Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study
Abstract
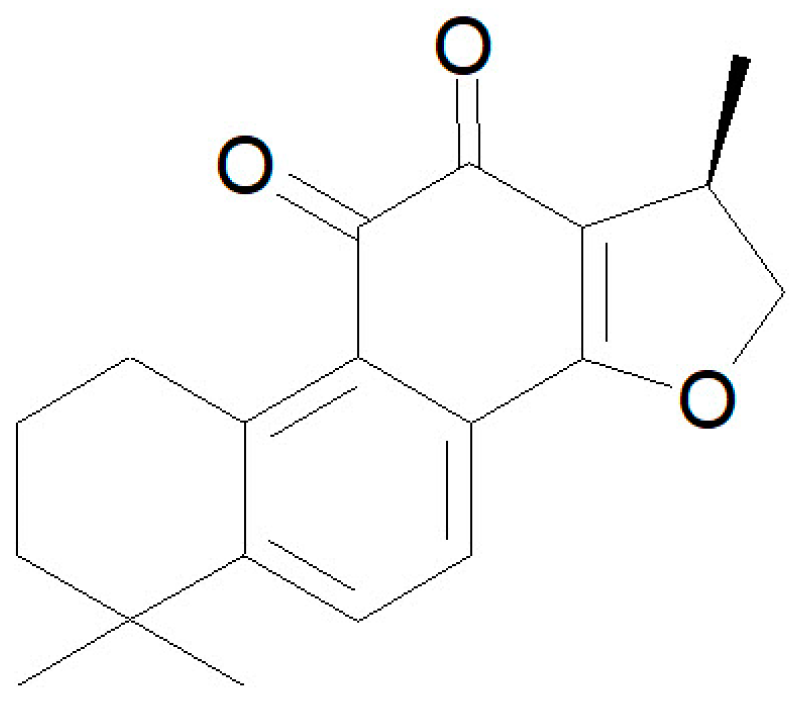
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Partition of CPT into Phospholipid Bilayers
2.3. Vesicle Contents Release
2.4. Differential Scanning Calorimetry
2.5. Fourier-Transform Infrared Spectroscopy
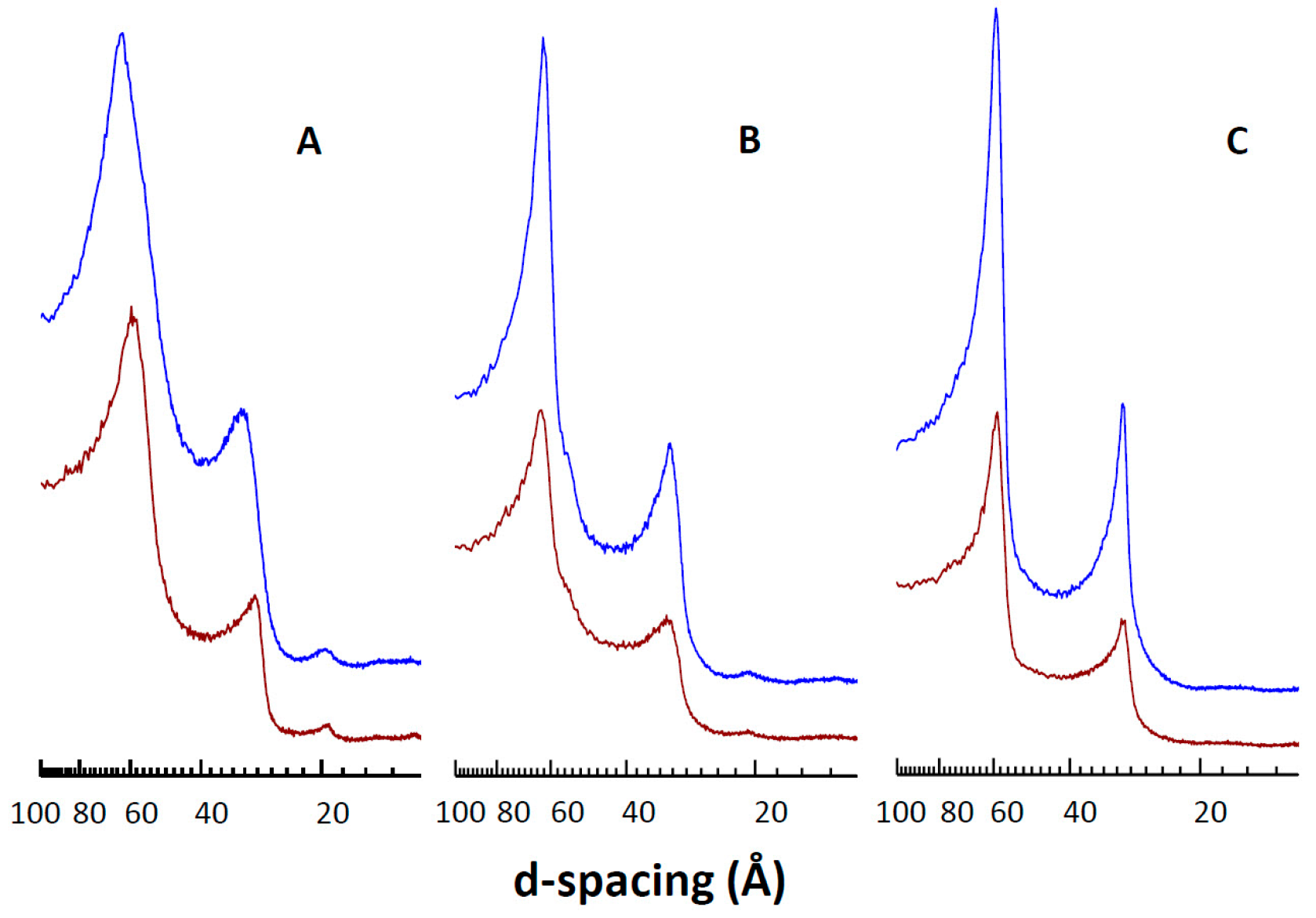
2.6. X-ray Scattering
2.7. Molecular Dynamics Simulations
3. Results and Discussion
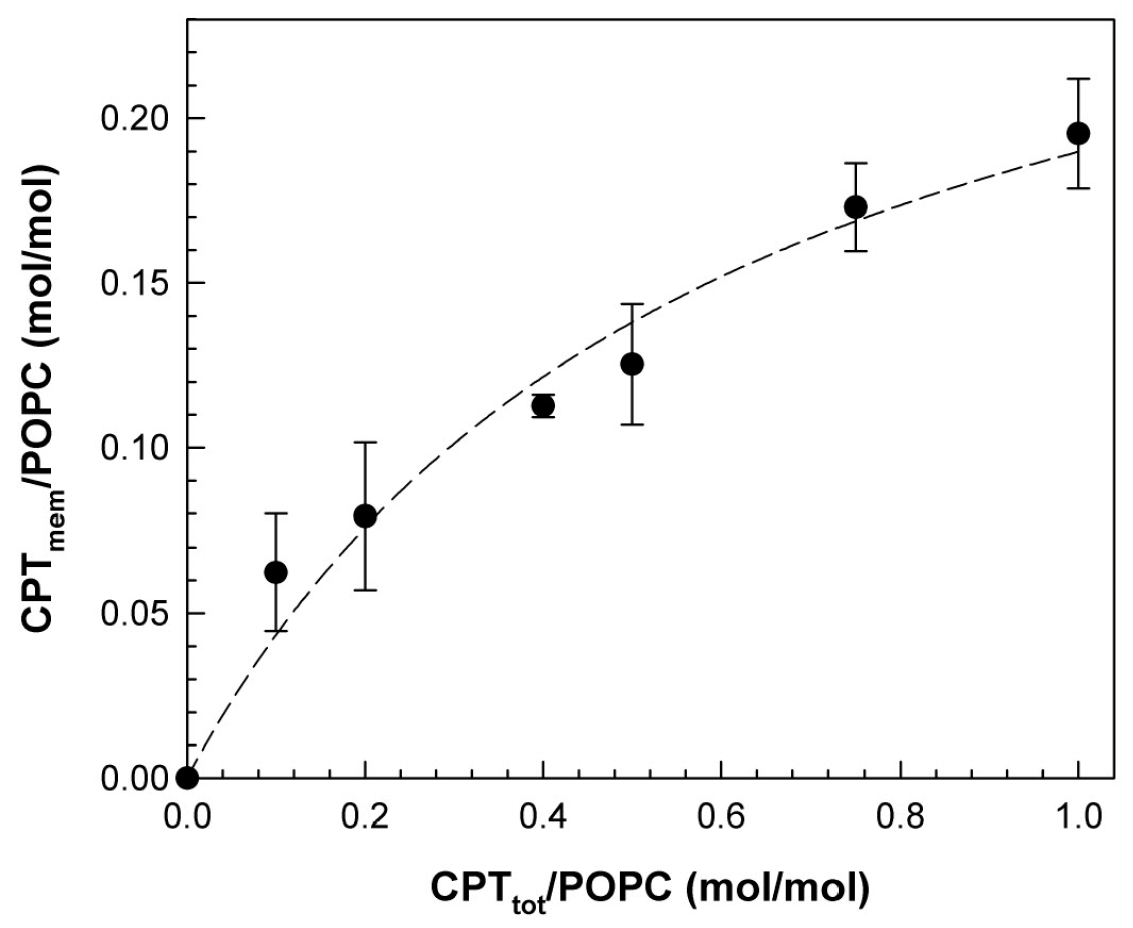
3.1. Partition of CPT into POPC Membranes
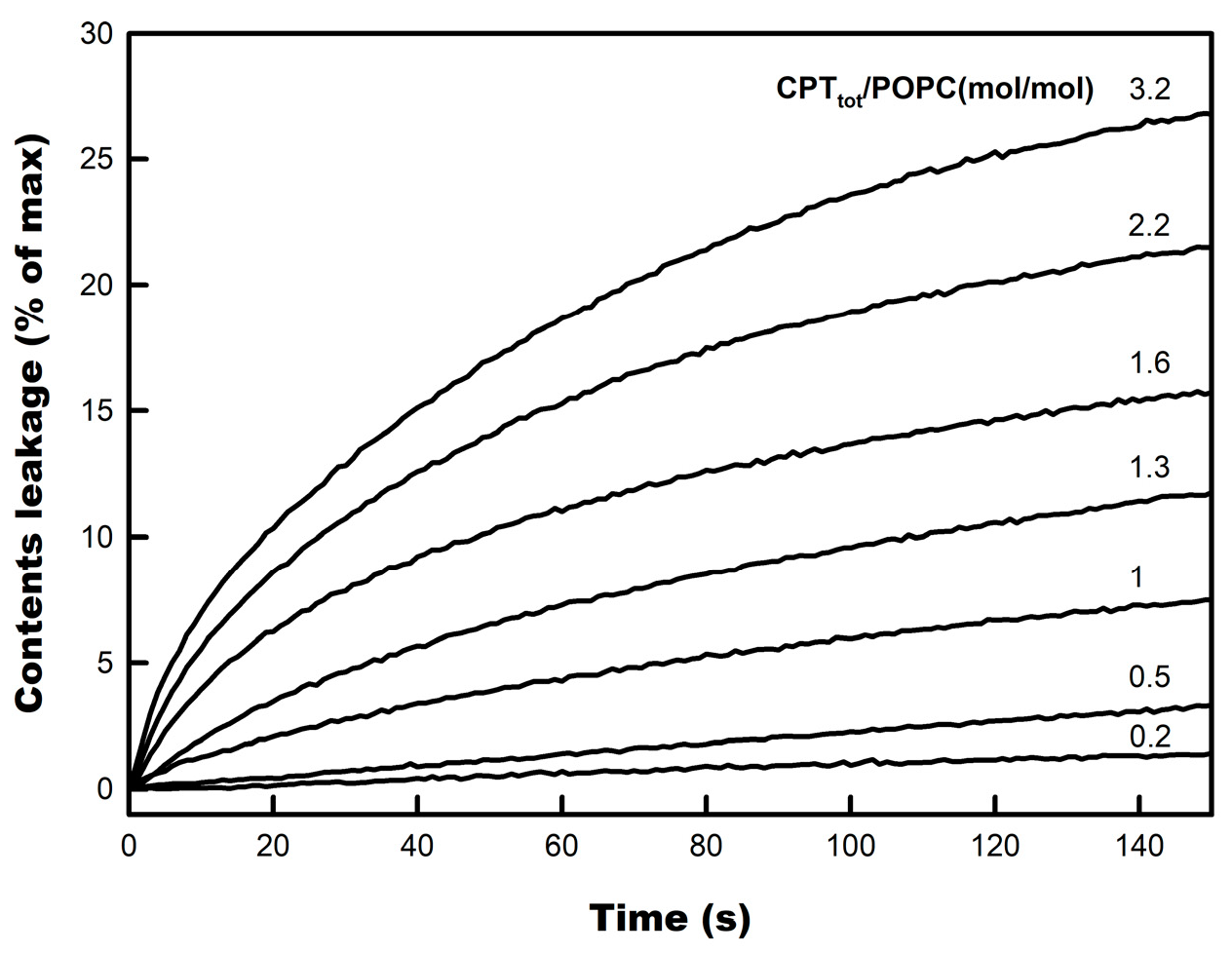
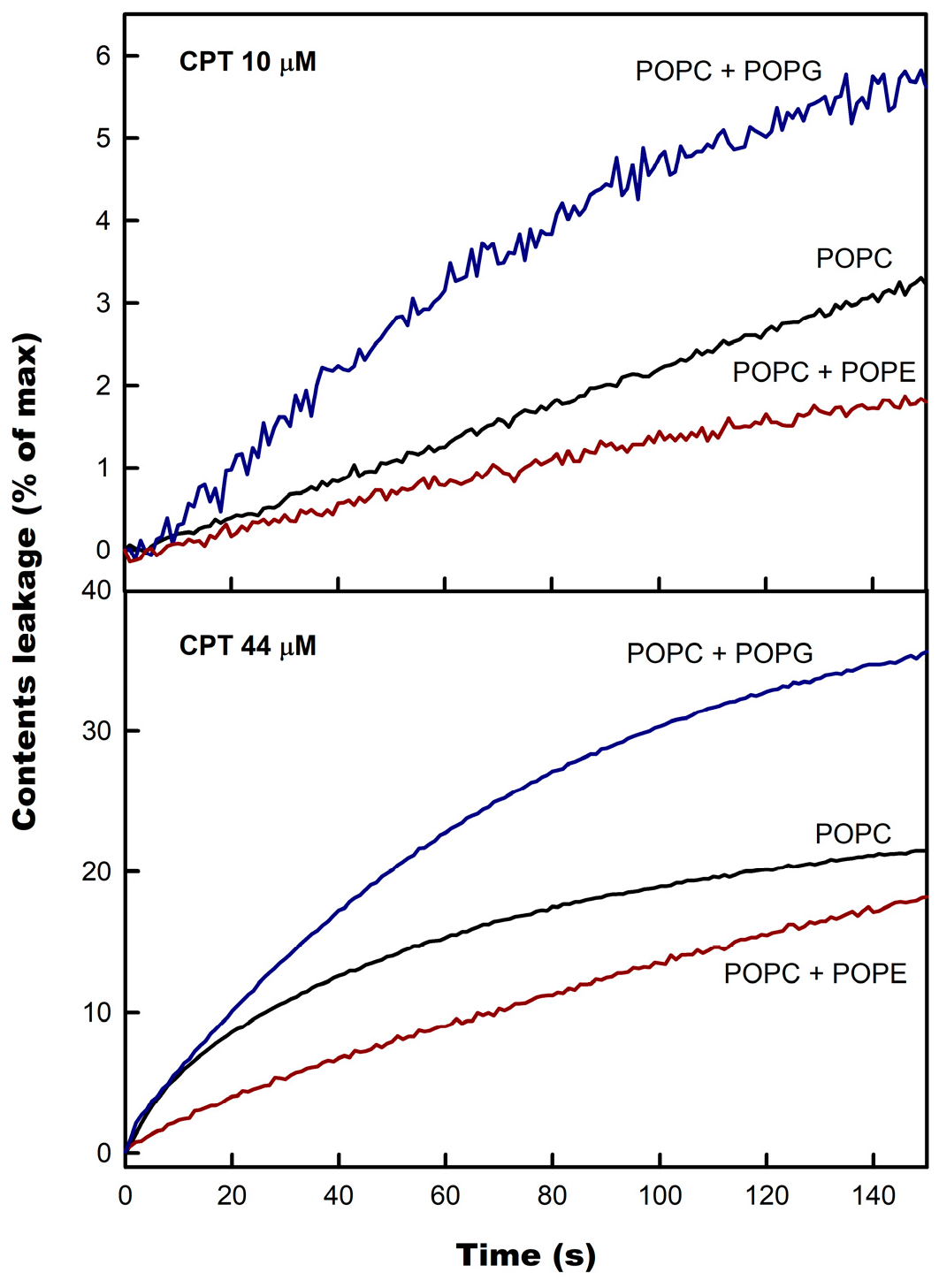
3.2. CPT-Induced Membrane Permeabilization
3.3. Modulation of DMPC Thermotropic Transitions by CPT
3.4. Effect of CPT on Acyl Chain and Polar Headgroup Regions of the DMPC Bilayer
3.5. Effect of CPT on DMPC Structural Parameters
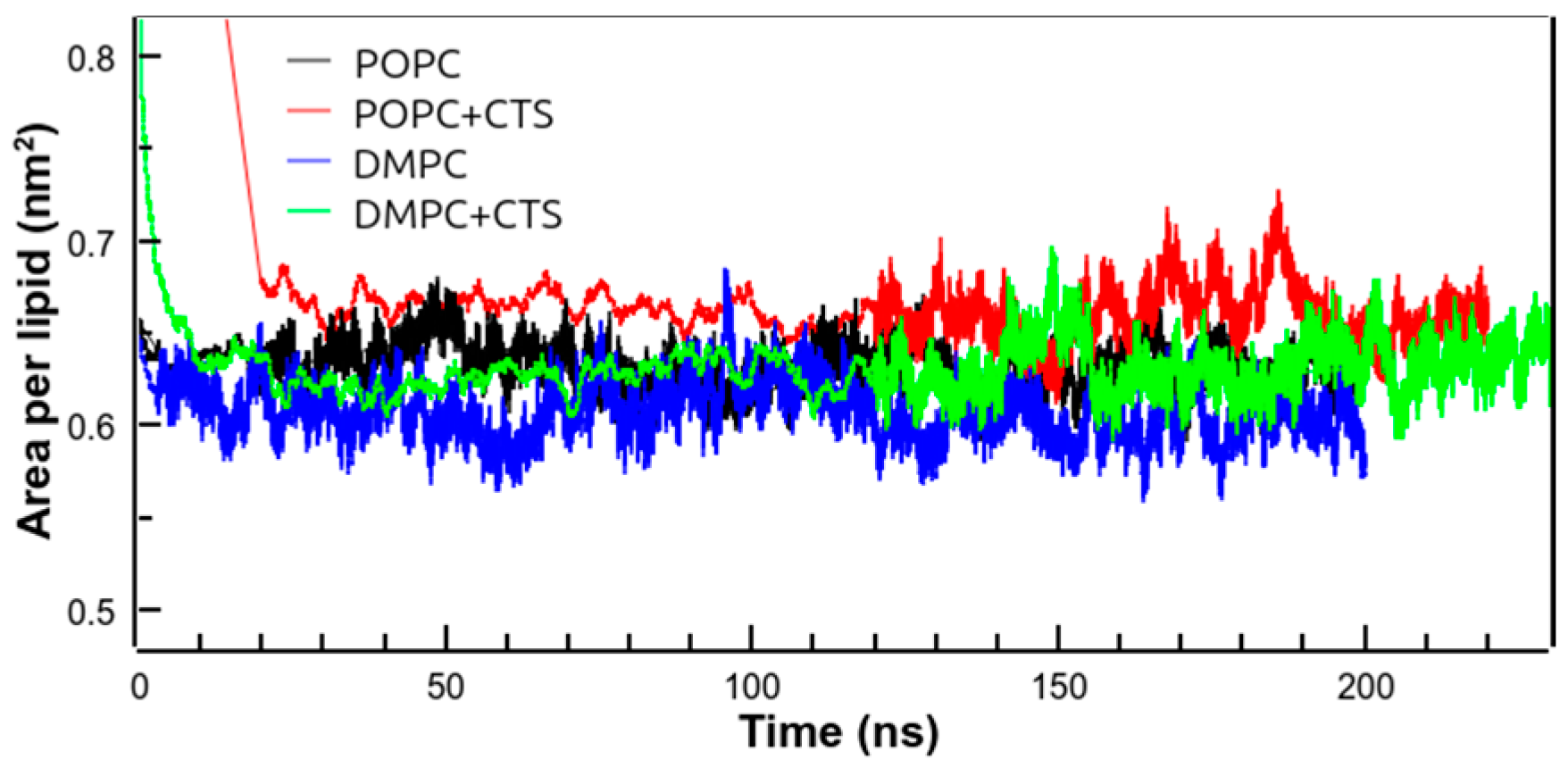
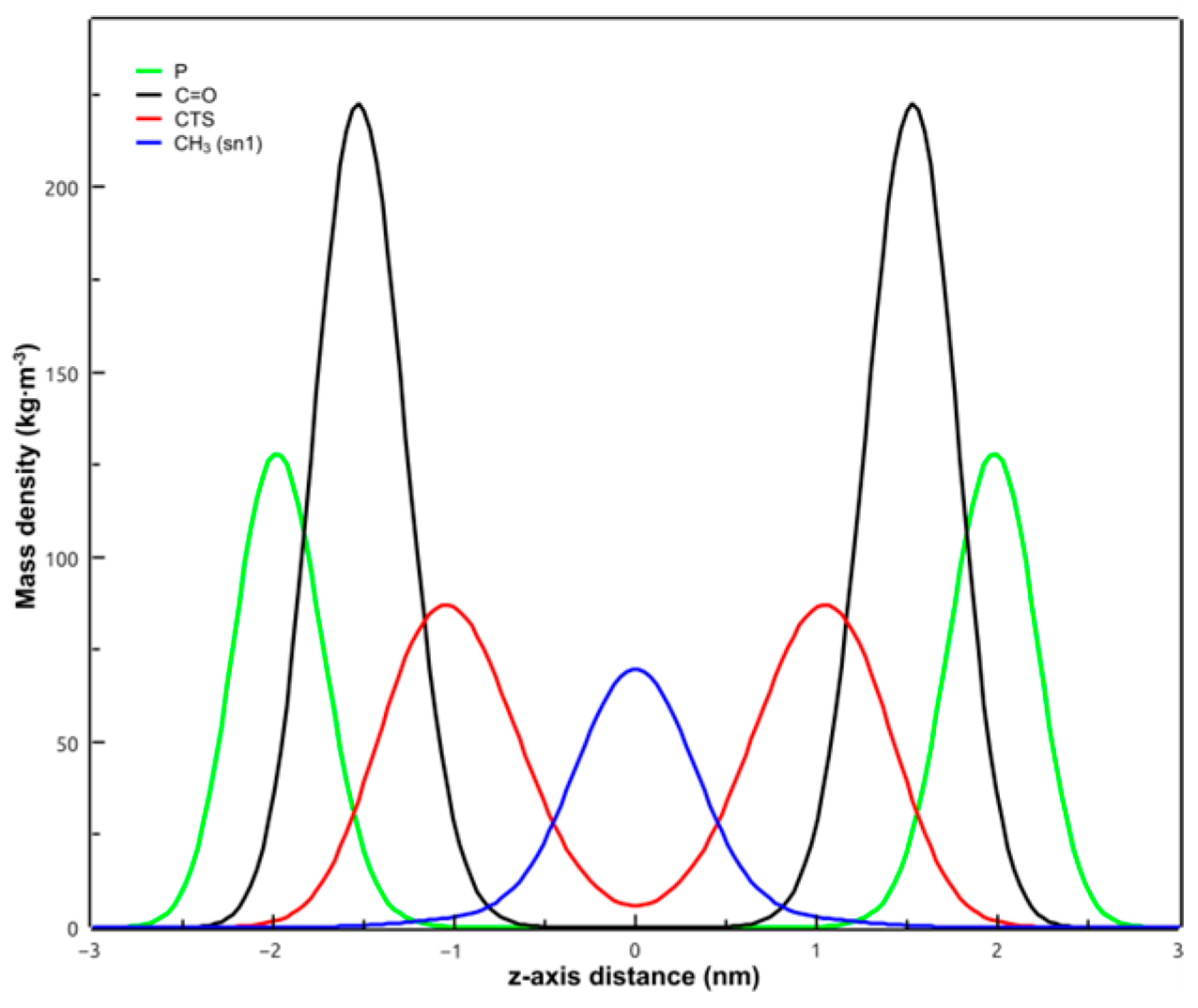
3.6. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 439237. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Zhong, C.; Chen, Z.; Zheng, Z.; Li, S.; Chen, B.; Wu, Q.; Yao, H. Tanshinone I: Pharmacological activities, molecular mechanisms against diseases and future perspectives. Phytomedicine 2023, 110, 154632. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, L.; Ding, F.; Yang, K.; Feng, Y.; Tang, H.; Xu, Q.M.; Feng, J.; Yang, S. Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. Sci. Rep. 2018, 8, 8460. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.Y.H.; Wu, Y.R.Y.H.; Li, B.; Yan, Z.Y. Cryptotanshinone: A review of its pharmacology activities and molecular mechanisms. Fitoterapia 2020, 145, 104633. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Saberifar, S.; Salami, S.; Hushmandi, K.; Najafi, M. Recent advances and future directions in anti-tumor activity of cryptotanshinone: A mechanistic review. Phyther. Res. 2021, 35, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Liu, X.; Gao, X. Pharmacological Properties of Tanshinones, the Natural Products from Salvia miltiorrhiza, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 87. [Google Scholar]
- Chen, W.; Lu, Y.; Chen, G.; Huang, S. Molecular Evidence of Cryptotanshinone for Treatment and Prevention of Human Cancer. Anticancer Agents Med. Chem. 2013, 13, 979–987. [Google Scholar] [CrossRef]
- Vundavilli, H.; Datta, A.; Sima, C.; Hua, J.; Lopes, R.; Bittner, M. Targeting oncogenic mutations in colorectal cancer using cryptotanshinone. PLoS ONE 2021, 16, e0247190. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Datta, A.; Sima, C.; Hua, J.; Lopes, R.; Bittner, M.L.; Miller, T.; Wilson-Robles, H.M. In Silico Modeling of the Induction of Apoptosis by Cryptotanshinone in Osteosarcoma Cell Lines. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 1683–1693. [Google Scholar] [CrossRef]
- Chen, B.C.; Ding, Z.S.; Dai, J.S.; Chen, N.P.; Gong, X.W.; Ma, L.F.; Qian, C.D. New Insights Into the Antibacterial Mechanism of Cryptotanshinone, a Representative Diterpenoid Quinone from Salvia miltiorrhiza Bunge. Front. Microbiol. 2021, 12, 647289. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Wang, T.; Li, N.; Mu, H.; Zhang, J.; Duan, J. Unveiling the mode of action of two antibacterial tanshinone derivatives. Int. J. Mol. Sci. 2015, 16, 17668–17681. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Noh, J.; Hong, S. Antibacterial Activities of Cryptotanshinone and Dihydrotanshinone I from a Medicinal Herb, Salvia miltiorrhiza Bunge. Biosci. Biotechnol. Biochem. 1999, 63, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Lee, J.H.; Choi, K.M.; Choi, S.M.; Park, J.H. Synergistic effect between cryptotanshinone and antibiotics against clinic methicillin and vancomycin-resistant Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2014, 2014, 450572. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.J.F.; van Gent, C.M.; Pries, C. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Coronel, J.R.; Aranda, F.J.; Teruel, J.A.; Marqués, A.; Manresa, Á.; Ortiz, A. Kinetic and Structural Aspects of the Permeabilization of Biological and Model Membranes by Lichenysin. Langmuir 2016, 32, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; van der Spoel, D.; Lindahl, E.; Hess, B.; the GROMACS Developent Team. GROMACS User Manual Version 2018. 2018. Available online: www.gromacs.org (accessed on 26 April 2024).
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Bjelkmar, P.; Larsson, P.; Cuendet, M.A.; Hess, B.; Lindahl, E. Implementation of the CHARMM Force Field in GROMACS: Analysis of Protein Stability Effects from Correction Maps, Virtual Interaction Sites, and Water Models. J. Chem. Theory Comput. 2010, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Schrödinger, L. PyMOL. 2015. Available online: https://github.com/schrodinger/pymol-open-source (accessed on 26 April 2024).
- Seelig, J. Deuterium magnetic resonance: Theory and application to lipid membranes. Q. Rev. Biophys. 1977, 10, 353–418. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Roux, B. Calculation of absolute protein-ligand binding free energy from computer simulations. Proc. Natl. Acad. Sci. USA 2005, 102, 6825–6830. [Google Scholar] [CrossRef] [PubMed]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Hub, J.S.; De Groot, B.L.; Van Der Spoel, D. g_wham—A Free Weighted Histogram Analysis Implementation Including Robust Error and Autocorrelation Estimates. J. Chem. Theory Comput. 2010, 6, 3713–3720. [Google Scholar] [CrossRef]
- Oberholzer, T.; Luisi, P.L. The Use of Liposomes for Constructing Cell Models. J. Biol. Phys. 2002, 28, 733–744. [Google Scholar] [CrossRef]
- Nasr, G.; Greige-Gerges, H.; Elaissari, A.; Khreich, N. Liposomal Membrane Permeability Assessment by Fluorescence Techniques: Main Permeabilizing Agents, Applications and Challenges; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 580, p. 119198. [Google Scholar]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Manna, M.; Mukhopadhyay, C. Molecular dynamics simulations of the interactions of kinin peptides with an anionic POPG bilayer. Langmuir 2011, 27, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- Nicol, F.; Nir, S.; Szoka, F.C. Effect of phospholipid composition on an amphipathic peptide-mediated pore formation in bilayer vesicles. Biophys. J. 2000, 78, 818. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Miao, Q.; Sun, Y.; Yang, F. tBid forms a pore in the liposome membrane. FEBS Lett. 2003, 555, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, A.; Aranda, F.J.; Espuny, M.J.; Teruel, J.A.; Marqués, A.; Manresa, A.; Ortiz, A. Mechanism of membrane permeabilization by a bacterial trehalose lipid biosurfactant produced by Rhodococcus sp. Langmuir 2009, 25, 7892–7898. [Google Scholar] [CrossRef]
- Sánchez, M.; Aranda, F.J.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, Á.; Ortiz, A. Permeabilization of biological and artificial membranes by a bacterial dirhamnolipid produced by Pseudomonas aeruginosa. J. Colloid Interface Sci. 2010, 341, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Heerklotz, H. Vesicle Leakage Reflects the Target Selectivity of Antimicrobial Lipopeptides from Bacillus subtilis. Biophys. J. 2015, 109, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Lipid intermolecular hydrogen bonding: Influence on structural organization and membrane function. Biochim. Biophys. Acta—Rev. Biomembr. 1987, 906, 353–404. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased Exposure of Anionic Phospholipids on the Surface of Tumor Blood Vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef]
- Battista, F.; Oliva, R.; Del Vecchio, P.; Winter, R.; Petraccone, L. Insights into the Action Mechanism of the Antimicrobial Peptide Lasioglossin III. Int. J. Mol. Sci. 2021, 22, 2857. [Google Scholar] [CrossRef] [PubMed]
- Rappolt, M.; Hickel, A.; Bringezu, F.; Lohner, K. Mechanism of the Lamellar/Inverse Hexagonal Phase Transition Examined by High Resolution X-ray Diffraction. Biophys. J. 2003, 84, 3111. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, A.; Aranda, F.J. Effects of dirhamnolipid on the structural properties of phosphatidylcholine membranes. Int. J. Pharm. 2006, 325, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Venable, R.M.; Krämer, A.; Pastor, R.W. Molecular Dynamics Simulations of Membrane Permeability. Chem. Rev. 2019, 119, 5954–5997. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nieh, M.P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta—Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Drabik, D.; Chodaczek, G.; Kraszewski, S.; Langner, M. Mechanical Properties Determination of DMPC, DPPC, DSPC, and HSPC Solid-Ordered Bilayers. Langmuir 2020, 36, 3826–3835. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Liu, Y.; Chu, N.; Petrache, H.I.; Tristram-Nagle, S.; Nagle, J.F. Structure of Fully Hydrated Fluid Phase DMPC and DLPC Lipid Bilayers Using X-ray Scattering from Oriented Multilamellar Arrays and from Unilamellar Vesicles. Biophys. J. 2005, 88, 2626. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Teruel, J.A.; Aranda, F.J.; Ortiz, A. On the Mechanism of Membrane Permeabilization by Tamoxifen and 4-Hydroxytamoxifen. Membranes 2023, 13, 292. [Google Scholar] [CrossRef]
- Comer, J.; Schulten, K.; Chipot, C. Calculation of Lipid-Bilayer Permeabilities Using an Average Force. J. Chem. Theory Comput. 2014, 10, 554–564. [Google Scholar] [CrossRef]
- Sajadi, F.; Rowley, C.N. Simulations of lipid bilayers using the CHARMM36 force field with the TIP3P-FB and TIP4P-FB water models. PeerJ 2018, 6, e5472. [Google Scholar] [CrossRef]

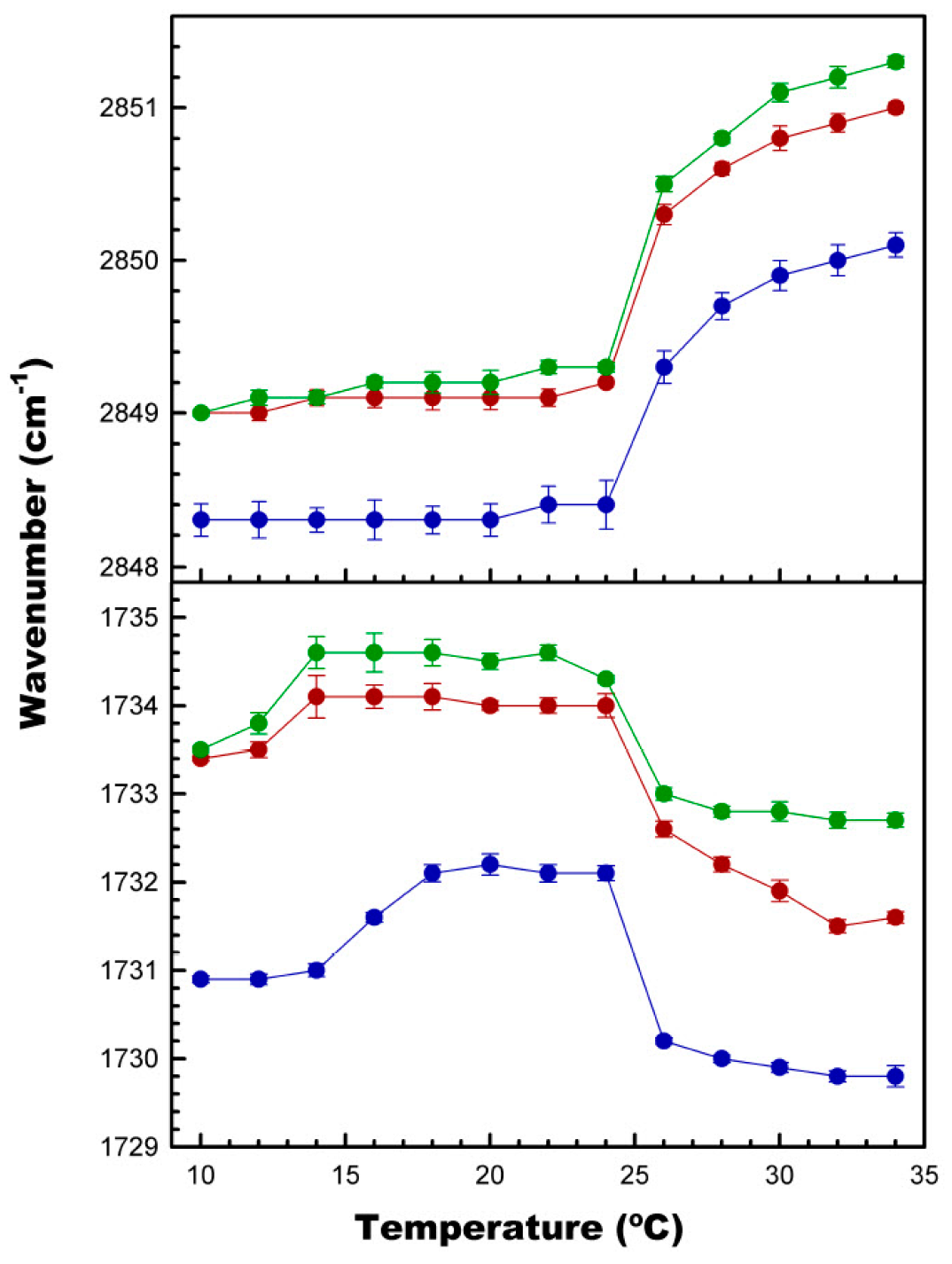
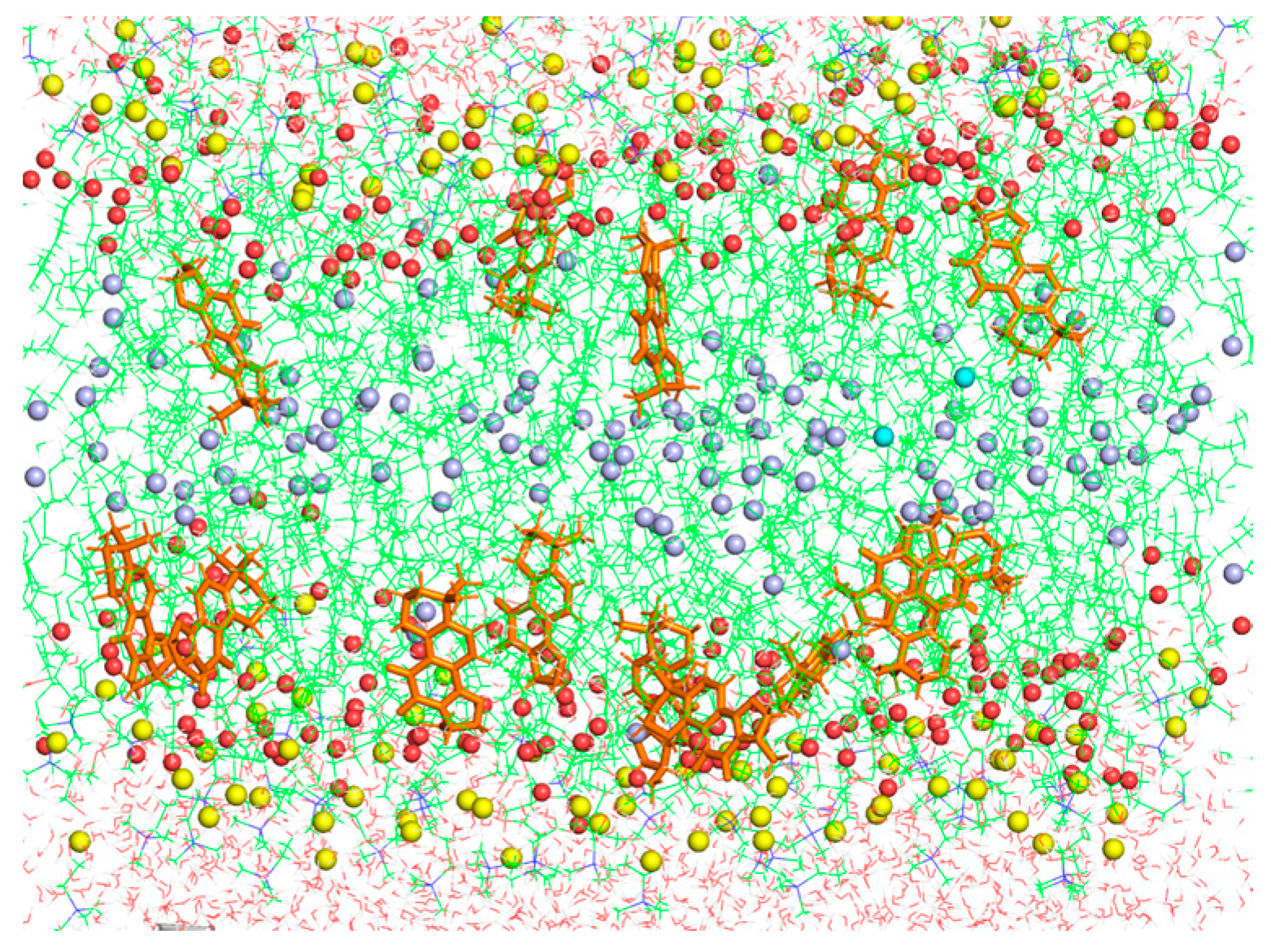
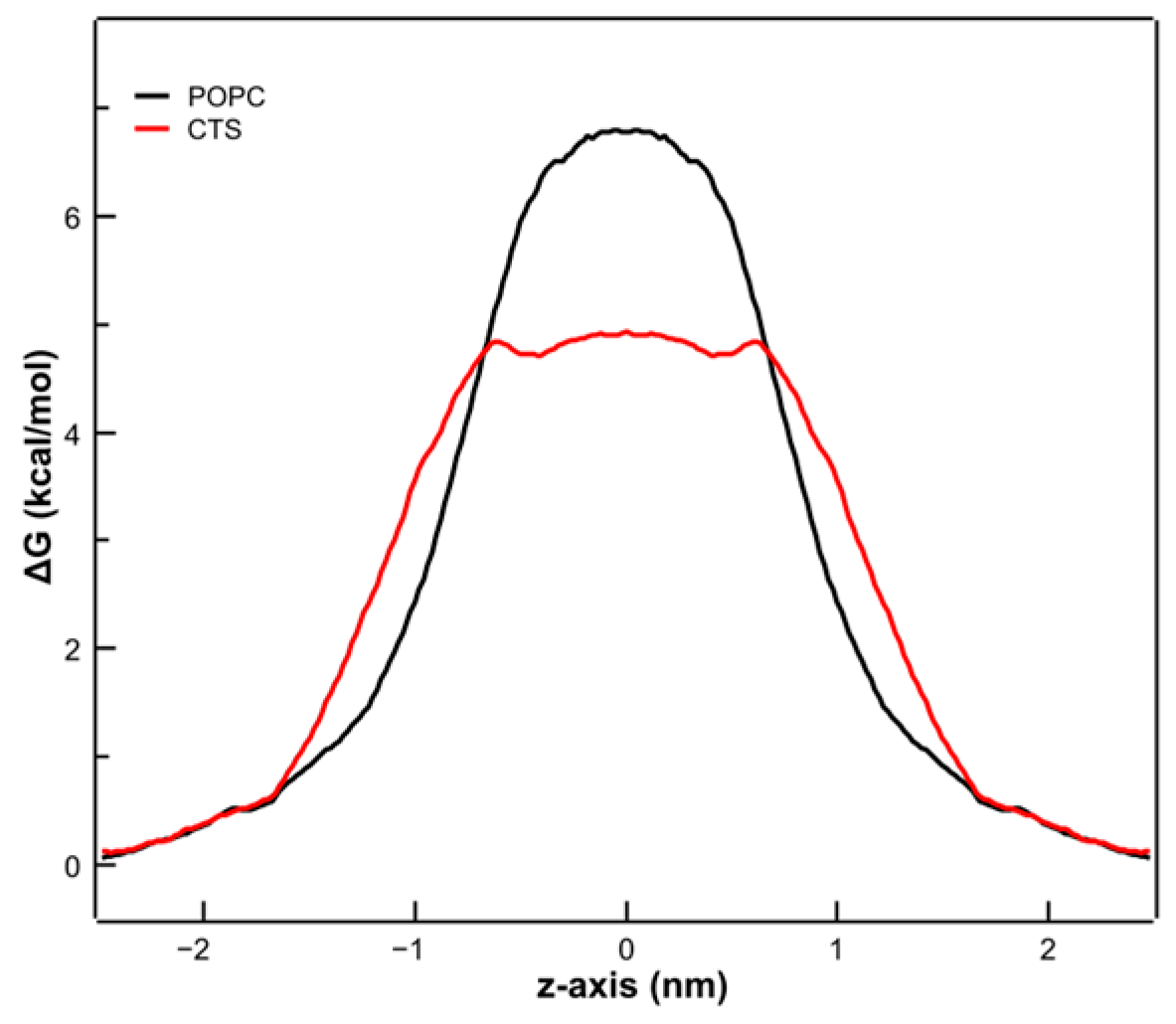
| Conditions | CH2 Symmetric Stretching | C=O Stretching |
|---|---|---|
| DMPC 12 °C | 2848.3 ± 0.12 | 1730.9 ± 0.06 |
| DMPC + CPT 12 °C | 2849.0 ± 0.05 | 1733.5 ± 0.09 |
| DMPC 34 °C | 2850.1 ± 0.08 | 1729.8 ± 0.12 |
| DMPC + CPT 34 °C | 2851.0 ± 0.04 | 1731.6 ± 0.06 |
| DPPC 24 °C | 2848.6 ± 0.11 | 1731.4 ± 0.04 |
| DPPC + CPT 24 °C | 2849.1 ± 0.03 | 1733.4 ± 0.03 |
| DPPC 50 °C | 2851.1 ± 0.03 | 1730.7 ± 0.13 |
| DPPC + CPT 50 °C | 2851.5 ± 0.03 | 1732.4 ± 0.10 |
| POPC 25 °C | 2848.9 ± 0.08 | 1726.9 ± 0.05 |
| POPC + CPT 25 °C | 2850.3 ± 0.06 | 1728.7 ± 0.07 |
| POPC | POPC + CPT | DMPC | DMPC + CPT | |
|---|---|---|---|---|
| Area per lipid (nm2) | 0.63 ± 0.01 | 0.66 ± 0.02 | 0.61 ± 0.01 | 0.64 ± 0.01 |
| Order parameter (SCD) | 0.14 ± 0.07 | 0.15 ± 0.07 | 0.20 ± 0.05 | 0.193 ± 0.05 |
| Thickness (nm) | 4.04 ± 0.07 | 3.93 ± 0.08 | 3.81 ± 0.06 | 3.65 ± 0.07 |
| Diffusion coefficient (108 cm2 s−1) | 5.45 ± 2.19 | 7.67 ± 2.16 | 7.3 ± 1.03 | 11.5 ± 3.2 |
| Cluster (% monomers) | - | 66.6 ± 0.9 | - | 65.6 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, J.; Aranda, F.J.; Teruel, J.A.; Ortiz, A. Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study. Membranes 2024, 14, 118. https://doi.org/10.3390/membranes14060118
Ortiz J, Aranda FJ, Teruel JA, Ortiz A. Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study. Membranes. 2024; 14(6):118. https://doi.org/10.3390/membranes14060118
Chicago/Turabian StyleOrtiz, Julia, Francisco J. Aranda, José A. Teruel, and Antonio Ortiz. 2024. "Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study" Membranes 14, no. 6: 118. https://doi.org/10.3390/membranes14060118
APA StyleOrtiz, J., Aranda, F. J., Teruel, J. A., & Ortiz, A. (2024). Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study. Membranes, 14(6), 118. https://doi.org/10.3390/membranes14060118






