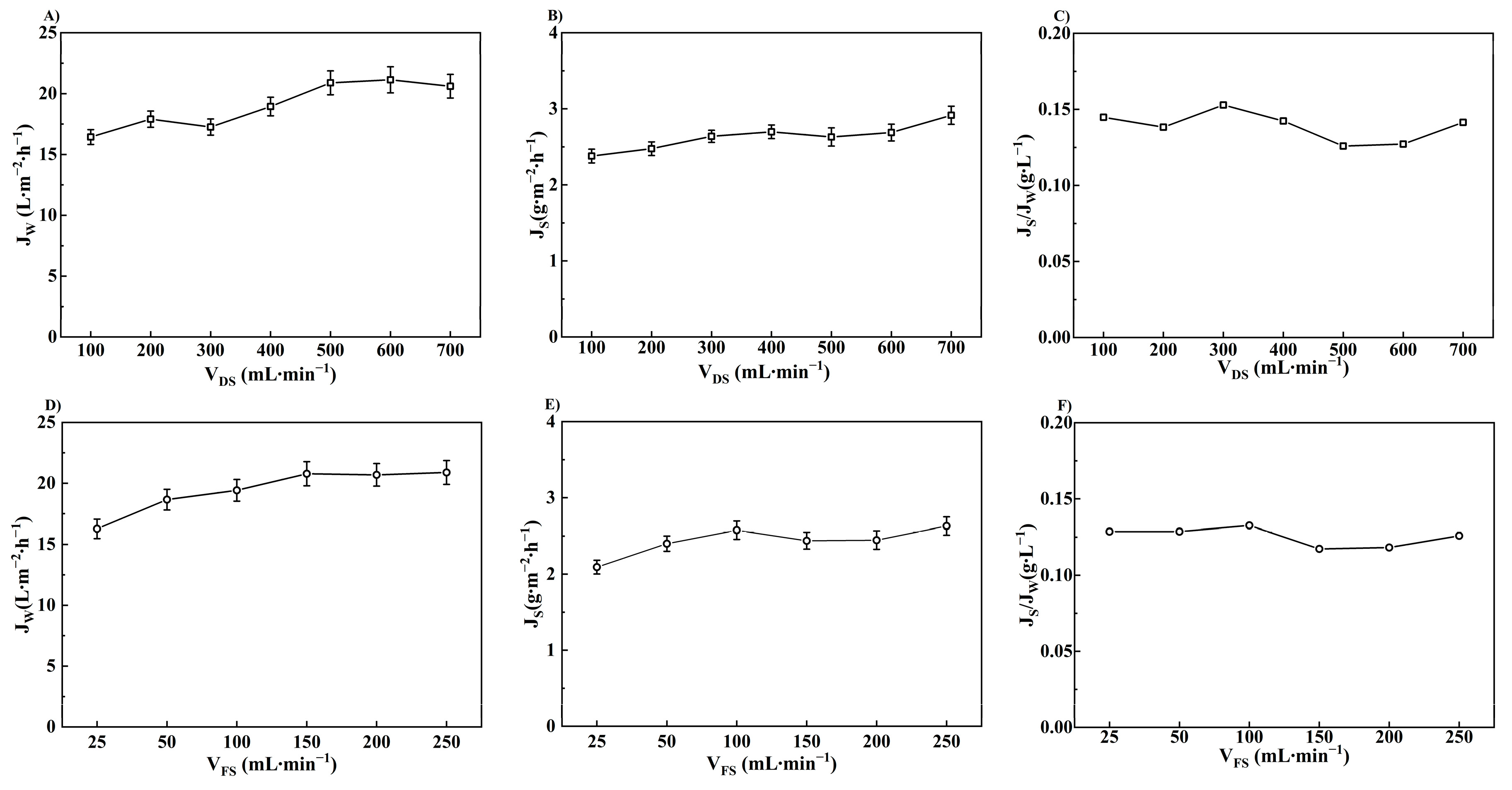
3.1.3. Effect of Flow Rate on FO Performance
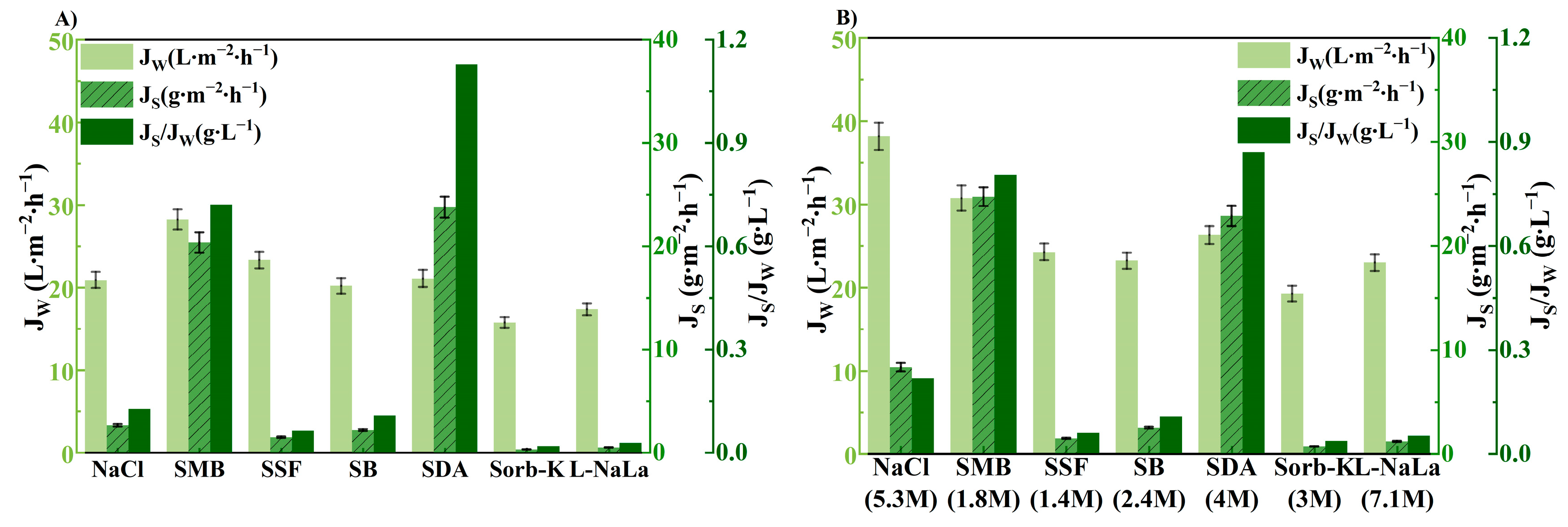
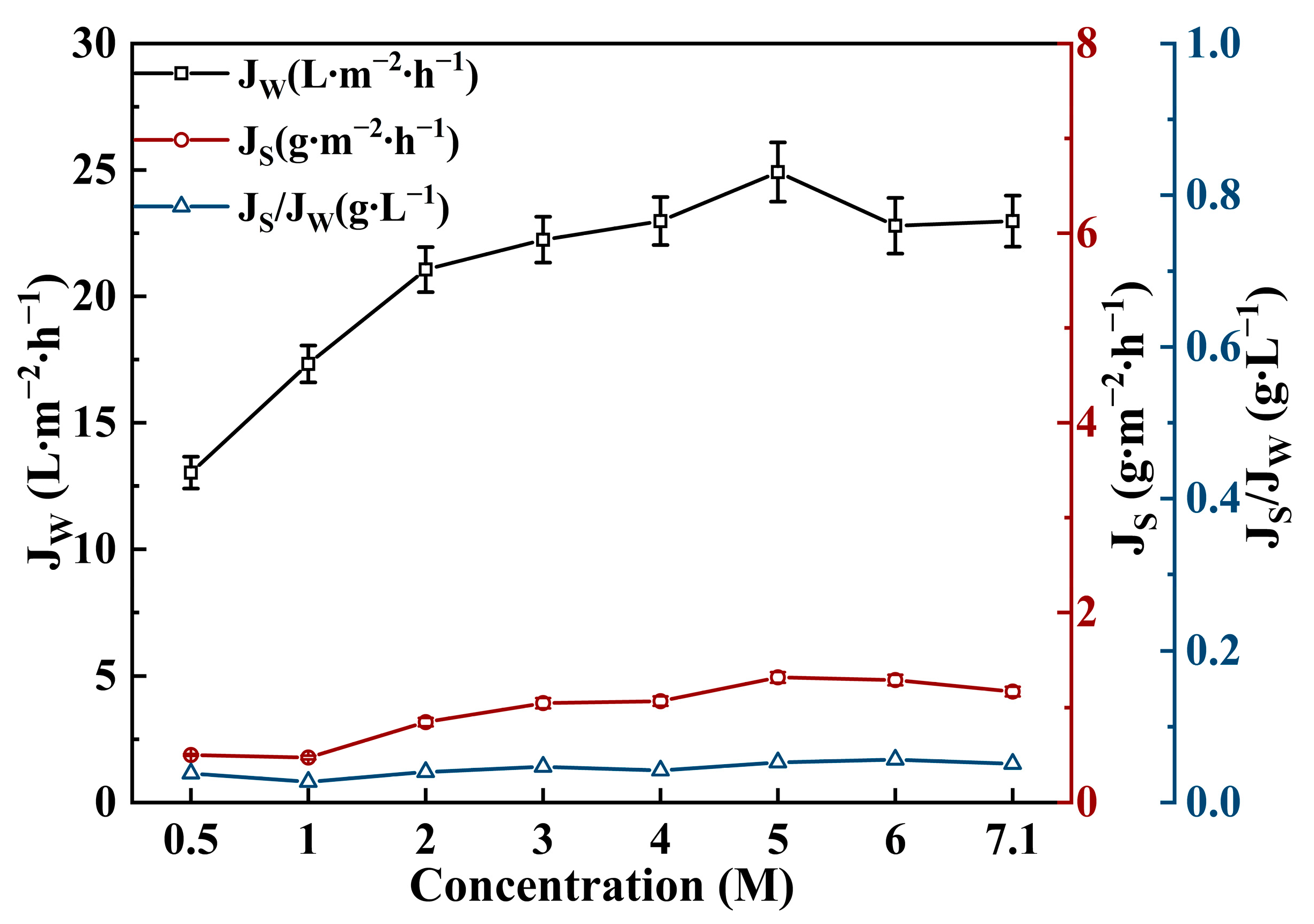
3.2. Effect of Draw Solution on FO Performance
3.3. Apple Juice Concentration Experiments
3.3.1. Comparative Study of FO and VE Concentration Experiments
3.3.2. Continuous FO Concentration Experiments
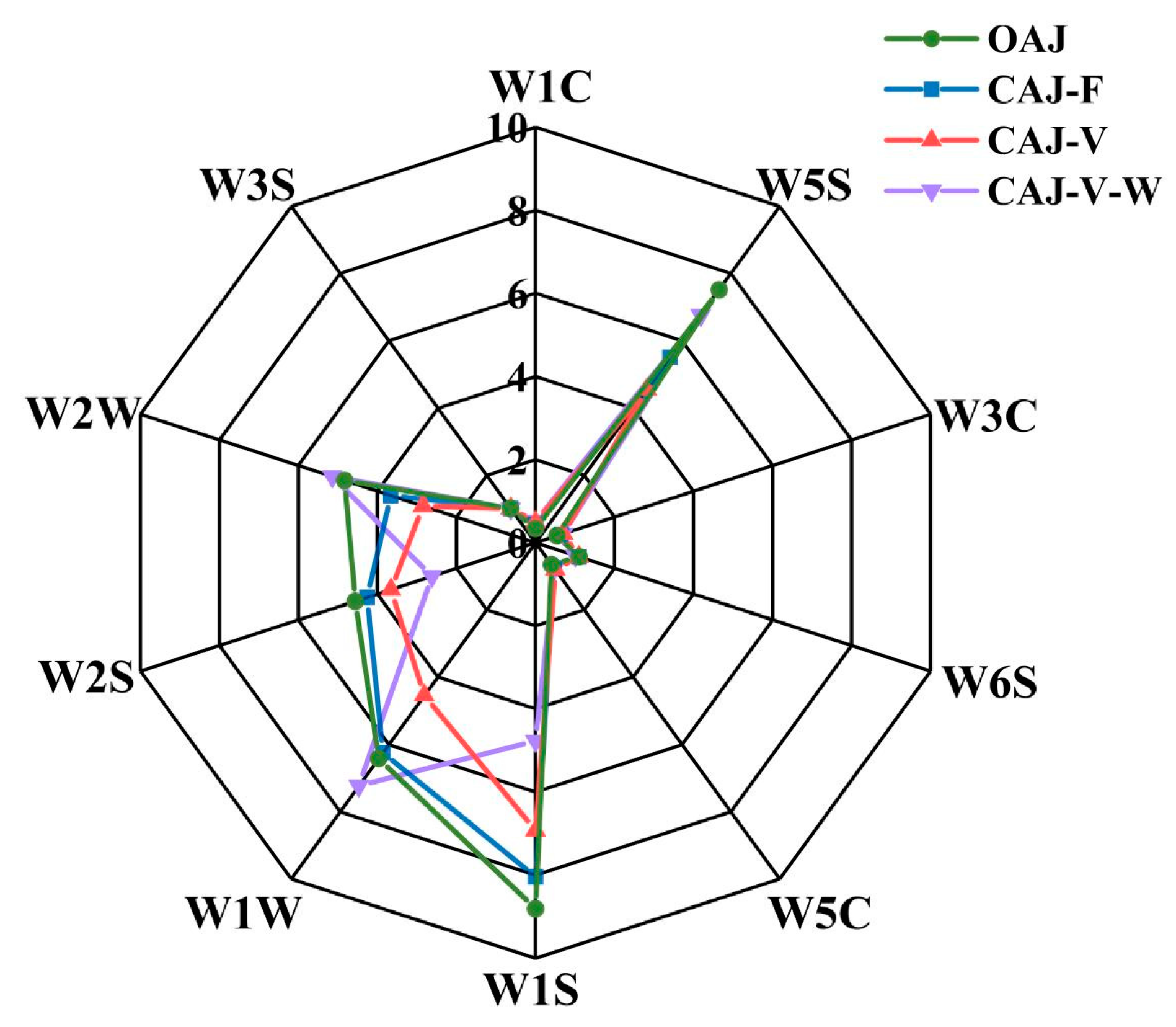
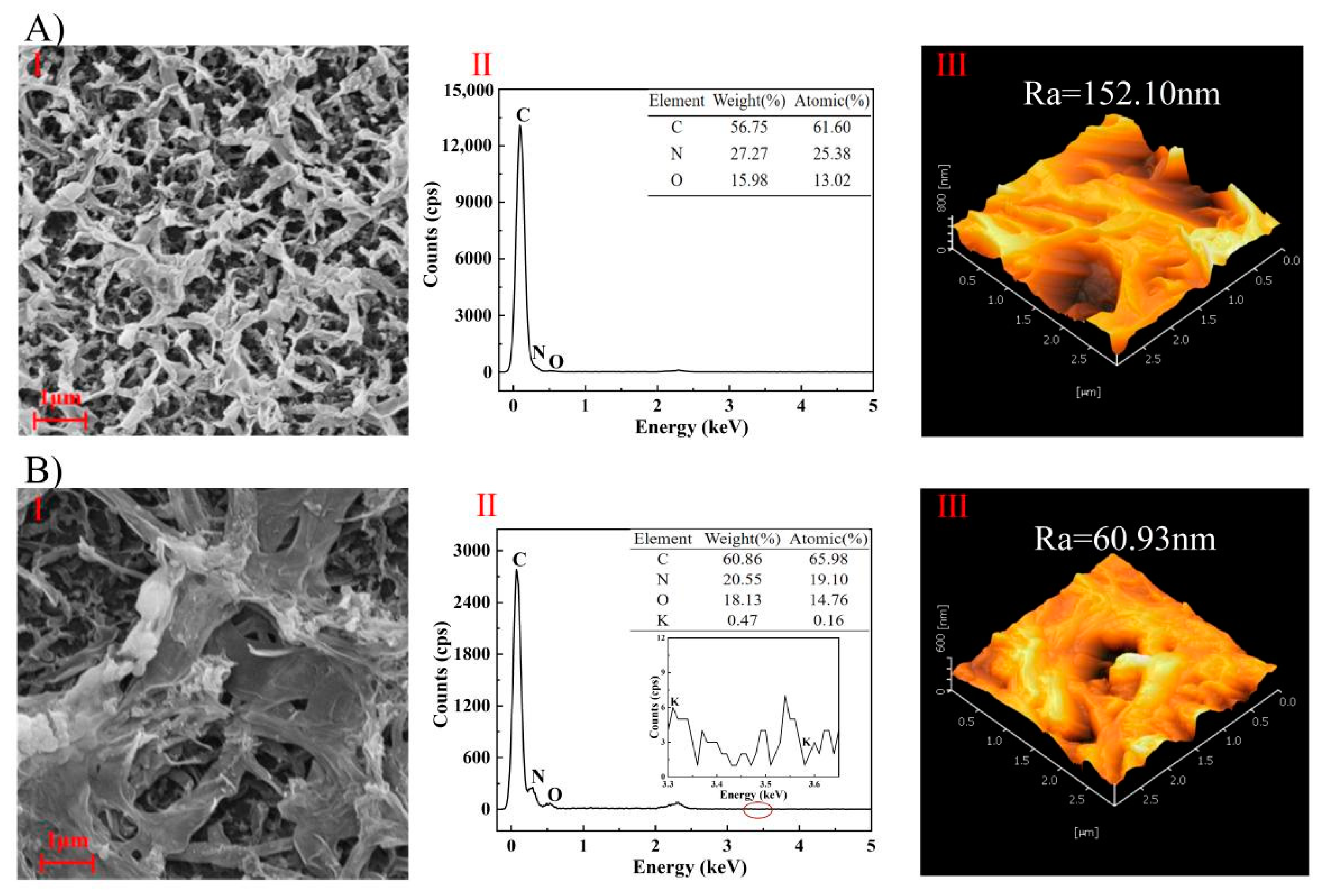
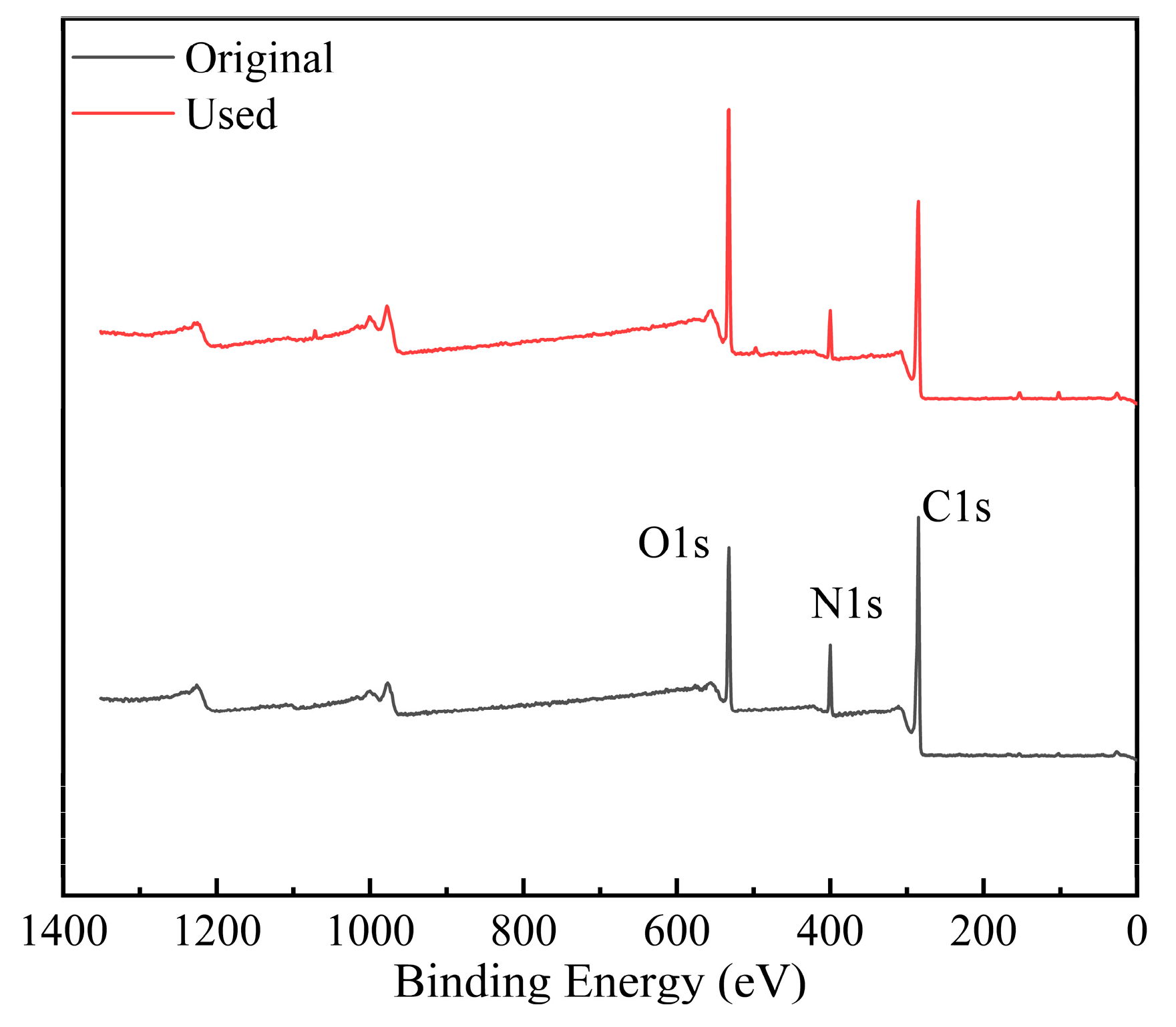
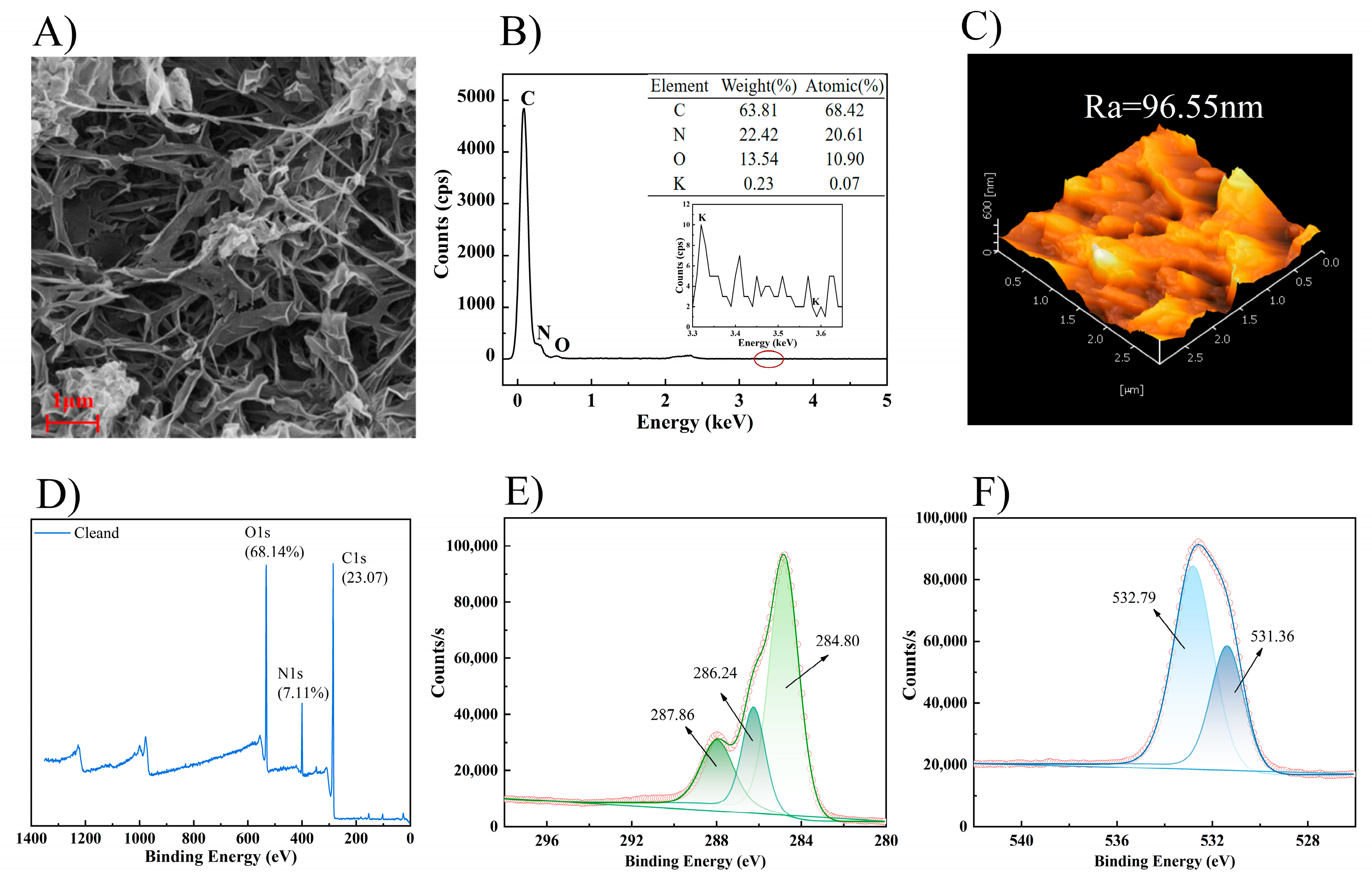
3.3.3. Membrane Autopsy Study
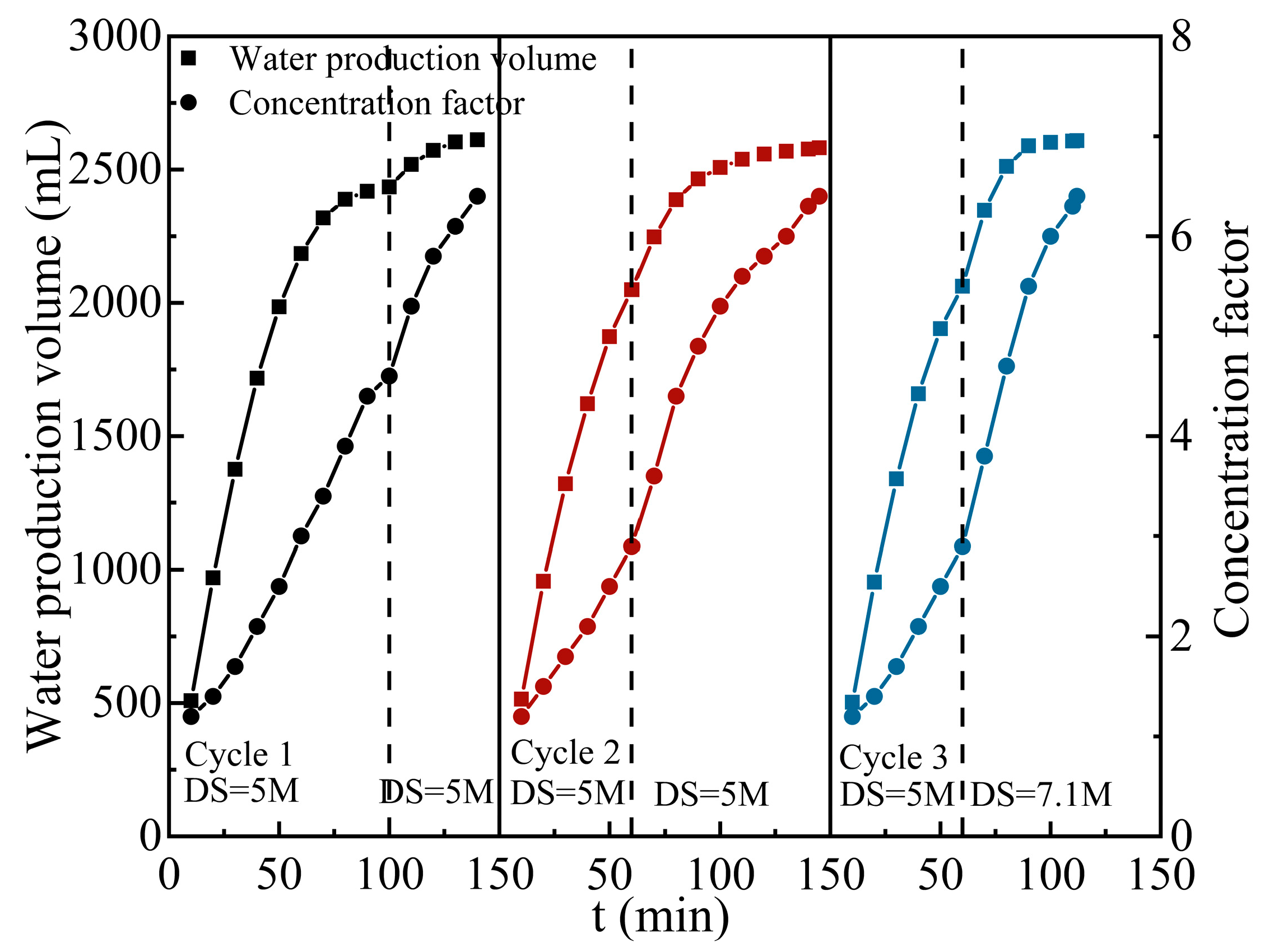
3.4. Regeneration of Draw Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAJ | concentrated apple juice |
| VE | vacuum evaporation |
| FO | forward osmosis |
| MD | membrane distillation |
| L-NaLa | sodium lactate |
| UP | ultrapure |
| DS | draw solution |
| Glu-K | Potassium gluconate |
| VMD | vacuum membrane distillation |
| PTFE | polytetrafluoroethylene |
| FS | feed solution |
| NaCl | sodium chloride |
| SSF | sodium sulfite |
| SMB | sodium metabisulfite |
| SB | sodium benzoate |
| SDA | sodium diacetate |
| Sorb-K | potassium sorbate |
| DPPH | 2,2-diphenyl-1-picryhydrazyl |
| SEM | scanning electron microscope |
| EDS | energy dispersive spectrometer |
| AFM | atomic force microscopy |
| XPS | X-ray photoelectron spectroscopy |
| TSS | total soluble solid |
| OAJ | original apple juice |
| CAJ-F | CAJ produced by FO |
| CAJ-V | CAJ produced by VE |
| ICP | internal concentration polarization |
| RSR | radicals scavenging rate |
| MOS | metal oxide semiconductor |
| CAJ-V-W | distillate produced in VE |
| TMC | trimesoyl chloride |
| MPD | m-Phenylenediamine |
References
- Cheng, J.; Wang, Q.; Yu, J. Life cycle assessment of potential environmental burden and human capital loss caused by apple production system in China. Environ. Sci. Pollut. Res. 2023, 30, 62015–62031. [Google Scholar] [CrossRef]
- Li, C.; Yuan, S.; Xie, Y.; Guo, Y.; Cheng, Y.; Yu, H.; Qian, H.; Yao, W. Transformation of fluopyram during enzymatic hydrolysis of apple and its effect on polygalacturonase and apple juice yield. Food Chem. 2021, 357, 129842. [Google Scholar] [CrossRef]
- Nijmeijer, K.; Oymaci, P.; Lubach, S.; Borneman, Z. Apple Juice, Manure and Whey Concentration with Forward Osmosis Using Electrospun Supported Thin-Film Composite Membranes. Membranes 2022, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qin, F.G.F.; Yuan, J.; Huang, S.; Jiang, R.; Shao, Y. Concentration of apple juice with an intelligent freeze concentrator. J. Food Eng. 2019, 256, 61–72. [Google Scholar] [CrossRef]
- Bozkir, H.; Baysal, T. Concentration of apple juice using a vacuum microwave evaporator as a novel technique: Determination of quality characteristics. J. Food Process Eng. 2017, 40, e12535. [Google Scholar] [CrossRef]
- Cheng, C.X.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of Mandarin (Citrus unshiu) juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102425. [Google Scholar] [CrossRef]
- Sampaio, K.L.; Garruti, D.S.; Franco, M.R.; Janzantti, N.S.; Da Silva, M.A. Aroma volatiles recovered in the water phase of cashew apple (Anacardium occidentale L.) juice during concentration. J. Sci. Food Agric. 2011, 91, 1801–1809. [Google Scholar] [CrossRef]
- Liu, X.; Mu, J.; Tan, D.; Mao, K.; Zhang, J.; Sadiq, F.A.; Sang, Y.; Zhang, A. Application of stable isotopic and mineral elemental fingerprints in identifying the geographical origin of concentrated apple juice in China. Food Chem. 2022, 391, 133269. [Google Scholar] [CrossRef]
- Trishitman, D.; Negi, P.S.; Rastogi, N.K. Concentration of pomegranate juice by forward osmosis or thermal evaporation and its shelf-life kinetic studies. Food Chem. 2022, 399, 133972. [Google Scholar] [CrossRef]
- Altaee, A.; Braytee, A.; Millar, G.J.; Naji, O. Energy efficiency of hollow fibre membrane module in the forward osmosis seawater desalination process. J. Membr. Sci. 2019, 587, 117165. [Google Scholar] [CrossRef]
- Singh, N.; Dhiman, S.; Basu, S.; Balakrishnan, M.; Petrinic, I.; Helix-Nielsen, C. Dewatering of sewage for nutrients and water recovery by Forward Osmosis (FO) using divalent draw solution. J. Water Process Eng. 2019, 31, 100853. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, K.; Gao, Y.; Li, G.; Li, Z.; Wang, Q.; Guo, L.; Liu, T.; Al-Namazi, M.A.; Li, S. Removal and Fouling Influence of Microplastics in Fertilizer Driven Forward Osmosis for Wastewater Reclamation. Membranes 2021, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Motsa, M.M.; Mamba, B.B.; Thwala, J.M.; Verliefde, A.R. Osmotic backwash of fouled FO membranes: Cleaning mechanisms and membrane surface properties after cleaning. Desalination 2017, 402, 62–71. [Google Scholar] [CrossRef]
- Wenten, I.G.; Khoiruddin, K.; Reynard, R.; Lugito, G.; Julian, H. Advancement of forward osmosis (FO) membrane for fruit juice concentration. J. Food Eng. 2020, 290, 110216. [Google Scholar] [CrossRef]
- Pei, J.; Gao, S.; Sarper, S.; Wang, H.; Chen, X.; Yu, J.; Yue, T.; Youravong, W.; Li, Z. Emerging forward osmosis and membrane distillation for liquid food concentration: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1910–1936. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Choi, J.; Hong, S. Evaluation on suitability of osmotic dewatering through forward osmosis (FO) for xylose concentration. Sep. Purif. Technol. 2017, 191, 225–232. [Google Scholar] [CrossRef]
- Corzo, B.; de la Torre, T.; Sans, C.; Ferrero, E.; Malfeito, J.J. Evaluation of draw solutions and commercially available forward osmosis membrane modules for wastewater reclamation at pilot scale. Chem. Eng. J. 2017, 326, 1–8. [Google Scholar] [CrossRef]
- An, X.; Hu, Y.; Wang, N.; Zhou, Z.; Liu, Z. Continuous Juice Concentration by Integrating Forward Osmosis with Membrane Distillation Using Potassium Sorbate Preservative as A Draw Solute. J. Membr. Ence 2018, 573, 192–199. [Google Scholar] [CrossRef]
- Long, Q.; Qi, G.; Wang, Y. Evaluation of Renewable Gluconate Salts as Draw Solutes in Forward Osmosis Process. ACS Sustain. Chem. Eng. 2015, 4, 85–93. [Google Scholar] [CrossRef]
- Milczarek, R.R.; Olsen, C.W.; Sedej, I. Quality of Watermelon Juice Concentrated by Forward Osmosis and Conventional Processes. Processes 2020, 8, 1568. [Google Scholar] [CrossRef]
- Morseletto, P. Targets for a circular economy. Resour. Conserv. Recycl. 2020, 153, 104553. [Google Scholar] [CrossRef]
- Giannetti, B.F.; Agostinho, F.; Eras, J.J.C.; Yang, Z.; Almeida, C. Cleaner production for achieving the sustainable development goals. J. Clean. Prod. 2020, 271, 122127. [Google Scholar] [CrossRef]
- Choi, B.G.; Kim, D.I.; Hong, S. Fouling evaluation and mechanisms in a FO-RO hybrid process for direct potable reuse. J. Membr. Sci. 2016, 520, 89–98. [Google Scholar] [CrossRef]
- Kim, J.E.; Phuntsho, S.; Chekli, L.; Choi, J.Y.; Shon, H.K. Environmental and economic assessment of hybrid FO-RO/NF system with selected inorganic draw solutes for the treatment of mine impaired water. Desalination 2018, 429, 96–104. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Minier-Matar, J.; Adham, S.; Nasser, M.S.; Judd, S. The status of forward osmosis technology implementation. Desalination 2019, 461, 10–21. [Google Scholar] [CrossRef]
- Cai, J.; Yin, H.; Guo, F. Transport analysis of material gap membrane distillation desalination processes. Desalination 2020, 481, 114361. [Google Scholar] [CrossRef]
- Wirth, D.; Cabassud, C. Water desalination using membrane distillation: Comparison between inside/out and outside/in permeation. Desalination 2002, 147, 139–145. [Google Scholar] [CrossRef]
- Yu, F.; Yu, Z.; Huang, X.; Gu, A.; Du, J.; Xie, S.; Liu, R.; Zou, D.; Fang, S.; Xie, M.; et al. Effective Membrane Distillation of Landfill Leachate Concentrate Using a Superhydrophobic SiO2/PVDF Membrane for Resource Recovery. ACS EST Water 2024, 4, 1711–1719. [Google Scholar] [CrossRef]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Ghaffour, N.; Vrouwenvelder, J.; Shon, H.K. Optimisation of a forward osmosis and membrane distillation hybrid system for the treatment of source-separated urine. Sep. Purif. Technol. 2018, 212, 368–375. [Google Scholar] [CrossRef]
- Li, M.; Li, K.; Wang, L.; Zhang, X. Feasibility of concentrating textile wastewater using a hybrid forward osmosis-membrane distillation (FO-MD) process: Performance and economic evaluation. Water Res. 2020, 172, 115488. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Hong, S. Treatment of industrial wastewater produced by desulfurization process in a coal-fired power plant via FO-MD hybrid process. Chemosphere 2018, 210, 44–51. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Benes, N.; Nijmeijer, A.; McCutcheon, J.R. Use of a Forward Osmosis–Membrane Distillation Integrated Process in the Treatment of High-Salinity Oily Wastewater. Ind. Eng. Chem. Res. 2019, 58, 956–962. [Google Scholar] [CrossRef]
- Zaragoza, G.; Andrés-Mañas, J.A.; Ruiz-Aguirre, A. Commercial scale membrane distillation for solar desalination. NPJ Clean Water 2018, 1, 20. [Google Scholar] [CrossRef]
- Alsaati, A.; Marconnet, A.M. Energy efficient membrane distillation through localized heating. Desalination 2018, 422, 99–107. [Google Scholar] [CrossRef]
- National Standards of People’s Republic of China. Apple Juice Concentrate: GB/T 18963-2012; Standards Press of China: Beijing, China, 2012. [Google Scholar]
- Li, Y.; Cai, R.; Fu, C.; Qi, L.; Yuan, Y.; Yue, T.; Ge, Q.; Zhao, Z.; Wang, Z. Degradation of Patulin in Apple Juice by Pulsed Light and its Effect on the Quality. Food Bioprocess Technol. 2022, 16, 870–880. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, Q.; Hu, K.; Zhang, R.; Yuan, Y.; He, S.; Zeng, Q.; Su, D. Effects of in vitro simulated digestion on the free and bound phenolic content and antioxidant activity of seven species of seaweeds. Int. J. Food Sci. Technol. 2020, 56, 2365–2374. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT—Food Sci. Technol. 2015, 2, 890–893. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Piekarska, A.; Mrugalska, B.; Konieczka, P.; Namieśnik, J.; Bartoszek, A. Phenolic composition and antioxidant properties of Polish blue-berried honeysuckle genotypes by HPLC-DAD-MS, HPLC postcolumn derivatization with ABTS or FC, and TLC with DPPH visualization. J. Agric. Food Chem. 2012, 60, 1755–1763. [Google Scholar] [CrossRef]
- Wu, H.; Yue, T.; Xu, Z.; Zhang, C. Sensor array optimization and discrimination of apple juices according to variety by an electronic nose. Anal. Methods 2017, 9, 921–928. [Google Scholar] [CrossRef]
- Deng, J.; Liu, C.; Xia, X.; Liao, Z.; Feng, S.; Liu, B.; Cheng, W.; Nie, X. Application of vacuum membrane distillation-Fenton oxidation process for deep purification of low-level radioactive organic wastewater. Sep. Purif. Technol. 2024, 337, 126360. [Google Scholar] [CrossRef]
- Tan, C.H.; Ng, H.Y. Revised external and internal concentration polarization models to improve flux prediction in forward osmosis process. Desalination 2013, 309, 125–140. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhang, Z.; Cui, Q.; Zhu, J. Effect of a novel hydrophilic double-skinned support layer on improving anti-fouling performance of thin-film composite forward osmosis membrane. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125081. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Wang, Y. Treatment of greywater by forward osmosis technology: Role of the operating temperature. Environ. Technol. 2019, 40, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Jalab, R.; Awad, A.M.; Nasser, M.S.; Hussein, I.A.; Almomani, F.; Minier-Matar, J.; Adham, S. Investigation of thin-film composite hollow fiber forward osmosis membrane for osmotic concentration: A pilot-scale study. Korean J. Chem. Eng. 2022, 39, 178–188. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Z.; Xiong, Q.; Wu, C.; Huang, J.; Zhou, R.; Jin, Y. Exploration of sodium lactate as the draw solute of forward osmosis for food processing. J. Food Eng. 2021, 296, 110465. [Google Scholar] [CrossRef]
- Song, Q.; Rune, C.J.; Thybo, A.K.; Clausen, M.P.; Orlien, V.; Giacalone, D. Sensory quality and consumer perception of high pressure processed orange juice and apple juice. LWT—Food Sci. Technol. 2022, 173, 114303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Liu, Y.; Xiao, Q.; Xu, S. Quantitative evaluation of concentration polarization under different operating conditions for forward osmosis process. Desalination 2016, 398, 106–113. [Google Scholar] [CrossRef]
- Zhang, K.; An, X.; Bai, Y.; Shen, C.; Jiang, Y.; Hu, Y. Exploration of food preservatives as draw solutes in the forward osmosis process for juice concentration. J. Membr. Sci. 2021, 635, 119495. [Google Scholar] [CrossRef]
- Chu, H.; Zhang, Z.; Zhong, H.; Yang, K.; Sun, P.; Liao, X.; Cai, M. Athermal Concentration of Blueberry Juice by Forward Osmosis: Food Additives as Draw Solution. Membranes 2022, 12, 808. [Google Scholar] [CrossRef]
- Xue, W.; Yamamoto, K.; Tobino, T.; Ratanatamskul, C. Modeling prediction of the process performance of seawater-driven forward osmosis for nutrients enrichment: Implication for membrane module design and system operation. J. Membr. Sci. 2016, 515, 7–21. [Google Scholar] [CrossRef]
- Soffer, L.J.; Dantes, D.A.; Newburger, R.; Sobotka, H. Metabolism of Sodium d-lactate: I. utilization of intravenously injected sodium d-lactate by normal persons. Arch. Intern. Med. 1937, 60, 876–881. [Google Scholar] [CrossRef]
- Mccutcheon, J.R.; Mcginnis, R.L.; Elimelech, M. Desalination by ammonia–carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Paravisini, L.; Peterson, D.G. Role of Reactive Carbonyl Species in non-enzymatic browning of apple juice during storage. Food Chem. 2018, 245, 1010–1017. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Yao, Y.; Wei, X.; Yue, T.; Meng, J. Formation of 5-hydroxymethylfurfural in industrial-scale apple juice concentrate processing. Food Control 2019, 102, 56–68. [Google Scholar] [CrossRef]
- Mangindaan, D.; Khoiruddin, K.; Wenten, I.G. Beverage dealcoholization processes: Past, present, and future. Trends Food Sci. Technol. 2018, 71, 36–45. [Google Scholar] [CrossRef]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; Van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. RCM 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Steinhaus, M.; Bogen, J.; Schieberle, P. Key aroma compounds in apple juice-changes during juice concentration. Dev. Food Sci. 2006, 43, 189–192. [Google Scholar] [CrossRef]
- Chou, S.; Shi, L.; Wang, R.; Tang, C.Y.; Qiu, C.; Fane, A.G. Characteristics and potential applications of a novel forward osmosis hollow fiber membrane. Desalination 2010, 261, 365–372. [Google Scholar] [CrossRef]
- Saha, N.K.; Balakrishnan, M.; Ulbricht, M. Sugarcane juice ultrafiltration: FTIR and SEM analysis of polysaccharide fouling. J. Membr. Sci. 2007, 306, 287–297. [Google Scholar] [CrossRef]
- Cai, M.; Hou, W.; Li, Z.; Lv, Y.; Sun, P. Understanding Nanofiltration Fouling of Phenolic Compounds in Model Juice Solution with Two Membranes. Food Bioprocess Technol. 2017, 10, 2123–2131. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zheng, T.; Mo, B.; Xu, H.; Huang, Y.; Wang, J.; Gao, C.; Gao, X. High Flux Nanofiltration Membranes with Double-Walled Carbon Nanotube (DWCNT) as the Interlayer. Membranes 2022, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Farah, E.-N.; Demet, U.; Erdoğan, H. Voltammetric determination of rutin in fruit juice samples using a 2 mercaptobenzothiazole coated pencil graphite electrode. J. Food Compos. Anal. 2021, 104, 104183. [Google Scholar] [CrossRef]
- Okoro, H.K.; Ndlwana, L.; Ikhile, M.I.; Barnard, T.G.; Ngila, J.C. Hyperbranched polyethylenimine-modified polyethersulfone (HPEI/PES) and nAg@HPEI/PES membranes with enhanced ultrafiltration, antibacterial, and antifouling properties. Heliyon 2021, 7, e07961. [Google Scholar] [CrossRef]
- Sousa, A.; Vareda, J.; Pereira, R.; Silva, C.; Câmara, J.S.; Perestrelo, R. Geographical differentiation of apple ciders based on volatile fingerprint. Food Res. Int. 2020, 137, 109550. [Google Scholar] [CrossRef] [PubMed]




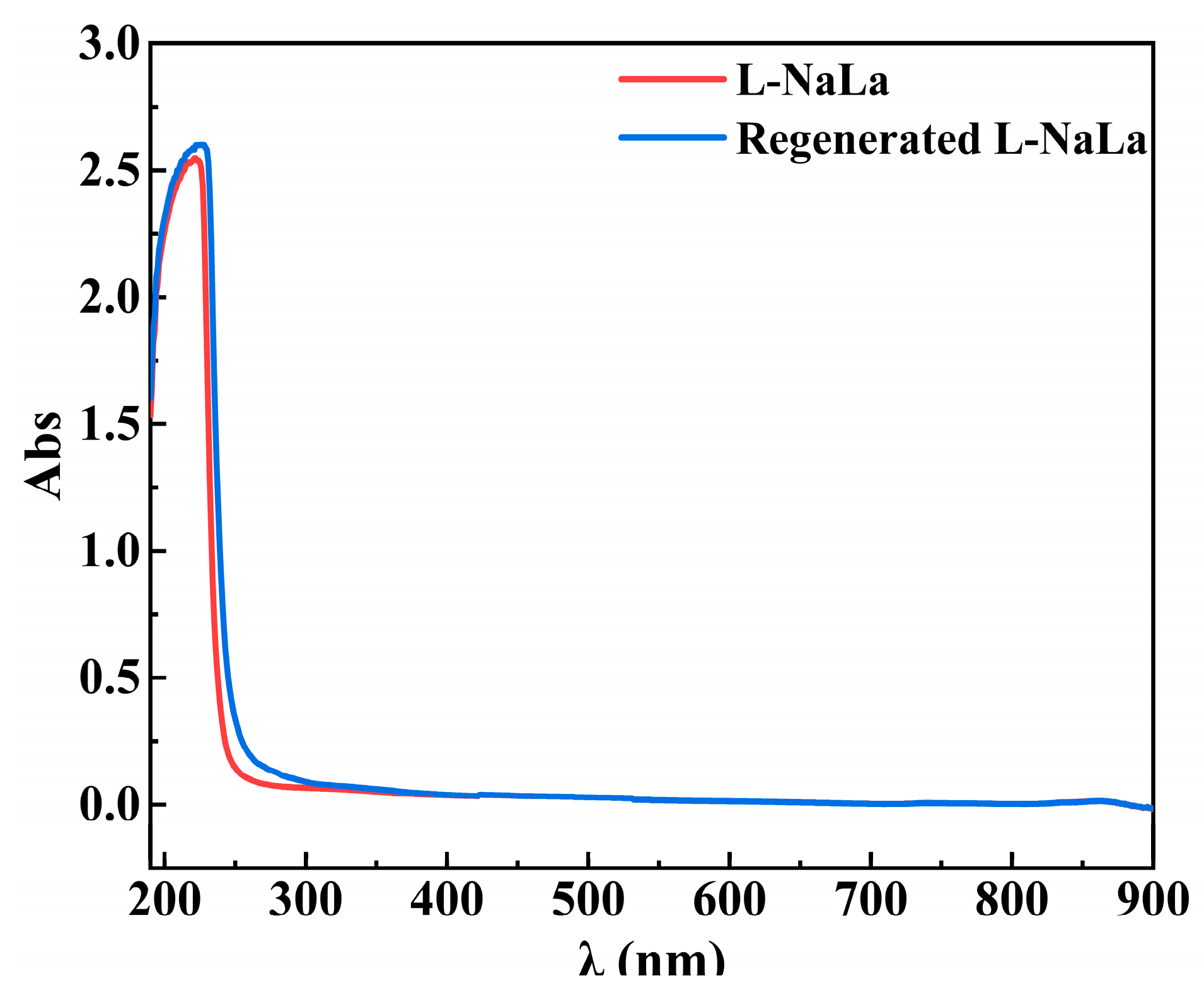
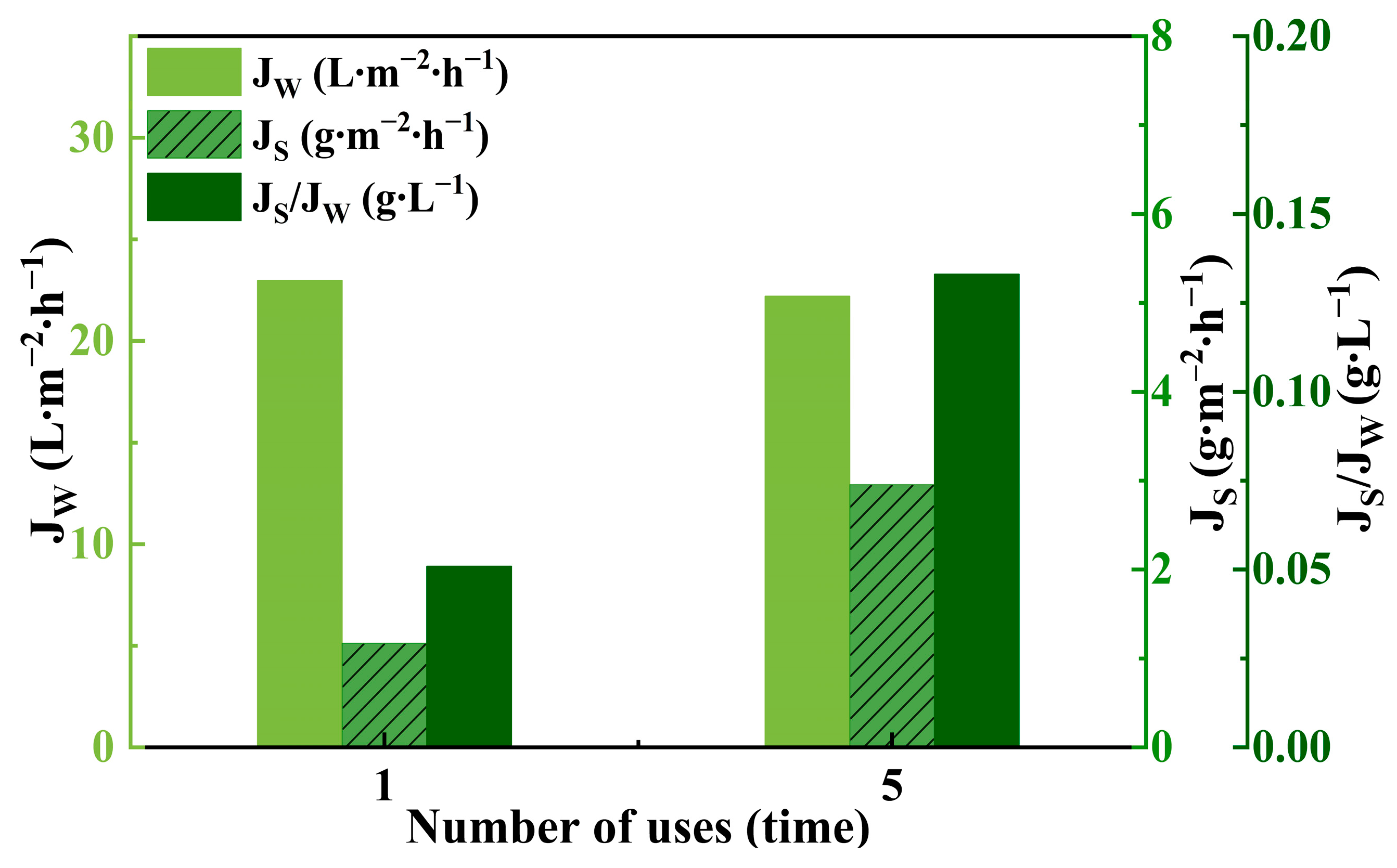
| Additive | MW (Da) | pH (1 M, 25.0 ± 0.5 °C) | Max Solubility in Water [50] (M) | Estimated Osmotic Pressure at Saturation Concentration a (mOsm∙kg−1) | Safety Limit b (g∙kg−1) |
|---|---|---|---|---|---|
| Sodium chloride (NaCl) | 58.4 | 6.5 | 5.3 | 9959 | N.A. |
| Sodium metabisulfite (SMB) | 190.0 | 4.5 | 1.8 | 6044 | 0.07 |
| Sodium sulfite (SSF) | 126.0 | 10.5 | 1.4 | 2708 | 0.07 |
| Sodium benzoate (SB) | 144.1 | 9.8 | 2.4 | 5122 | 0.20 |
| Sodium diacetate (SDA) | 142.0 | 5.0 | 4.0 | 12,000 | 0.50 |
| potassium sorbate (Sorb-K) | 150.2 | 10 | 3.0 | 6504 | 1.00 |
| Sodium lactate (L-NaLa) | 112.1 | 7.2 | 7.1 | 14,782 | N.A. |
| Element | OAJ | CAJ-F | CAJ-V | Benefits |
|---|---|---|---|---|
| pH | 3.802 | 3.994 | 3.658 |
|
| Total acid (%) | 0.266 | 0.206 | 0.300 |
|
| Total sugar (g∙L−1) | 91.03 | 91.03 | 85.67 | Energy supply |
| Total phenolic (g∙L−1) | 0.087 | 0.086 | 0.115 |
|
| Total flavone (g∙L−1) | 0.031 | 0.033 | 0.035 |
|
| DPPH RSR (%) | 84.78 | 84.47 | 81.13 | Antioxidant |
| Sensor Name | Type of Substance |
|---|---|
| W1C | Aromatic |
| W5S | Broad range |
| W3C | Aromatic |
| W6S | Hydrogen |
| W5C | Arom–aliph |
| W1S | Broad methane |
| W1W | Sulphur–organic |
| W2S | Broad alcohol |
| W2W | Sulph–chlor |
| W3S | Methane–aliph |
| Sample | C (Atomic%) | N (Atomic%) | O (Atomic%) |
|---|---|---|---|
| Original | 69.81 | 10.23 | 19.96 |
| Used | 64.29 | 7.11 | 28.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, C.; Deng, J.; Zhang, P.; Feng, S.; Chen, Y. Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution. Membranes 2024, 14, 106. https://doi.org/10.3390/membranes14050106
Zhao Y, Liu C, Deng J, Zhang P, Feng S, Chen Y. Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution. Membranes. 2024; 14(5):106. https://doi.org/10.3390/membranes14050106
Chicago/Turabian StyleZhao, Yuhang, Chang Liu, Jianju Deng, Panpan Zhang, Shiyuan Feng, and Yu Chen. 2024. "Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution" Membranes 14, no. 5: 106. https://doi.org/10.3390/membranes14050106
APA StyleZhao, Y., Liu, C., Deng, J., Zhang, P., Feng, S., & Chen, Y. (2024). Green and Sustainable Forward Osmosis Process for the Concentration of Apple Juice Using Sodium Lactate as Draw Solution. Membranes, 14(5), 106. https://doi.org/10.3390/membranes14050106







