Mo-BiVO4 Photocatalytically Modified Ceramic Ultrafiltration Membranes for Enhanced Water Treatment Efficiency
Abstract
:1. Introduction
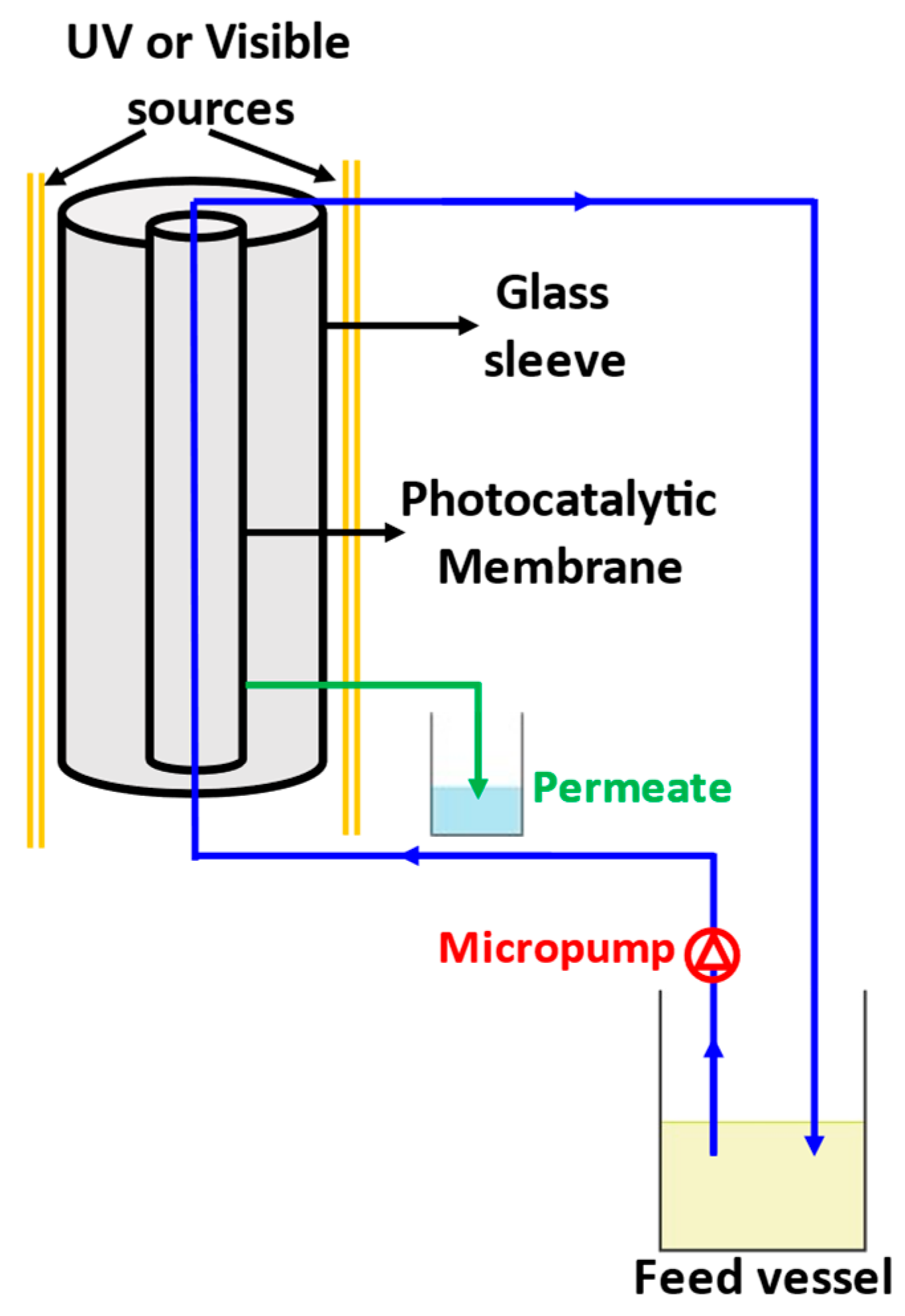
2. Materials and Methods
3. Results
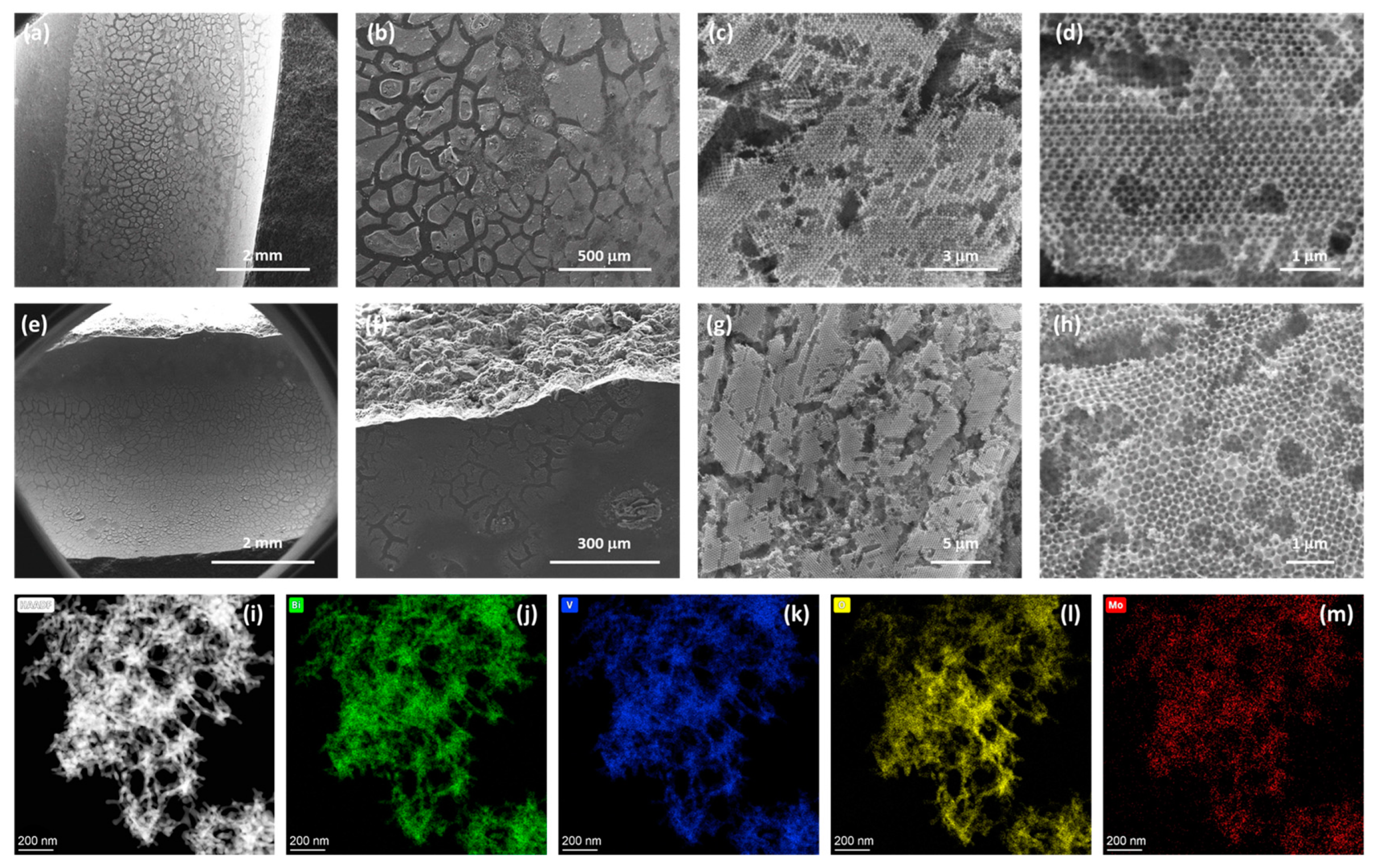
3.1. Morphological Analysis
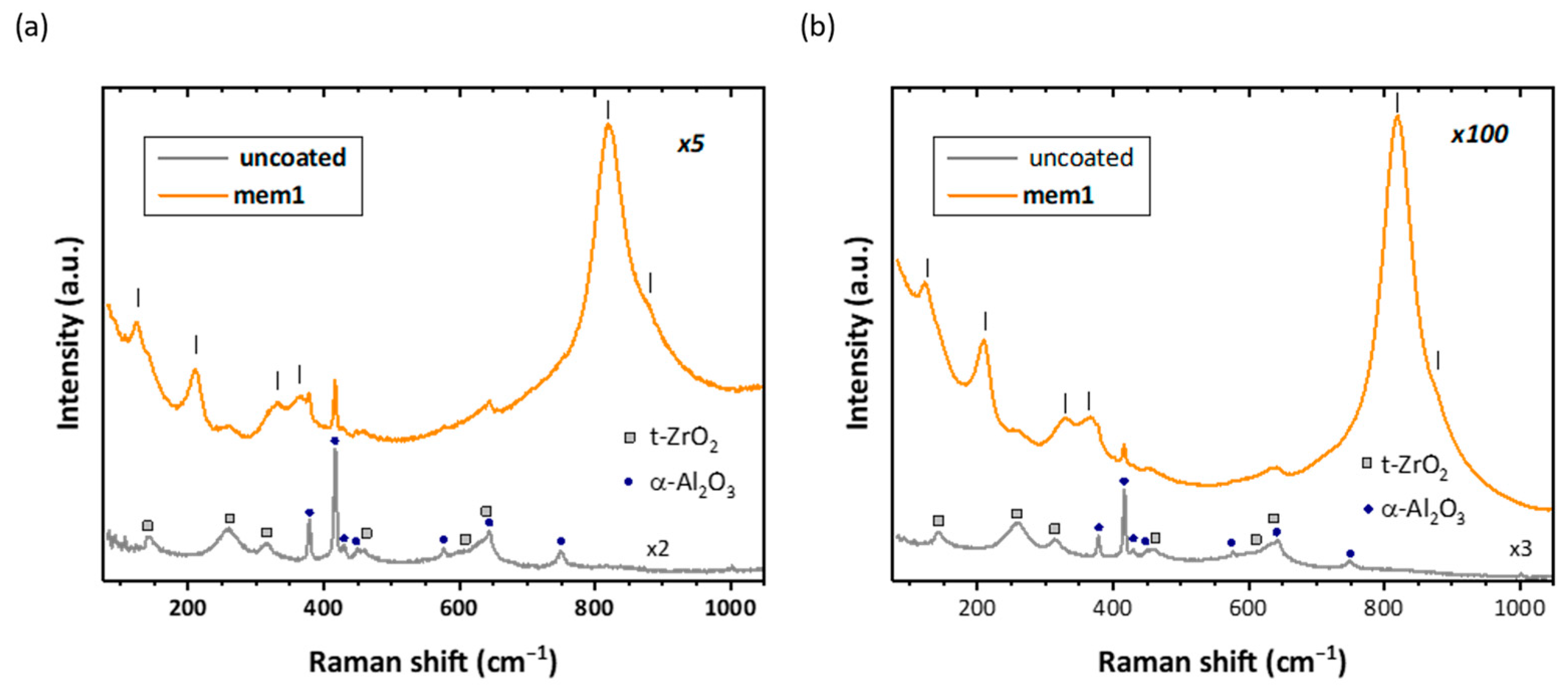
3.2. Raman Analysis
3.3. Optical Properties
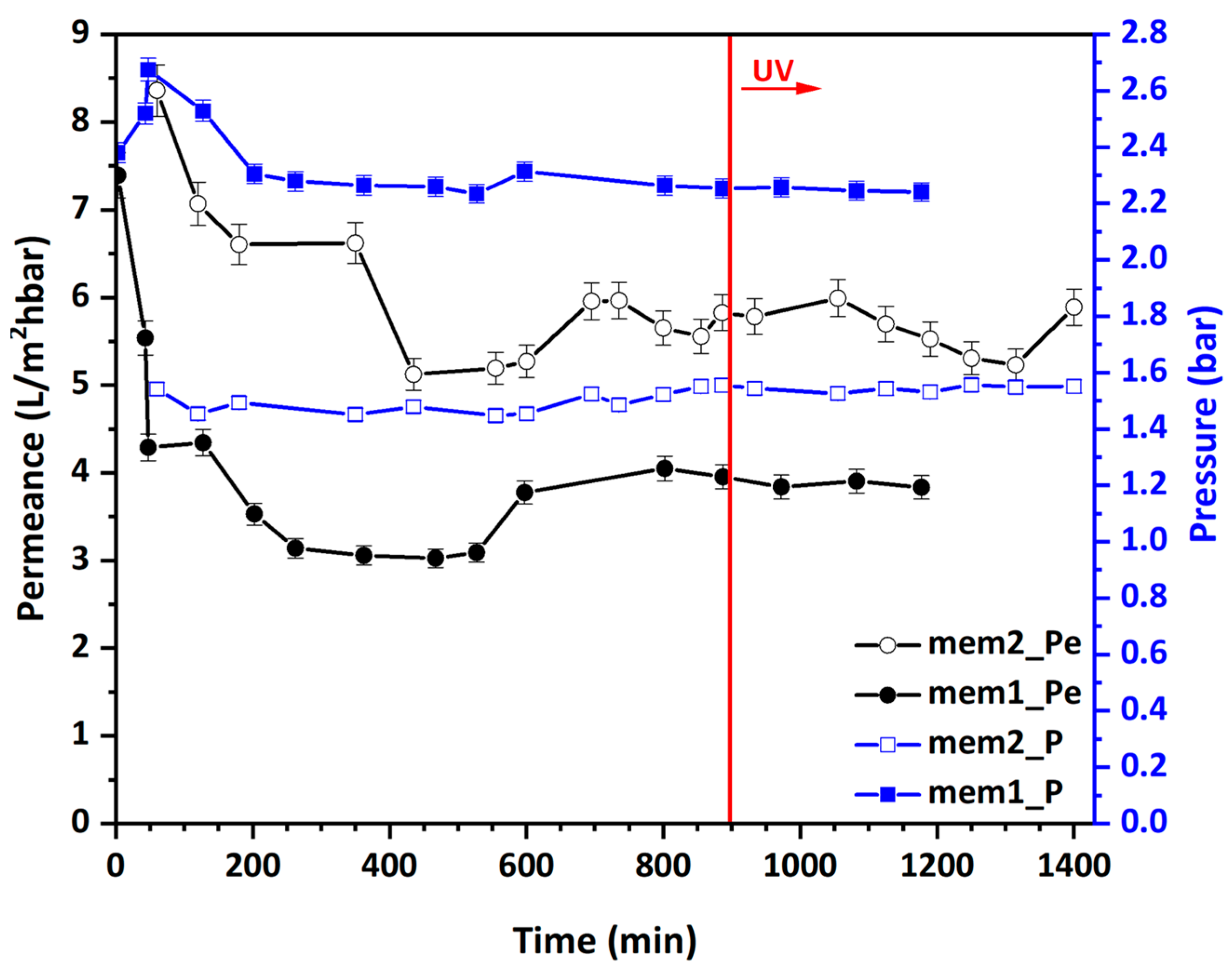
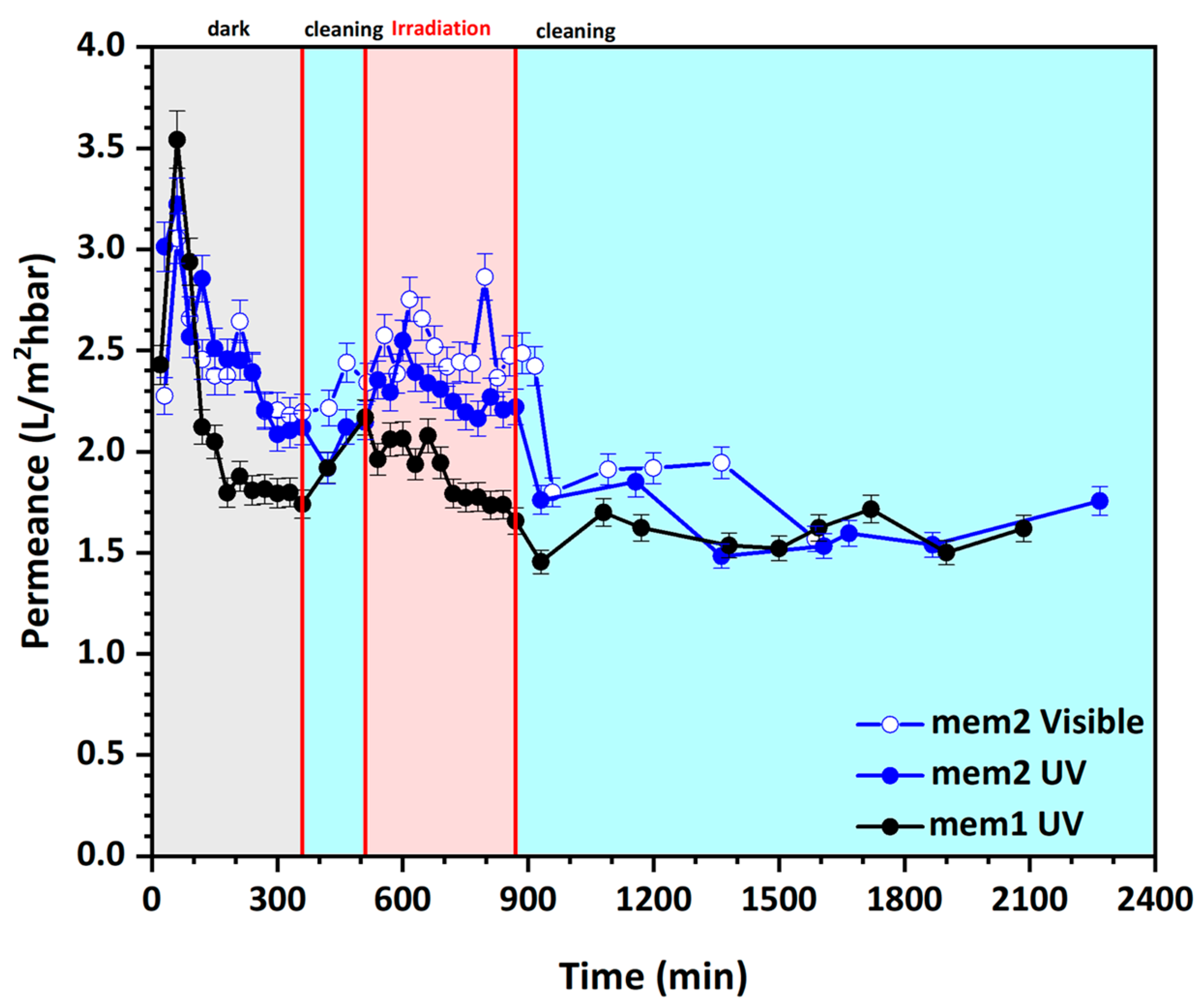
3.4. Study of Water Permeance of Photocatalytic Membranes
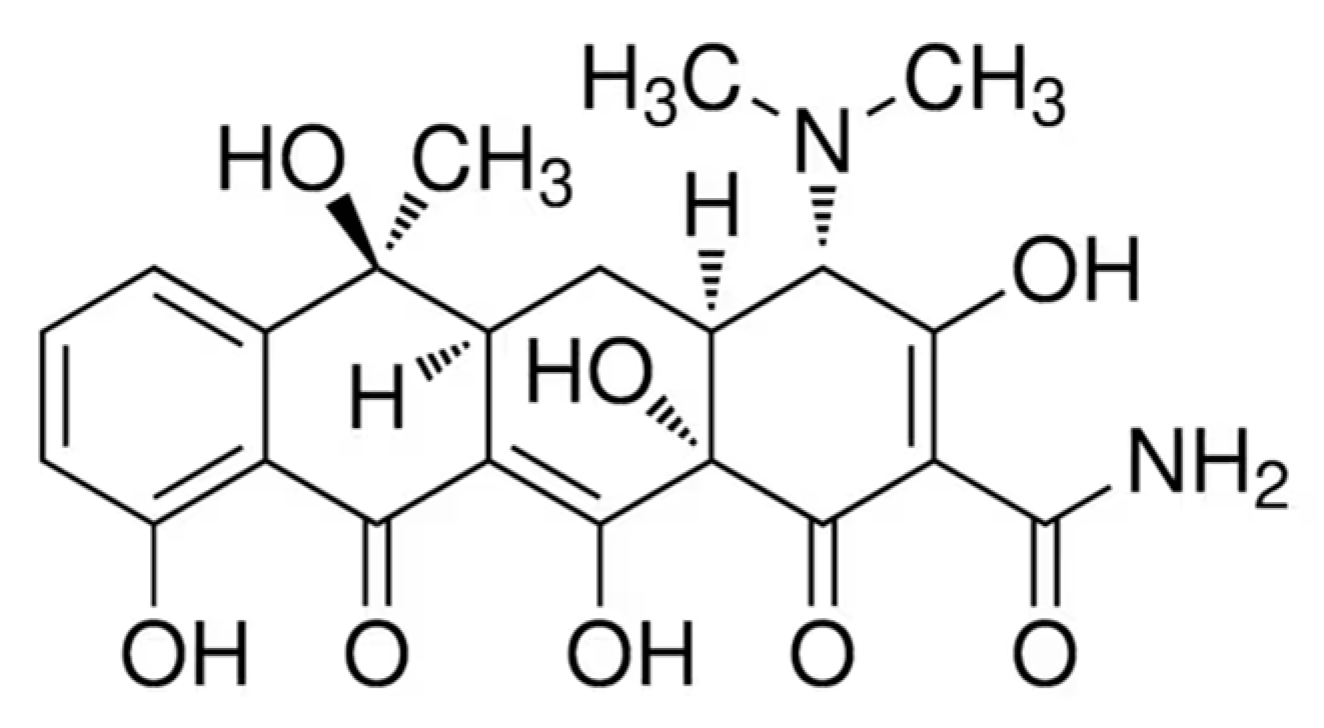
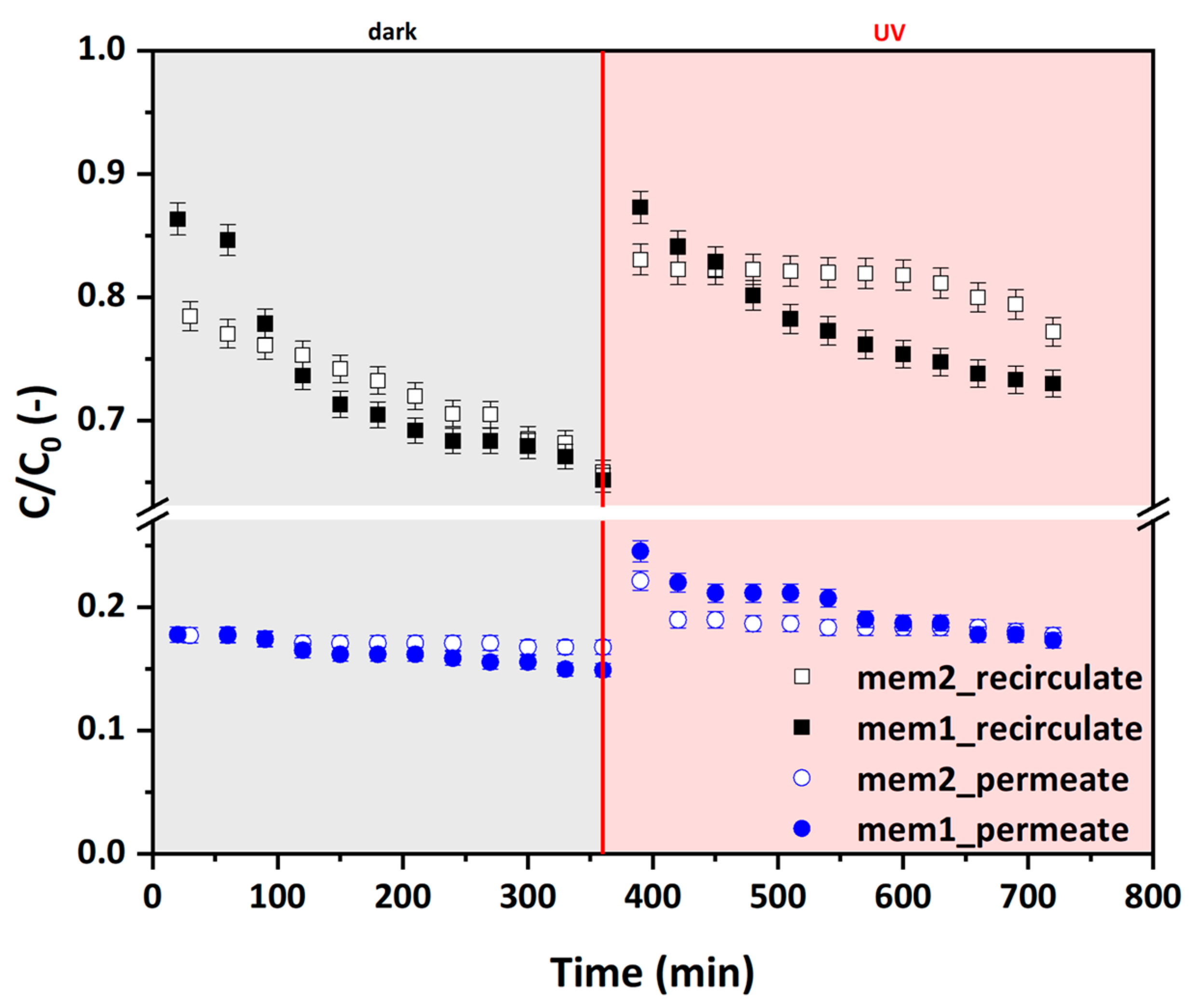
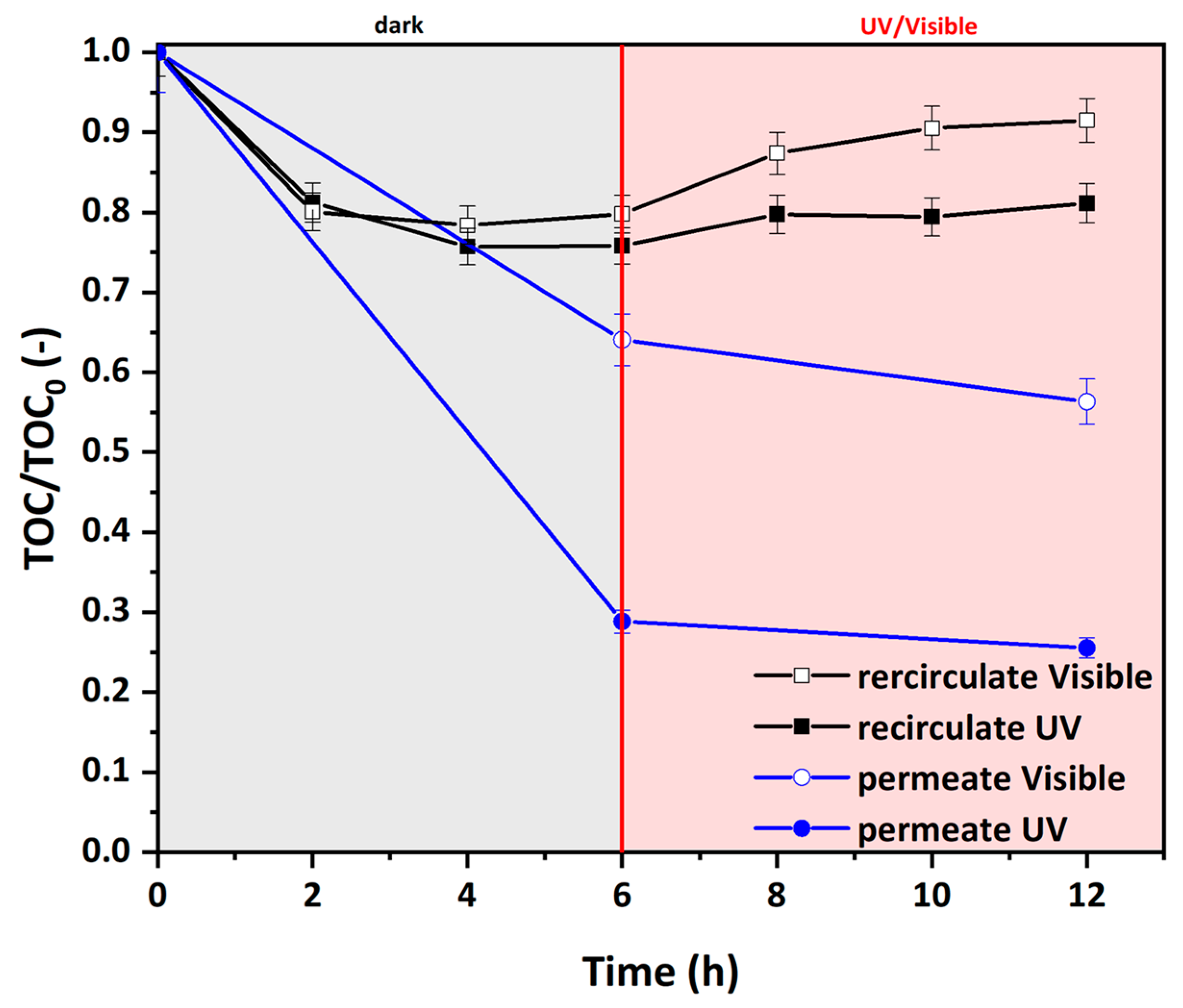
3.5. Photocatalytic Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Zhang, S.; Wang, P.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Urea catalytic oxidation for energy and environmental applications. Chem. Soc. Rev. 2024, 53, 1552–1591. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-T.; Xu, G.-D.; Chen, L.-J.; Jia, J.-H.; Peng, Y.-W. Catalytic advanced oxidation processes (AOPS) in water treatment by covalent organic frameworks-based materials: A review. Res. Chem. Intermed. 2021, 47, 3109–3130. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kong, D.; Liu, D.; Pan, L.; Chen, Y.; Zhang, X.; Zou, J.-J. Bimetallic phosphide decorated Mo–BiVO4 for significantly improved photoelectrochemical activity and stability. RSC Adv. 2019, 9, 15629. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, Y.; Zhang, W.; Zhang, Z.; Hua, R. Preparation and photocatalytic properties of Mo-doped BiVO4. J. Mater. Sci. Mater. Electron. 2019, 30, 19335–19342. [Google Scholar] [CrossRef]
- Pylarinou, M.; Sakellis, E.; Tsipas, P.; Romanos, G.E.; Gardelis, S.; Dimoulas, A.; Likodimos, V. Mo-BiVO4/Ca-BiVO4 Homojunction nanostructure-based inverse opals for photoelectrocatalytic pharmaceutical degradation under visible light. ACS Appl. Nano Mater. 2023, 6, 6759–6771. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, C.-Y.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic enhancement in BiVO4 photonic crystals for efficient water splitting. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, K.; Cheng, H.; Zhang, L. A green desulfurization technique: Utilization of flue gas SO2 to produce H2 via a photoelectrochemical process based on Mo-doped BiVO4. Front. Chem. 2017, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Perkins, C.L.; Lin, Q.; Law, M. Textured nanoporous Mo:BiVO4 photoanodes with high charge transport and charge transfer quantum efficiencies for oxygen evolution. Energy Environ. Sci. 2016, 9, 1412–1429. [Google Scholar] [CrossRef]
- Ta, C.X.M.; Akamoto, C.; Furusho, Y.; Amano, F. A macroporous-structured WO3/Mo-doped BiVO4 photoanode for vapor-fed water splitting under visible light irradiation. ACS Sustain. Chem. Eng. 2020, 8, 9456–9463. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211–212, 304–316. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid ultrafiltration/photocatalytic membranes for efficient water treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Han, H.; Dai, R.; Wang, Z. Fabrication of high-performance thin-film composite nanofiltration membrane by dynamic calcium-carboxyl intra-bridging during post-treatment. Membranes 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Barquín, C.; Vital-Grappin, A.; Kumakiri, I.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Performance of TiO2-based tubular membranes in the photocatalytic degradation of organic compounds. Membranes 2023, 13, 448. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Ye, J.; Mai, Y.; Wang, C.; Yang, Y.; Li, Y.; Besenbacher, F.; Niemantsverdriet, H.; Rosei, F.; et al. A modular tubular flow system with replaceable photocatalyst membranes for scalable coupling and hydrogenation. Angew. Chem. Int. Ed. 2023, 62, e202302979. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Abdallah, H.; Khairy, S.A. Low-cost photocatalytic membrane modified with green heterojunction TiO2/ZnO nanoparticles prepared from waste. Sci. Rep. 2023, 13, 22150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Wu, G.-R.; Sun, L.-J.; Xu, J.-K.; Gao, S.-Q.; Tan, X.-S.; Lin, Y.-W. Efficient degradation of tetracycline antibiotics by engineered myoglobin with high peroxidase activity. Molecules 2022, 27, 8660. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Naing, H.H.; Wang, K.; Li, Y.; Mishra, A.K.; Zhang, G. Sepiolite supported BiVO4 nanocomposites for efficient photocatalytic degradation of organic pollutants: Insight into the interface effect towards separation of photogenerated charges. Sci. Total Environ. 2020, 722, 137825. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mandal, M.K.; Pandey, S.; Kumar, R.; Dubey, K.K. Visible-light-driven photocatalytic degradation of tetracycline using heterostructured Cu2O−TiO2 nanotubes, kinetics, and toxicity evaluation of degraded products on cell lines. ACS Omega 2022, 7, 33572–33586. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Olatunji, O.S. Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle-like SnO2 nanoparticles anchored on exfoliated g-C3N4. Environ. Sci. Eur. 2022, 34, 5. [Google Scholar] [CrossRef]
- Krakkóa, D.; Heieren, B.T.; Illés, Á.; Kvamme, K.; Dóbéc, S.; Záray, G. (V)UV degradation of the antibiotic tetracycline: Kinetics, transformation products and pathway. Process Saf. Environ. Prot. 2022, 163, 395–404. [Google Scholar] [CrossRef]
- Liaqat, M.; Khalid, N.R.; Tahir, M.B.; Znaidia, S.; Alrobei, H.; Alzaid, M. Visible light induced photocatalytic activity of MnO2/BiVO4 for the degradation of organic dye and tetracycline. Ceram. Int. 2023, 49, 10455–10461. [Google Scholar] [CrossRef]
- Phillips, K.R.; Shirman, T.; Shirman, E.; Shneidman, A.V.; Kay, T.M.; Aizenberg, J. Nanocrystalline precursors for the co-assembly of crack-free metal oxide inverse opals. Adv. Mater. 2018, 30, 1706329. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Krishnan, R.S. Raman effect of corundum. J. Chem. Phys. 1967, 47, 1009–1012. [Google Scholar] [CrossRef]
- Kadleíková, M.; Breza, J.; Veselý, M. Raman spectra of synthetic sapphire. Microelectron. J. 2001, 32, 955–958. [Google Scholar] [CrossRef]
- Labropoulos, A.; Romanos, G.E.; Kouvelos, E.; Falaras, P.; Likodimos, V.; Francisco, M.; Kroon, M.C.; Iliev, B.; Adamova, G.; Schubert, T.J.S. Alkyl-methyl-imidazolium tricyanomethanide ionic liquids under extreme confinement onto nanoporous ceramic membranes. J. Phys. Chem. C 2013, 117, 10114–10127. [Google Scholar] [CrossRef]
- Cava, S.; Tebcherani, S.M.; Souza, I.A.; Pianaro, S.A.; Paskocimas, C.A.; Longo, E.; Varela, J.A. Structural characterization of phase transition of Al2O3 nanopowders obtained by polymeric precursor method. Mater. Chem. Physics 2007, 103, 394–399. [Google Scholar] [CrossRef]
- Tzialla, O.; Labropoulos, A.; Panou, A.; Sanopoulou, M.; Kouvelos, E.; Athanasekou, C.; Beltsios, K.; Likodimos, V.; Falaras, P.; Romanos, G. Phase behavior and permeability of alkyl-methyl-imidazolium tricyanomethanide ionic liquids supported in nanoporous membranes. Sep. Purif. Technol. 2014, 135, 22–34. [Google Scholar] [CrossRef]
- Quintard, P.E.; Barbéris, P.; Mirgorodsky, A.P.; Merle-Méjean, T. Comparative lattice-dynamical study of the Raman spectra of monoclinic and tetragonal phases of zirconia and hafnia. J. Am. Ceram. Soc. 2002, 85, 1745–1749. [Google Scholar] [CrossRef]
- Tabares, J.A.M.; Anglada, M.J. Quantitative analysis of monoclinic phase in 3Y-TZP by Raman spectroscopy. J. Am. Ceram. Soc. 2010, 93, 1790–1795. [Google Scholar] [CrossRef]
- Pellicer-Porres, J.; Vázquez-Socorro, D.; López-Moreno, S.; Muñoz, A.; Rodríguez-Hernández, P.; Martínez-García, D.; Achary, S.N.; Rettie, A.J.E.; Mullins, C.B. Phase transition systematics in BiVO4 by means of high-pressure–high-temperature Raman experiments. Phys. Rev. B 2018, 98, 214109. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E.; Eckert, H.; Jefferson, D.A. Vanadium(V) environments in bismuth vanadates: A structural investigation using Raman spectroscopy and solid state 51V NMR. J. Solid State Chem. 1991, 90, 194–210. [Google Scholar] [CrossRef]
- Zhou, D.; Pang, L.-X.; Guo, J.; Wang, H.; Yao, X.; Randall, C. Phase evolution, phase transition, Raman spectra, infrared spectra, and microwave dielectric properties of low temperature firing (K0.5xBi1−0.5x)(MoxV1−x)O4 ceramics with scheelite related structure. Inorg. Chem. 2011, 50, 12733–12738. [Google Scholar] [CrossRef]
- Lai, F.-D. Composite thin films of (ZrO2)x-(Al2O3)1−x for high transmittance attenuated phase shifting mask in ArF optical lithography. J. Vac. Sci. Technol. B 2004, 22, 1174–1178. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Pavitra, V.; Ahmad, P.; Syed, A.; Nagaraju, G. Photocatalytic degradation of an organic dye using Ag doped ZrO2 nanoparticles: Milk powder facilitated eco-friendly synthesis. J. King Saud Univ. Sci. 2020, 32, 1872–1878. [Google Scholar] [CrossRef]
- Wu, H.; Irani, R.; Zhang, K.; Jing, L.; Dai, H.; Chung, H.Y.; Abdi, F.F.; Ng, Y.H. Unveiling carrier dynamics in periodic porous BiVO4 photocatalyst for enhanced solar water splitting. ACS Energy Lett. 2021, 6, 3400–3407. [Google Scholar] [CrossRef]
- Kumari, L.; Xu, J.M.; Leblanc, R.M.; Wang, D.Z.; Li, Y.; Guo, H.; Zhang, J. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 2009, 9, 3874–3880. [Google Scholar] [CrossRef]
- Iurina, V.I.; Neshchimenko, V.V.; Mikhailov, M.M.; Li, C. Color centers induced by proton exposure in aluminum oxide hollow particles. AIP Conf. Proc. 2018, 2051, 20108. [Google Scholar]
- Yaroshchuk, A.E. Non-steric mechanisms of nanofiltration: Superposition of Donnan and dielectric exclusion. Sep. Purif. Technol. 2001, 22–23, 143–158. [Google Scholar] [CrossRef]
- Epsztein, R.; Shaulsky, E.; Dizge, N.; Warsinger, D.M.; Elimelech, M. Role of ionic charge density in Donnan exclusion of monovalent anions by nanofiltration. Environ. Sci. Technol. 2018, 52, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulos, G.V.; Arfanis, M.K.; Sánchez Pérez, J.A.; Agüera, A.; Cadena Aponte, F.X.; Markellou, E.; Romanos, G.E.; Falaras, P. Novel pilot-scale photocatalytic nanofiltration reactor for agricultural wastewater treatment. Membranes 2023, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Vangeli, O.C.; Romanos, G.E.; Beltsios, K.G.; Fokas, D.; Athanasekou, C.P.; Kanellopoulos, N.K. Development and characterization of chemically stabilized ionic liquid membranes-Part I: Nanoporous ceramic supports. J. Membr. Sci. 2010, 365, 366–377. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding photoelectrocatalytic degradation of tetracycline over three-dimensional coral-like ZnO/BiVO4 nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Debnath, B.; Majumdar, M.; Bhowmik, M.; Bhowmik, K.L.; Debnath, A.; Roy, D.N. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J. Environ. Manag. 2020, 261, 110235. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-R.; Huang, C.-H. Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere 2010, 79, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ji, Y.; Wang, F.; Lei, H.; Gu, Z. Adsorption of tetracycline onto alumina: Experimental research and molecular dynamics simulation. Desalination Water Treat. 2016, 57, 5174–5182. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, G.V.; Pylarinou, M.; Sakellis, E.; Katsaros, F.K.; Likodimos, V.; Romanos, G.E. Mo-BiVO4 Photocatalytically Modified Ceramic Ultrafiltration Membranes for Enhanced Water Treatment Efficiency. Membranes 2024, 14, 112. https://doi.org/10.3390/membranes14050112
Theodorakopoulos GV, Pylarinou M, Sakellis E, Katsaros FK, Likodimos V, Romanos GE. Mo-BiVO4 Photocatalytically Modified Ceramic Ultrafiltration Membranes for Enhanced Water Treatment Efficiency. Membranes. 2024; 14(5):112. https://doi.org/10.3390/membranes14050112
Chicago/Turabian StyleTheodorakopoulos, George V., Martha Pylarinou, Elias Sakellis, Fotios K. Katsaros, Vlassis Likodimos, and George Em. Romanos. 2024. "Mo-BiVO4 Photocatalytically Modified Ceramic Ultrafiltration Membranes for Enhanced Water Treatment Efficiency" Membranes 14, no. 5: 112. https://doi.org/10.3390/membranes14050112
APA StyleTheodorakopoulos, G. V., Pylarinou, M., Sakellis, E., Katsaros, F. K., Likodimos, V., & Romanos, G. E. (2024). Mo-BiVO4 Photocatalytically Modified Ceramic Ultrafiltration Membranes for Enhanced Water Treatment Efficiency. Membranes, 14(5), 112. https://doi.org/10.3390/membranes14050112









