Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
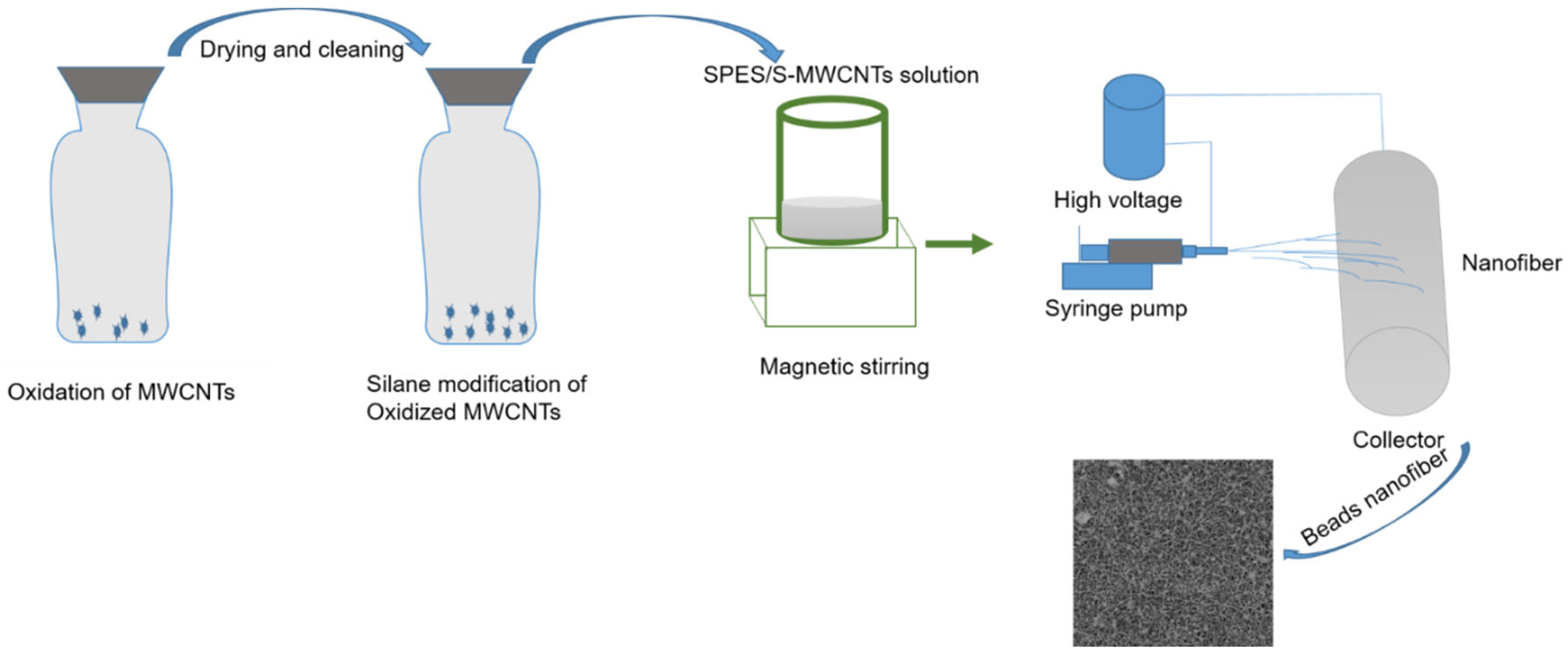
2.1. Oxidation Process of MWCNTs
2.2. Electrospun Membrane Fabrication
2.3. Membrane Characterization
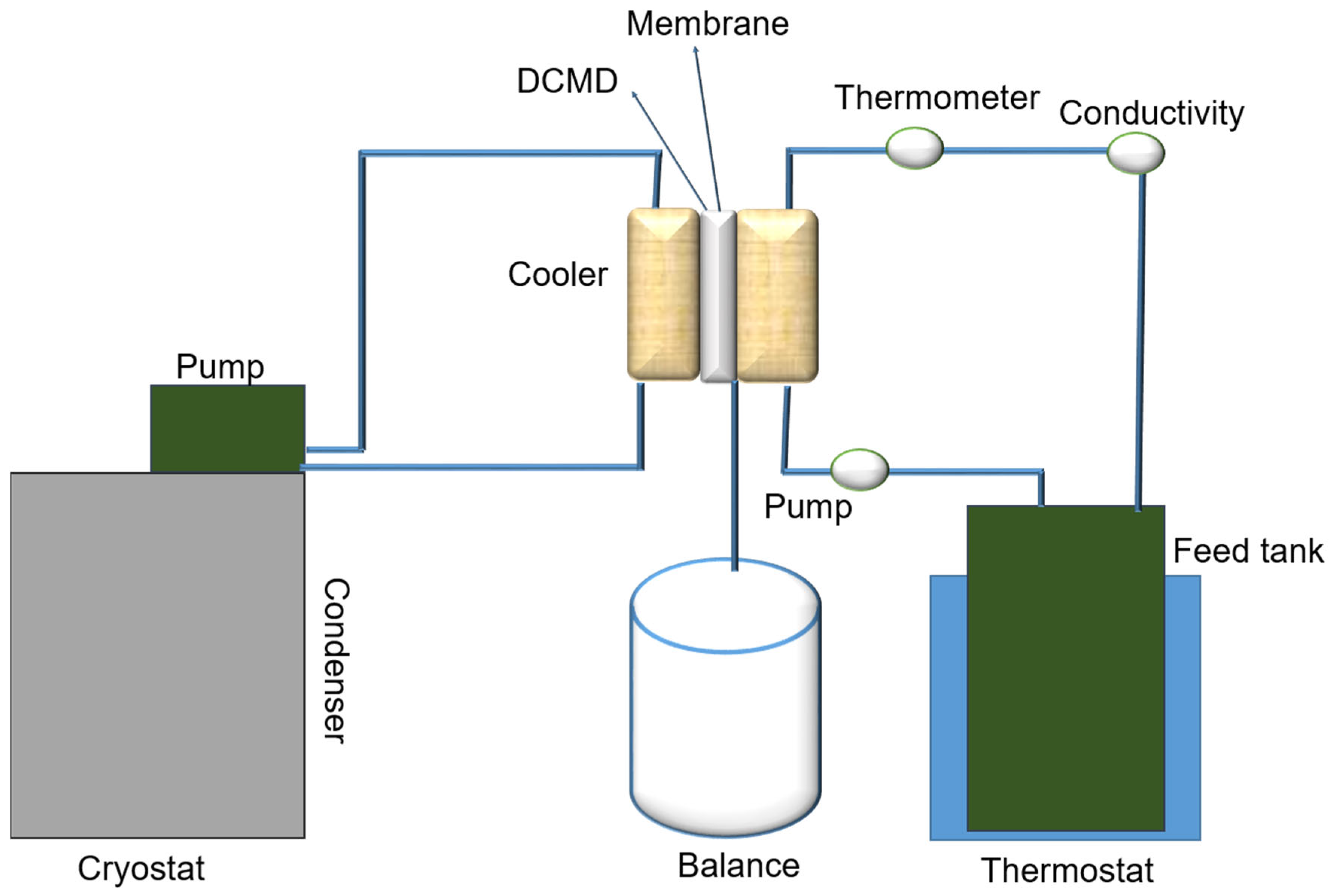
2.4. Direct Contact Membrane Distillation Set-Up
3. Results and Discussion
3.1. Membrane Characterization
3.2. X-ray Diffraction
3.3. Tensile Strength Analysis
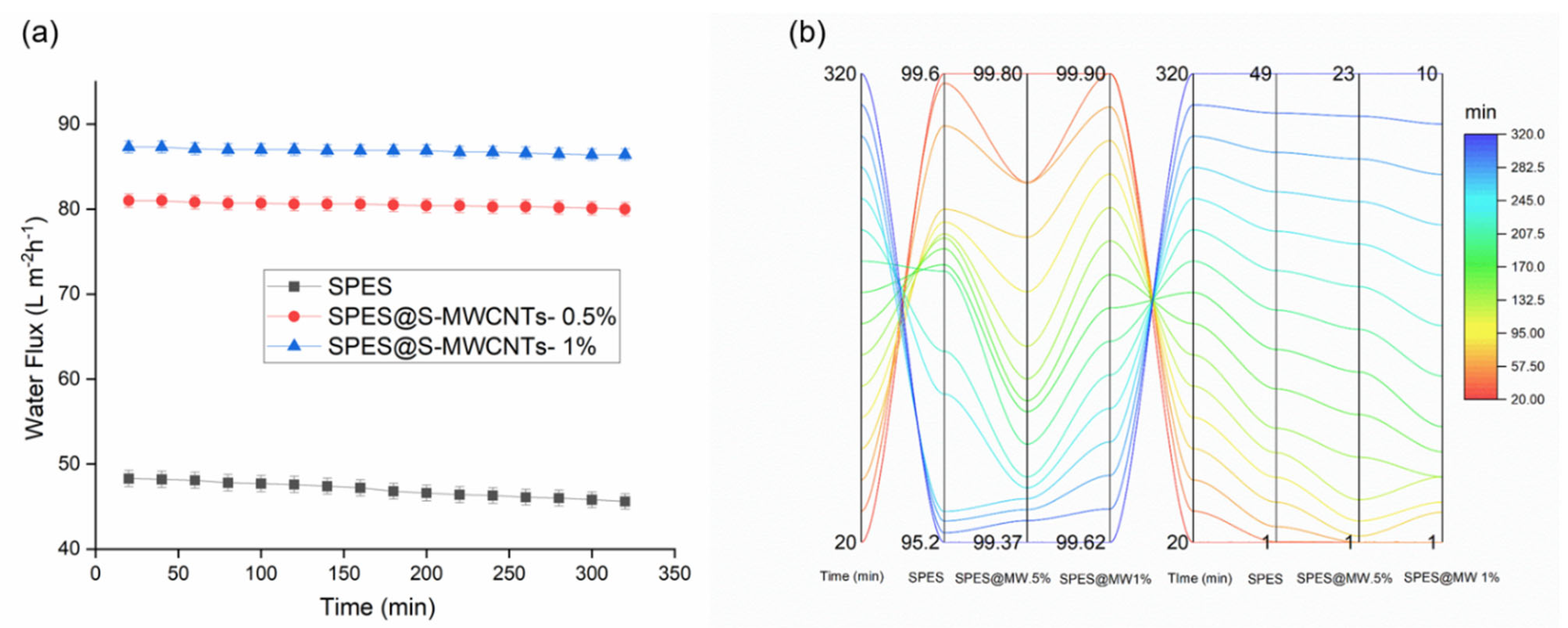
3.4. Direct Contact Membrane Distillation Performance
3.5. Comparison with Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Zhu, H.; Chen, X.; Zheng, S.; Liang, F.; Yang, F.; Yang, S.; Zhang, Y. Full-Coverage Spongy HEAA/PES Composite Ultrafiltration Membrane with High Selectivity and Antifouling Performances. ACS Appl. Polym. Mater. 2023, 5, 2727–2738. [Google Scholar] [CrossRef]
- Talukder, M.E.; Alam, F.; Pervez, M.N.; Jiangming, W.; Hassan, F.; Stylios, G.K.; Naddeo, V.; Song, H. New generation washable PES membrane face mask for virus filtration. Nanocomposites 2022, 8, 12–23. [Google Scholar] [CrossRef]
- Abdul-Hussein, S.T.; Al-Furaiji, M.H.; Meskher, H.; Ghernaout, D.; Fal, M.; Alotaibi, A.M.; Alsalhy, Q.F. Prospects of forward osmosis-based membranes for seawater mining: Economic analysis, limitations and opportunities. Desalination 2024, 579, 117477. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef]
- Anvari, A.; Yancheshme, A.A.; Rekaabdar, F.; Hemmati, M.; Tavakolmoghadam, M.; Safekordi, A. PVDF/PAN blend membrane: Preparation, characterization and fouling analysis. J. Polym. Environ. 2017, 25, 1348–1358. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances on protein separation and purification methods. Adv. Colloid Interface Sci. 2020, 284, 102254. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Alternative heating techniques in membrane distillation: A review. Desalination 2020, 496, 114713. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zeng, X.; Huang, S.; Zhang, H.; Qin, X. Improved desalination properties of hydrophobic GO-incorporated PVDF electrospun nanofibrous composites for vacuum membrane distillation. Sep. Purif. Technol. 2020, 230, 115889. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.-G.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Qasim, M.; Samad, I.U.; Darwish, N.A.; Hilal, N. Comprehensive review of membrane design and synthesis for membrane distillation. Desalination 2021, 518, 115168. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V.; Figoli, A. Ag nanoparticles immobilized sulfonated polyethersulfone/polyethersulfone electrospun nanofiber membrane for the removal of heavy metals. Sci. Rep. 2022, 12, 5814. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhong, J.; Ma, R.; Xu, X.; Wu, H.; Yu, Y. Engineering beads-on-string structural electrospun nanofiber Janus membrane with multi-level roughness for membrane distillation. Desalination 2022, 539, 115950. [Google Scholar] [CrossRef]
- Pervez, M.N.; Mishu, M.R.; Talukder, M.E.; Stylios, G.K.; Buonerba, A.; Hasan, S.W.; Naddeo, V. Electrospun nanofiber membranes for the control of micro/nanoplastics in the environment. Water Emerg. Contam. Nanoplastics 2022, 1, 1–6. [Google Scholar] [CrossRef]
- Razman, K.K.; Hanafiah, M.M.; Mohammad, A.W.; Agashichev, S.; Sgouridis, S.; AlMarzooqi, F. Environmental performance of a photovoltaic brackish water reverse osmosis for a cleaner desalination process: A case study. Sci. Total Environ. 2023, 896, 165244. [Google Scholar] [CrossRef]
- Huang, J.J.; Tian, Y.; Wang, R.; Tian, M.; Liao, Y. Fabrication of bead-on-string polyacrylonitrile nanofibrous air filters with superior filtration efficiency and ultralow pressure drop. Sep. Purif. Technol. 2020, 237, 116377. [Google Scholar] [CrossRef]
- Lee, E.-J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Giagnorio, M.; Wu, C.; Luo, Y.; Hélix-Nielsen, C.; Yu, P.; Zhang, W. Beaded electrospun polyvinylidene fluoride (PVDF) membranes for membrane distillation (MD). J. Membr. Sci. 2022, 661, 120850. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Enhancement in hydrophilicity of different polymer phase-inversion ultrafiltration membranes by introducing PEG/Al2O3 nanoparticles. Sep. Purif. Technol. 2014, 128, 45–57. [Google Scholar] [CrossRef]
- Li, M.; Gao, X.; Wang, X.; Chen, S.; Yu, J. Wettable and flexible silica nanofiber/bead-based membranes for separation of oily wastewater. ACS Appl. Nano Mater. 2021, 4, 2952–2962. [Google Scholar] [CrossRef]
- Pervez, M.N.; Hossain, M.Y.; Talukder, M.E.; Faisal, A.M.; Hasan, K.F.; Islam, M.; Ahmed, F.; Cai, Y.; Stylios, G.K.; Naddeo, V.; et al. Nanomaterial-based smart and sustainable protective textiles. In Protective Textiles from Natural Resources; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Gao, Z.; Stylios, G.K.; Hassan, M.M.; Naddeo, V. Chitosan-functionalized sodium alginate-based electrospun nanofiber membrane for As (III) removal from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 106693. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Pan, Z. Porous bead-on-string poly (lactic acid) fibrous membranes for air filtration. J. Colloid Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef]
- Talukder, M.E.; Alam, F.; Talukder, M.R.; Mishu MM, R.; Pervez, M.N.; Song, H.; Naddeo, V. Fabrication of a polyethersulfone/polyethyleneimine porous membrane for sustainable separation of proteins in water media. Environ. Sci. Water Res. Technol. 2023, 9, 2323–2337. [Google Scholar] [CrossRef]
- Talukder, M.E.; Alam, F.; Mishu MM, R.; Pervez, M.N.; Song, H.; Russo, F.; Naddeo, V. Sustainable Membrane Technologies for By-Product Separation of Non-Pharmaceutical Common Compounds. Water 2022, 14, 4072. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Venault, A.; Chang, K.-Y.; Maggay, I.V.; Chang, Y. Assessment of the DCMD performances of poly (vinylidene difluoride) vapor-induced phase separation membranes with adjusted wettability via formation process parameter manipulation. Desalination 2023, 560, 116682. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fibers Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Pervez, M.N.; Yeo, W.S.; Mishu MM, R.; Talukder, M.E.; Roy, H.; Islam, M.S.; Naddeo, V. Electrospun nanofiber membrane diameter prediction using a combined response surface methodology and machine learning approach. Sci. Rep. 2023, 13, 9679. [Google Scholar] [CrossRef]
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Schutz, J.; Wang, L.; Padhye, R. Electrospun bilayer nanomembrane with hierarchical placement of bead-on-string and fibers for low resistance respiratory air filtration. Sep. Purif. Technol. 2019, 224, 247–254. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, F.; Zhao, X. Constructing spherical-beads-on-string structure of electrospun membrane to achieve high vapor flux in membrane distillation. Water Res. 2024, 256, 121605. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Fabrication of bioinspired composite nanofiber membranes with robust superhydrophobicity for direct contact membrane distillation. Environ. Sci. Technol. 2014, 48, 6335–6341. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Mirza, N.R.; Huang, Z.; Zhang, J.; Zheng, Y.-M.; Xiang, J.; Xie, Z. Enhanced desalination performance of aluminium fumarate MOF-incorporated electrospun nanofiber membrane with bead-on-string structure for membrane distillation. Desalination 2021, 520, 115338. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, C.; Wang, X.; Liu, Y.; Hu, H.; Guo, Y.; Ma, K.; Fei, B.; Xin, J.H. Beads-on-string structured nanofibers for smart and reversible oil/water separation with outstanding antifouling property. ACS Appl. Mater. Interfaces 2016, 8, 25612–25620. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.I.; Willott, J.D.; de Vos, W.M. Enhancing the Separation Performance of Aqueous Phase Separation-Based Membranes through Polyelectrolyte Multilayer Coatings and Interfacial Polymerization. ACS Appl. Polym. Mater. 2021, 3, 3560–3568. [Google Scholar] [CrossRef]
- Wen, X.; He, C.; Hai, Y.; Ma, R.; Sun, J.; Yang, X.; Qi, Y.; Wei, H.; Chen, J. Fabrication of an antifouling PES ultrafiltration membrane via blending SPSF. RSC Adv. 2022, 12, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.E.; Hasan, K.F.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Pervez, M.N.; Talukder, M.E.; Mishu, M.R.; Buonerba, A.; Del Gaudio, P.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Figoli, A.; et al. One-Step Fabrication of Novel Polyethersulfone-Based Composite Electrospun Nanofiber Membranes for Food Industry Wastewater Treatment. Membranes 2022, 12, 413. [Google Scholar] [CrossRef]
- Yao, N.; Chau, J.; Elele, E.; Khusid, B.; Sirkar, K.K.; Dehn, D.J. Characterization of microporous ECTFE membrane after exposure to different liquid media and radiation. J. Membr. Sci. 2017, 532, 89–104. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Shi, L.; Cheng, B.; Yin, X.; Zhuang, X.; Di, Y. Enhancing proton conductivity of proton exchange membrane with SPES nanofibers containing porous organic cage. Polym. Adv. Technol. 2020, 31, 1571–1580. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the fabrication and application of electrospun nanofiber composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Shabani, I.; Hasani-Sadrabadi, M.M.; Haddadi-Asl, V.; Soleimani, M. Nanofiber-based polyelectrolytes as novel membranes for fuel cell applications. J. Membr. Sci. 2011, 368, 233–240. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Li, X.; Zhuang, X.; Cheng, B. Preparation and characterization of proton exchange membranes with through-membrane proton conducting channels. Ionics 2017, 23, 2359–2366. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, N.; Zhao, Y.; Gao, L.; Liu, W.; Liu, Y.; Kang, W. Dendritic sulfonated polyethersulfone nanofiber membrane@ LaCoO3 nanowire-based composite solid electrolytes with facilitated ion transport channels for high-performance all-solid-state lithium metal batteries. J. Mater. Chem. A 2023, 11, 2780–2792. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Art to use electrospun nanofbers/nanofber based membrane in waste water treatment, chiral separation and desalination. J. Membr. Sci. Res. 2019, 5, 100–125. [Google Scholar]
- Yun, K.M.; Suryamas, A.B.; Iskandar, F.; Bao, L.; Niinuma, H.; Okuyama, K. Morphology optimization of polymer nanofiber for applications in aerosol particle filtration. Sep. Purif. Technol. 2010, 75, 340–345. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Zhao, M.; Zhang, L.; Ji, X.; Sun, H.; Sun, Y.; Ma, Z.; Xue, J.; Gao, X. Fabrication of a cation-exchange membrane via the blending of SPES/N-phthaloyl chitosan/MIL-101 (Fe) using response surface methodology for desalination. Membranes 2022, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, S.; Kahrizi, M.; Fadaie, N.; Hadadpour, S.; Ramavandi, B.; Gonzales, R.R. Developing a thin film composite membrane with hydrophilic sulfonated substrate on nonwoven backing fabric support for forward osmosis. Membranes 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- do Amaral Montanheiro, T.L.; Cristovan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Tursi, A.; Beneduci, A.; Nicotera, I.; Simari, C. MWCNTs Decorated with TiO2 as Highly Performing Filler in the Preparation of Nanocomposite Membranes for Scalable Photocatalytic Degradation of Bisphenol A in Water. Nanomaterials 2023, 13, 2325. [Google Scholar] [CrossRef]
- An, A.K.; Lee, E.-J.; Guo, J.; Jeong, S.; Lee, J.-G.; Ghaffour, N. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Sci. Rep. 2017, 7, srep41562. [Google Scholar]
- Elmarghany, M.R.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Sabry, M.N.; Matsuyama, H.; Nady, N. Triple-layer nanocomposite membrane prepared by electrospinning based on modified PES with carbon nanotubes for membrane distillation applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, A.; Hussain, S.; Imran, M. Systematic exploration of electrospun polyvinylidene fluoride (PVDF)/multi-walled carbon nanotubes’(MWCNTs) composite nanofibres for humidity sensing application. J. Taibah Univ. Sci. 2021, 15, 257–266. [Google Scholar] [CrossRef]
- Song, J.; Deng, Q.; Huang, M.; Kong, Z. Carbon nanotube enhanced membrane distillation for salty and dyeing wastewater treatment by electrospinning technology. Environ. Res. 2022, 204, 111892. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C.; Kumar, P.S.; Kweon, J. Diethylenetriaminepentaacetic acid-functionalized multi-walled carbon nanotubes/titanium oxide-PVDF nanofiber membrane for effective separation of oil/water emulsion. Sep. Purif. Technol. 2021, 257, 117926. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Nxumalo, E.N.; Verliefde, A.R.; Mhlanga, S.D.; Onyango, M.S. f-MWCNTs/AgNPs-coated superhydrophobic PVDF nanofibre membrane for organic, colloidal, and biofouling mitigation in direct contact membrane distillation. J. Environ. Chem. Eng. 2020, 8, 103654. [Google Scholar] [CrossRef]
- Irfan, M.; Irfan, M.; Idris, A.; Alsubaie, A.S.; Mahmoud, K.H.; Yusof, N.M.; Akhtar, N. Dual Optimized Sulfonated Polyethersulfone and Functionalized Multiwall Carbon Tube Based Composites High Fouling Resistance Membrane for Protein Separation. Membranes 2022, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Morawska, K.; Malinowski, S.; Wardak, C. Chloride ion-selective electrode with solid-contact based on polyaniline nanofibers and multiwalled carbon nanotubes nanocomposite. Membranes 2022, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, Z.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Ultra-fine electrospun nanofibrous membranes for multicomponent wastewater treatment: Filtration and adsorption. Sep. Purif. Technol. 2020, 242, 116794. [Google Scholar] [CrossRef]
- Nayak, V.; Shivanna, J.M.; Ramu, S.; Radoor, S.; Balakrishna, R.G. Efficacy of electrospun nanofiber membranes on fouling mitigation: A review. ACS Omega 2022, 7, 43346–43363. [Google Scholar] [CrossRef]
- Shabani, I.; Haddadi-Asl, V.; Soleimani, M.; Seyedjafari, E.; Hashemi, S.M. Ion-exchange polymer nanofibers for enhanced osteogenic differentiation of stem cells and ectopic bone formation. ACS Appl. Mater. Interfaces 2013, 6, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, J.; Zheng, J.; Zhang, S.; Dou, L. Nanofiber mats electrospun from composite proton exchange membranes prepared from poly (aryl ether sulfone) s with pendant sulfonated aliphatic side chains. RSC Adv. 2014, 4, 25195–25200. [Google Scholar] [CrossRef]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar]
- Sandhu, H.; Gangacharyulu, D. An experimental study on stability and some thermophysical properties of multiwalled carbon nanotubes with water–ethylene glycol mixtures. Part. Sci. Technol. 2016, 35, 547–554. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Guo, H.; Liu, Y.; Wang, Y.; Yin, H.; Li, X.; Song, J.; Nghiem, L.D.; He, T. Scaling mitigation in membrane distillation: From superhydrophobic to slippery. Desalination 2019, 466, 36–43. [Google Scholar] [CrossRef]
- Kharraz, J.A.; An, A.K. Patterned superhydrophobic polyvinylidene fluoride (PVDF) membranes for membrane distillation: Enhanced flux with improved fouling and wetting resistance. J. Membr. Sci. 2020, 595, 117596. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, W.; Gao, H.; Wu, C.; Gray, S.; Lu, X. In-situ construction of superhydrophobic PVDF membrane via NaCl-H2O induced polymer incipient gelation for membrane distillation. Sep. Purif. Technol. 2021, 274, 11776. [Google Scholar] [CrossRef]


| Sample | Viscosity (mPa S−1) | Electric Conductivity (µS cm−1) | Diameter (nm) | Thickness (mm) | Pore Size (µm) | Porosity (%) |
|---|---|---|---|---|---|---|
| SPES | 2410 | 1.6 | 67.5 ± 10.01 | 0.4 | 4.75 ± 0.9 | 64.4 |
| SPES@S-MWCNTs (0.5%) | 2461 | 1.7 | 75.8± 11.54 | 0.45 | 4.3 ± 0.8 | 73.9 |
| SPES@S-MWCNTs (1%) | 2456 | 1.75 | 83.8± 12.14 | 0.5 | 3.9 ± 0.7 | 79.1 |
| Material | Membrane Preparation Process | CA (°) | ∆T (°C) | NaCL Concentration (wt%) | Flux (L m−2 h−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|
| PAN/PS/PMDS | Electrospinning | 148.5 | 40 | 3.5 | 27.7 | 100 | [12] |
| PVDF | CF4 plasma (15 min)/electrospinning | 148.5 | 40 | 15.354 mg L−1 | 15.3 | 100 | [64] |
| PVDF-nanofiber | Electrospinning | 148 | 35 | 100 g L−1 | 10.5 | 99.99 | [17] |
| CNT/PcH membrane | Electrospinning | 158.5 | 50 | 70 g L−1 | 29.5 | 100 | [66] |
| PVDF | Fluorination | 155 | 40 | Seawater | 19.5 | 99.7 | [67] |
| PVDF | NIPS | 155.3 | 55 | 3.5 | 54.5 | 99.98 | [68] |
| SPES@MWCNTs | Fluorination/Electrospinning | 145 | 50 | 3.5 | 87.3 | 99.8 | This work |
| Pure SPES | Electrospinning | 70 | 50 | 3.5 | 48.4 | 95.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talukder, M.E.; Talukder, M.R.; Pervez, M.N.; Song, H.; Naddeo, V. Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation. Membranes 2024, 14, 120. https://doi.org/10.3390/membranes14060120
Talukder ME, Talukder MR, Pervez MN, Song H, Naddeo V. Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation. Membranes. 2024; 14(6):120. https://doi.org/10.3390/membranes14060120
Chicago/Turabian StyleTalukder, Md Eman, Md. Romon Talukder, Md. Nahid Pervez, Hongchen Song, and Vincenzo Naddeo. 2024. "Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation" Membranes 14, no. 6: 120. https://doi.org/10.3390/membranes14060120
APA StyleTalukder, M. E., Talukder, M. R., Pervez, M. N., Song, H., & Naddeo, V. (2024). Bead-Containing Superhydrophobic Nanofiber Membrane for Membrane Distillation. Membranes, 14(6), 120. https://doi.org/10.3390/membranes14060120









