Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater
Abstract
1. Introduction
2. Materials and Methods
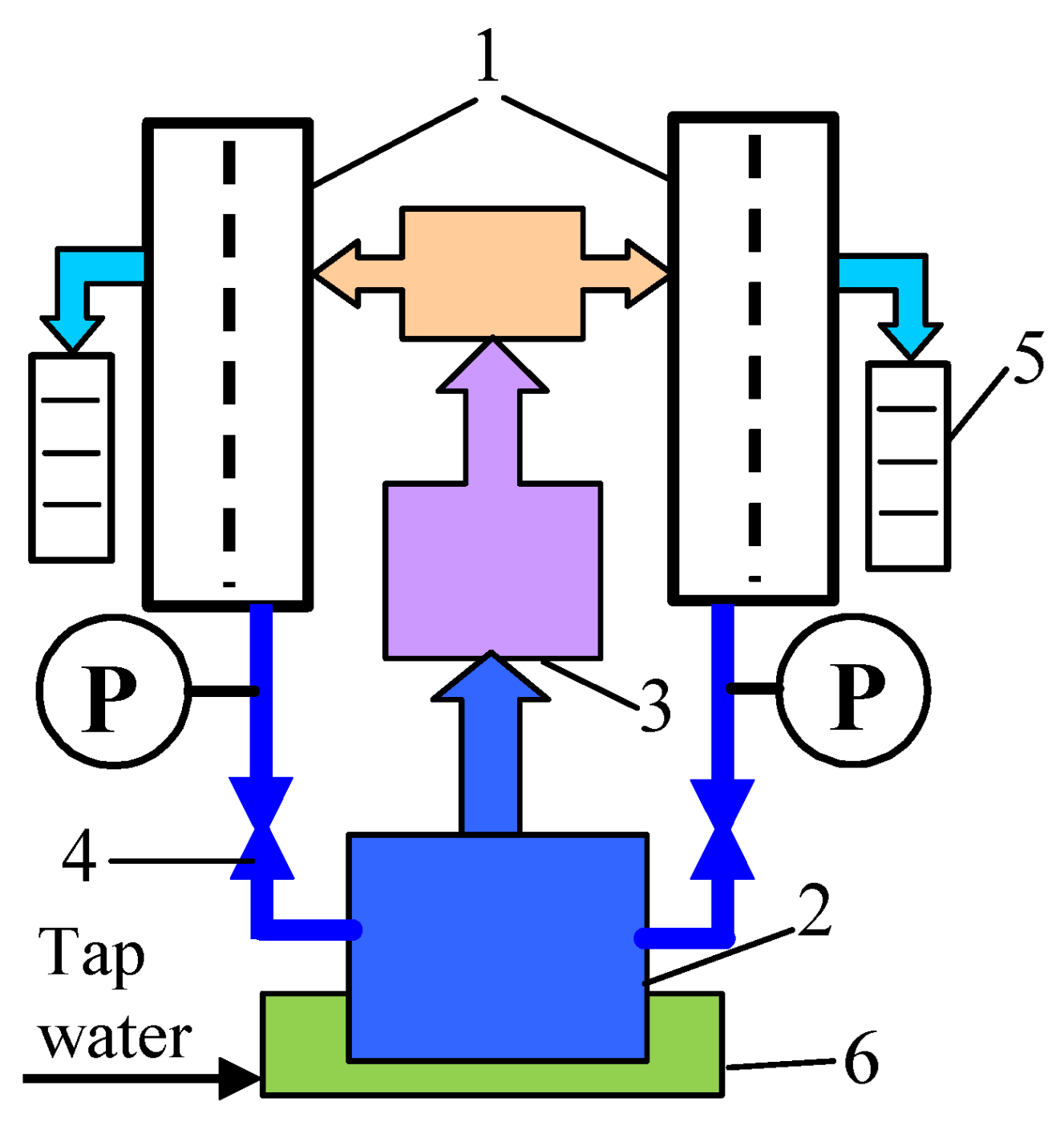
2.1. UF Installation
2.2. Working Solutions
2.3. Analytical Methods
3. Results and Discussion
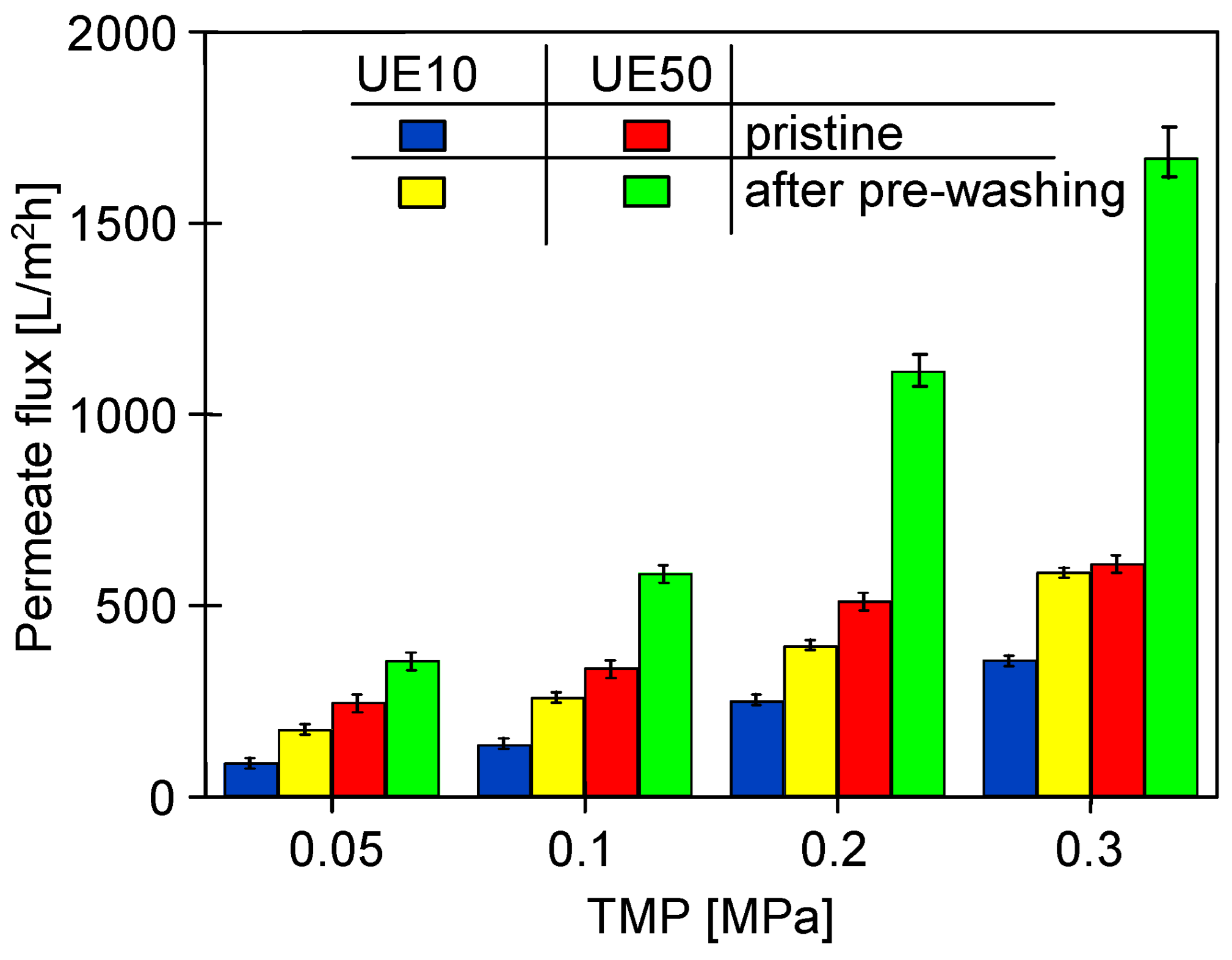
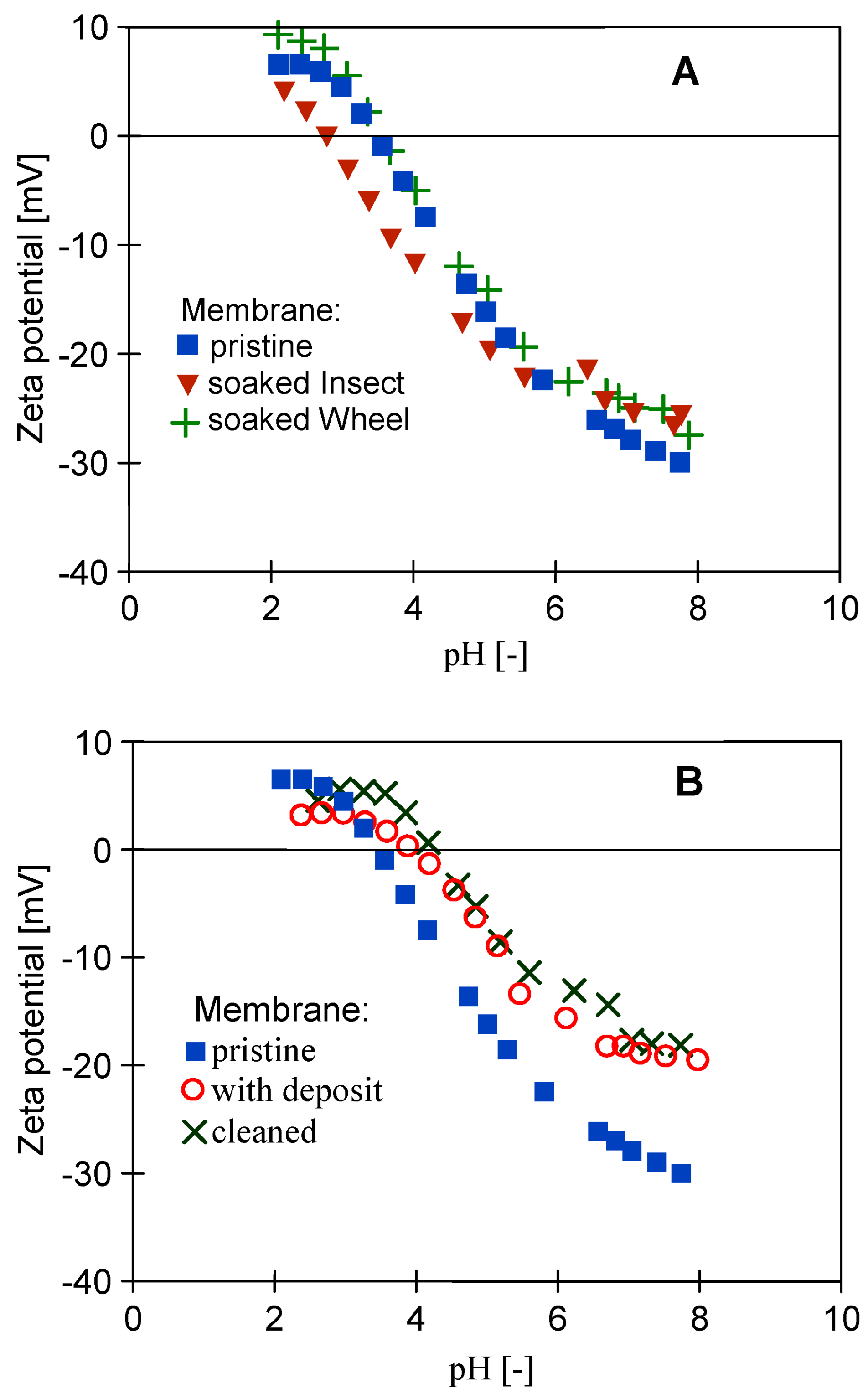
3.1. The Influence of Cleaning Agents on the Performance PES Membranes
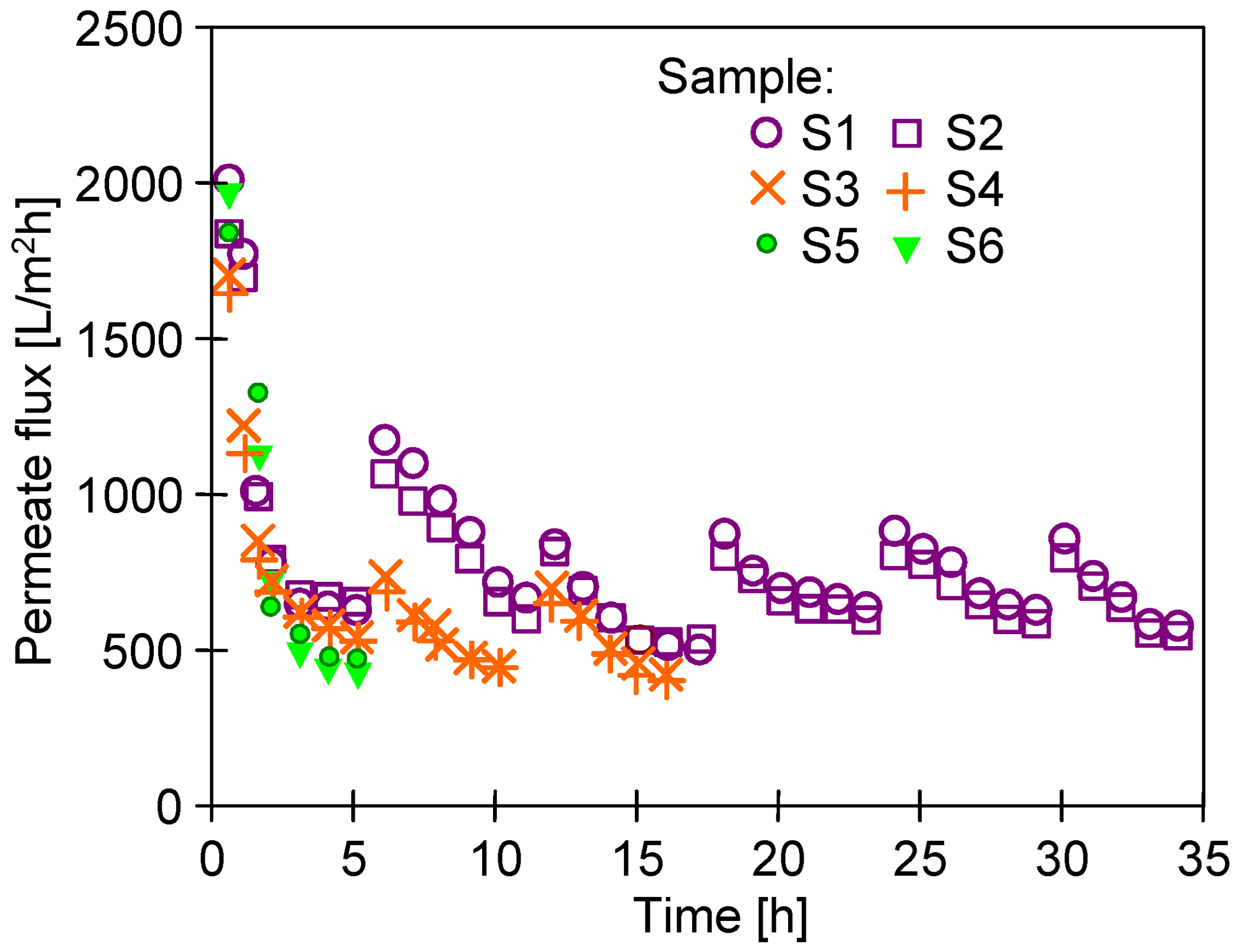
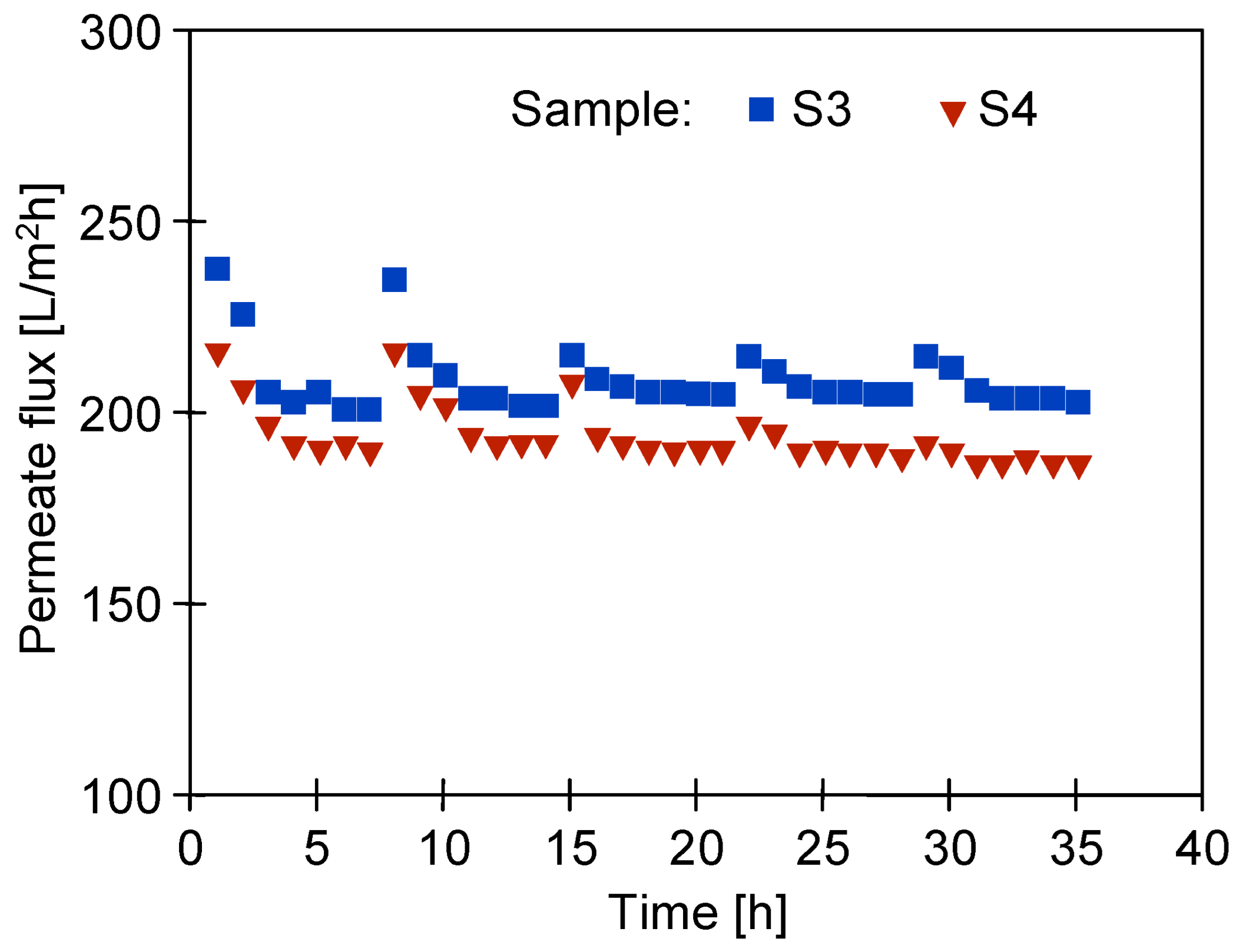
3.2. Long-Term UF with Periodical Alkaline Rinsing
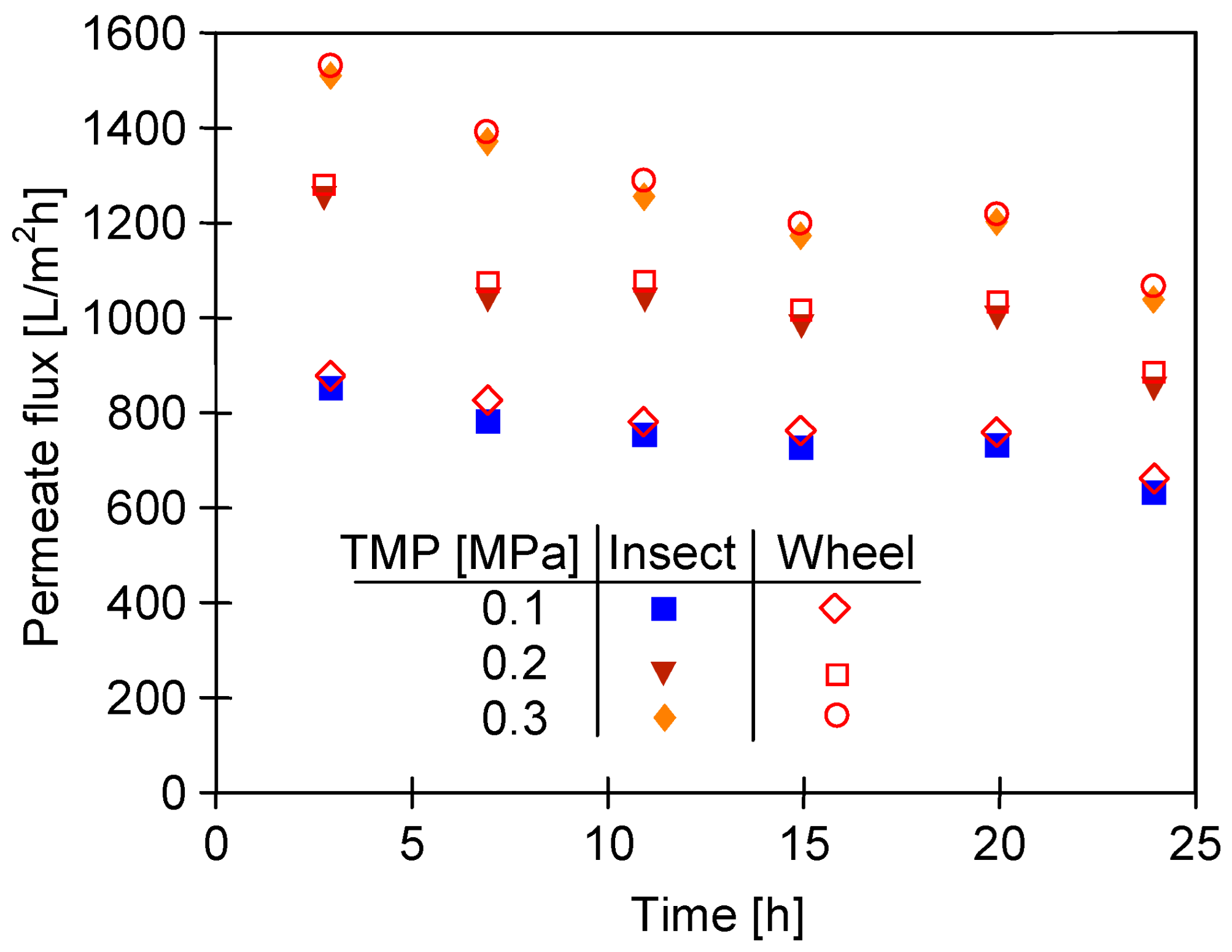
3.3. Filtration of Alkaline Solutions
3.4. Long-Term Tests of Membrane Aging
3.5. Treatment of Synthetic Car wash Wastewater
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Veit, M.T.; Novais, Í.G.V.; Juchen, P.T.; Palácio, S.M.; da Cunha Gonçalves, G.; Zanette, J.C. Automotive Wash Effluent Treatment Using Combined Process of Coagulation/Flocculation/Sedimentation–Adsorption. Water Air Soil Pollut. 2020, 231, 494. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Mohamed, R.M.S.R.; Rahman, M.A.A.; Johari, M.R.; Kassim, A.H.M. Treatment of Wastewater from Car Washes Using Natural Coagulation and Filtration System. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012046. [Google Scholar] [CrossRef]
- Li, T.; Xue-Jun, T.; Fu-Yi, C.; Qi, Z.; Jun, Y. Reuse of Carwash Wastewater with Hollow Fiber Membrane Aided by Enhanced Coagulation and Activated Carbon Treatments. Water Sci. Technol. 2007, 56, 111–118. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. Car Wash Wastewater Reclamation. Full-Scale Application and Upcoming Features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.-S.Z.; Amin, N.K.; Fouad, Y.O. Treatment of real wastewater produced from mobil car wash station using electrocoagulation technique. Environ. Monit. Assess. 2015, 187, 628. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Umar, M.; Karim, S.S.; Amaduddin, M.; Akhtar, K.; Shah, F.; Hussain, A. Design of a car wash waste water treatment process for local car wash stations. J. Pak. Inst. Chem. Eng. 2017, 45, 83–95. [Google Scholar]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Kuan, W.-H.; Ke, L.-W.; Wu, J.-M. A Study of Car Wash Wastewater Treatment by Cyclo-Flow Filtration. Water 2022, 14, 1476. [Google Scholar] [CrossRef]
- Zaneti, R.N.; Etchepare, R.; Rubio, J. Car wash wastewater treatment and water reuse—A case study. Water Sci. Technol. 2013, 67, 82–88. [Google Scholar] [CrossRef]
- Pinto, A.C.S.; de Barros Grossi, L.; de Melo, R.A.C.; de Assis, T.M.; Ribeiro, V.M.; Amaral, M.C.S.; de Souza Figueiredo, K.C. Carwash Wastewater Treatment by Micro and Ultrafiltration Membranes: Effects of Geometry, Pore Size, Pressure Difference and Feed Flow Rate in Transport Properties. J. Water Process Eng. 2017, 17, 143–148. [Google Scholar] [CrossRef]
- Uçar, D. Membrane processes for the reuse of car washing wastewater. J. Water Reuse Desalin. 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Polyethersulfone membrane fouling mitigation during ultrafiltration of wastewaters from car washes. Desalination 2024, 574, 117254. [Google Scholar] [CrossRef]
- Brinck, J.; Jönsson, A.-S.; Jönsson, B.; Lindau, J. Influence of pH on the adsorptive fouling of ultrafiltration membranes by fatty acid. J. Membr. Sci. 2000, 164, 187–194. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.; Díez, M.A.; García, R.; Riera, F.A. Membrane technology for the recovery of detergent compounds: A review. J. Ind. Eng. Chem. 2012, 18, 1859–1873. [Google Scholar] [CrossRef]
- Malczewska, B.; Żak, A. Structural Changes and Operational Deterioration of the UF Polyethersulfone (PES) Membrane Due to Chemical Cleaning. Sci. Rep. 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Velizarov, S.; Van der Bruggen, B. Stability of Polyethersulfone Membranes to Oxidative Agents: A review. Polym. Degrad. Stab. 2018, 157, 15–33. [Google Scholar] [CrossRef]
- Rabiller-Baudry, M.; Bouzin, A.; Hallery, C.; Girard, J.; Leperoux, C. Evidencing the Chemical Degradation of a Hydrophilised PES Ultrafiltration Membrane Despite Protein Fouling. Sep. Purif. Technol. 2015, 147, 62–81. [Google Scholar] [CrossRef]
- Li, K.; Su, Q.; Li, S.; Wen, G.; Huang, T. Aging of PVDF and PES Ultrafiltration Membranes by Sodium Hypochlorite: Effect of Solution pH. J. Environ. Sci. 2021, 104, 444–455. [Google Scholar] [CrossRef]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of Polyethersulfone Ultrafiltration Membranes Under Long-Term Exposures to Alkaline and Acidic Cleaning Solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Gryta, M.; Wożniak, P. The Resistance of Polyethersulfone Membranes on the Alkaline Cleaning Solutions. Membranes 2024, 14, 27. [Google Scholar] [CrossRef]
- Manios, T.K.; Pihlajamäki, A.; Mattia, D.; Bird, M.R. Effect of fouling and cleaning upon the surface charge and porosity of polyethersulphone membranes during coffee brew decaffeination. Food Bioprod. Process. 2023, 141, 60–72. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barredo-Damas, S.; Iborra-Clar, A.; Pascual-Garrido, J.; Iborra-Clar, M.I. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability 2021, 13, 13682. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef]
- Susanto, H.; Robbani, M.H.; Istirokhatun, T.; Firmansyah, A.A.; Rhamadhan, R.N. Preparation of Low-Fouling Polyethersulfone Ultrafiltration Membranes by Incorporating High-Molecular-Weight Chitosan with the Help of a Surfactant. S. Afr. J. Chem. Eng. 2020, 33, 133–140. [Google Scholar] [CrossRef]
- Hanafi, Y.; Loulergue, P.; Ababou-Girard, S.; Meriadec, C.; Rabiller-Baudry, M.; Baddari, K.; Szymczyk, A. Electrokinetic analysis of PES/PVP membranes aged by sodium hypochlorite solutions at different pH. J. Membr. Sci. 2016, 501, 24–32. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Ahmed, S.I.A.; Souaya, E.R.; Badawy, M.I.; Ulbricht, M. Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep. Purif. Technol. 2017, 175, 36–46. [Google Scholar] [CrossRef]
- Chheang, M.; Hongprasith, N.; Ratanatawanate, C.; Lohwacharin, J. Effects of Chemical Cleaning on the Ageing of Polyvinylidene Fluoride Microfiltration and Ultrafiltration Membranes Fouled with Organic and Inorganic Matter. Membranes 2022, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Luo, J.; Wei, J.; Woodley, J.; Daugaard, A.E.; Pinelo, M. Commercial Polysulfone Membranes Pretreated with Ethanol and NaOH: Effects on Permeability, Selectivity and Antifouling Properties. Sep. Purif. Technol. 2019, 219, 82–89. [Google Scholar] [CrossRef]
- Pellegrin, B.; Prulho, R.; Rivaton, A.; Thérias, S.; Jean-Luc Gardette, J.-L.; Gaudichet-Maurin, E.; Causserand, C. Multi-Scale Analysis of Hypochlorite Induced PES/PVP Ultrafiltration Membranes Degradation. J. Membr. Sci. 2013, 447, 287–296. [Google Scholar] [CrossRef]
- Teella, A.; Zydney, A.L.; Zhou, H.; Olsen, C.; Robinson, C. Effects of Chemical Sanitization Using NaOH on the Properties of Polysulfone and Polyethersulfone Ultrafiltration Membranes. Biotechnol. Prog. 2015, 3, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Su, S.; Gao, S.; Tian, J. Reconstruction of the Polyamide Film in Nanofiltration Membranes Via the Post-Treatment with a Ternary Mixture of Ethanol-Water-NaOH: Mechanism and Effect. Desalination 2022, 519, 115317. [Google Scholar] [CrossRef]
- Thushanthan, K.; Aponsu, G.M.D.N.; Gamage, J.C.P.H. Experimental Study on Tensile Strength Degradation of Carbon Fiber Reinforced Polymer (CFRP) Composite in Alkaline Environment. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–29 July 2022. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R.; Nyström, M. The chemical cleaning of polymeric UF membranes fouled with spent sulphite liquor over multiple operational cycles. J. Membr. Sci. 2003, 216, 67–79. [Google Scholar] [CrossRef]
- Ecolab. ULTRASIL™ Membrane Care Program. Available online: https://www.gejoma.nl/artikel/7087/ecolab-ultrasil-11-225kg.html (accessed on 7 March 2024).
- Weis, A.; Bird, M.R. The influence of multiple fouling and cleaning cycles upon the membrane processing of lignosulphonates. Food Bioprod. Process. 2001, 79, 184–187. [Google Scholar] [CrossRef]
- Mhlongo, S.A.; Sibali, L.L.; Ndibewu, P.P. Some Aspects of the Synthesis, Characterization and Modification of Poly(ether)sulfone Polymeric Membrane for Removal of Persistent Organic Pollutants in Wastewater Samples. J. Water Chem. Technol. 2023, 45, 388–401. [Google Scholar] [CrossRef]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Winnicki, T. Separation of anionic surfactants on ultrafiltration membranes. Desalination 2004, 162, 33–40. [Google Scholar] [CrossRef]
- Rong, C.; Wang, T.; Luo, Z.; Hu, Y.; Kong, Z.; Qin, Y.; Hanaoka, T.; Ito, M.; Kobayashi, M.; Li, Y.-Y. Pilot plant demonstration of temperature impacts on the methanogenic performance and membrane fouling control of the anaerobic membrane bioreactor in treating real municipal wastewater. Bioresour. Technol. 2022, 354, 127167. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Dong, S.; Ji, W.; Nie, J.; Zhu, L.; Du, B. In-Situ Healing of Damaged Polyethersulfone Ultrafiltration Membranes with Microgels. J. Membr. Sci. 2022, 647, 120313. [Google Scholar] [CrossRef]
- Kourde-Hanafi, Y.; Loulergue, P.; Szymczyk, A.; Van der Bruggen, B.; Nachtnebel, M.; Rabiller-Baudry, M.; Audic, J.-L.; Pölt, P.; Baddari, K. Influence of PVP Content on Degradation of PES/PVP Membranes: Insights from Characterization of Membranes with Controlled Composition. J. Membr. Sci. 2017, 533, 261–269. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, C.; Jiang, X.; Zhou, Y.; Lin, X.; Zhao, X.; He, Q.; Chai, H.; Pang, X.; Ma, J. Dopamine-triggered one-step codeposition of zwitterionic surfactants for anti-fouling polyethersulfone ultrafiltration membrane modification. Appl. Surf. Sci. 2022, 598, 153871. [Google Scholar] [CrossRef]
- Urbański, R.; Góralska, E.; Bart, H.-J.; Szymanowski, J. Ultrafiltration of Surfactant Solutions. J. Colloid Interface Sci. 2002, 253, 419–426. [Google Scholar] [CrossRef] [PubMed]



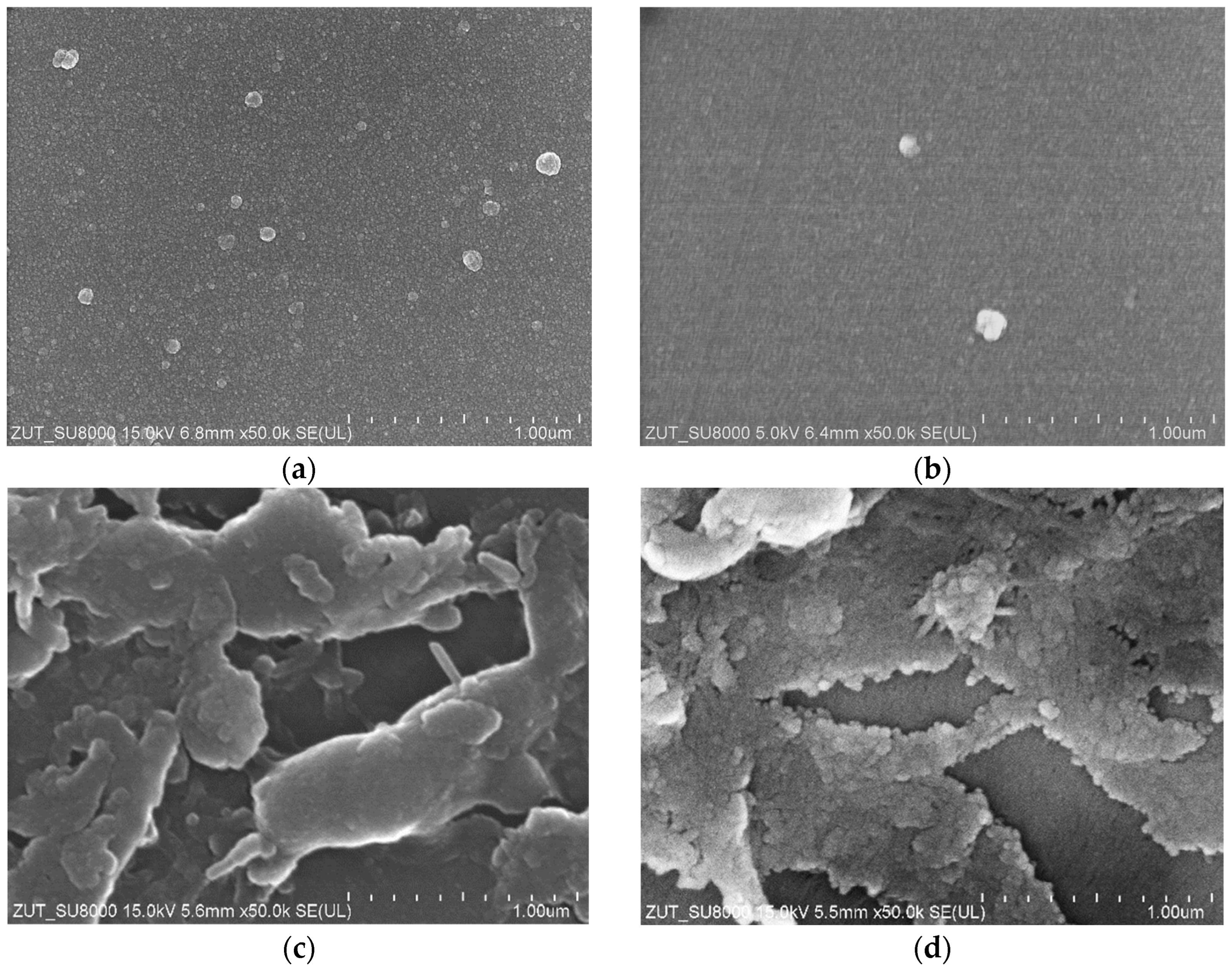
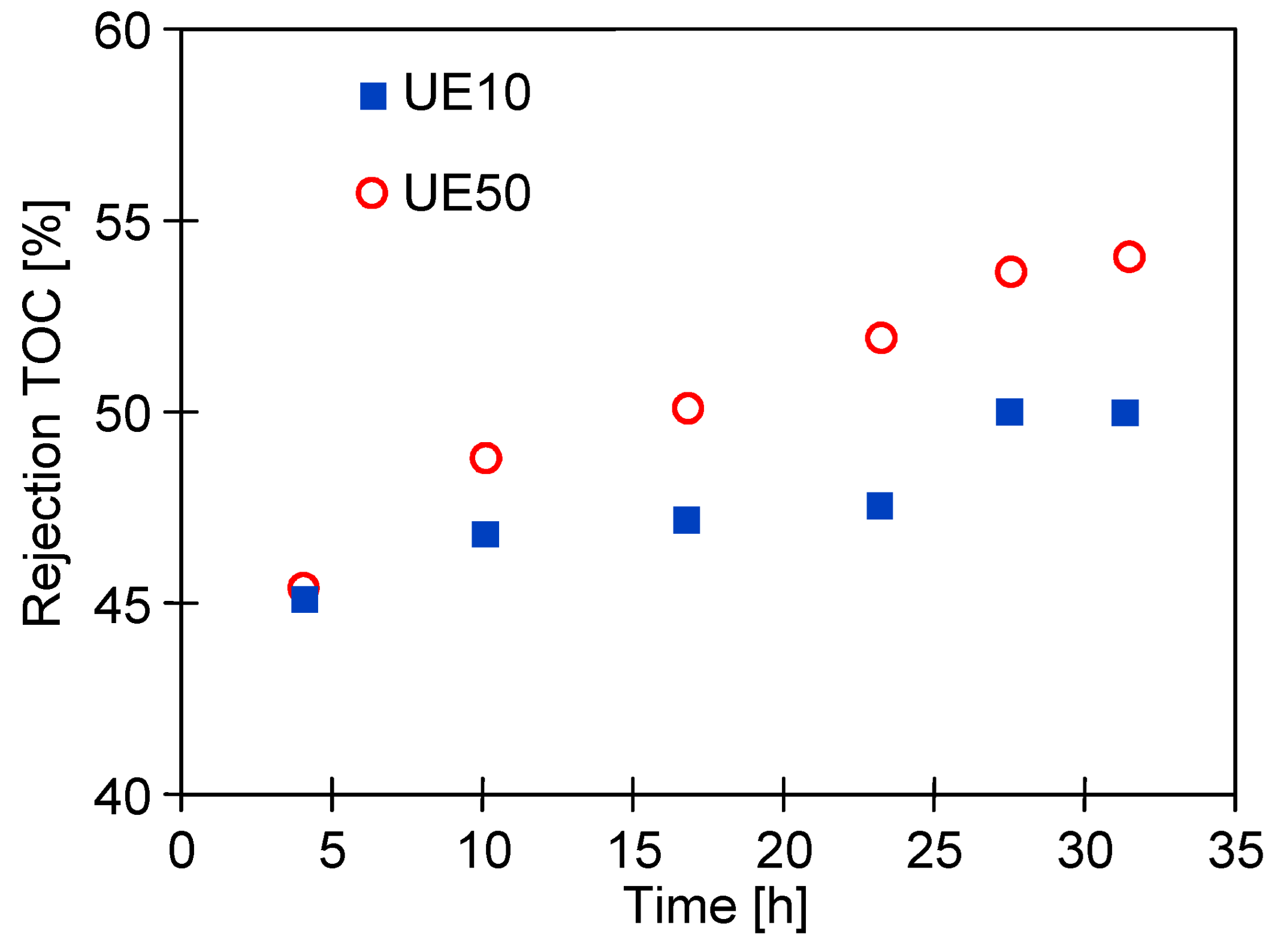
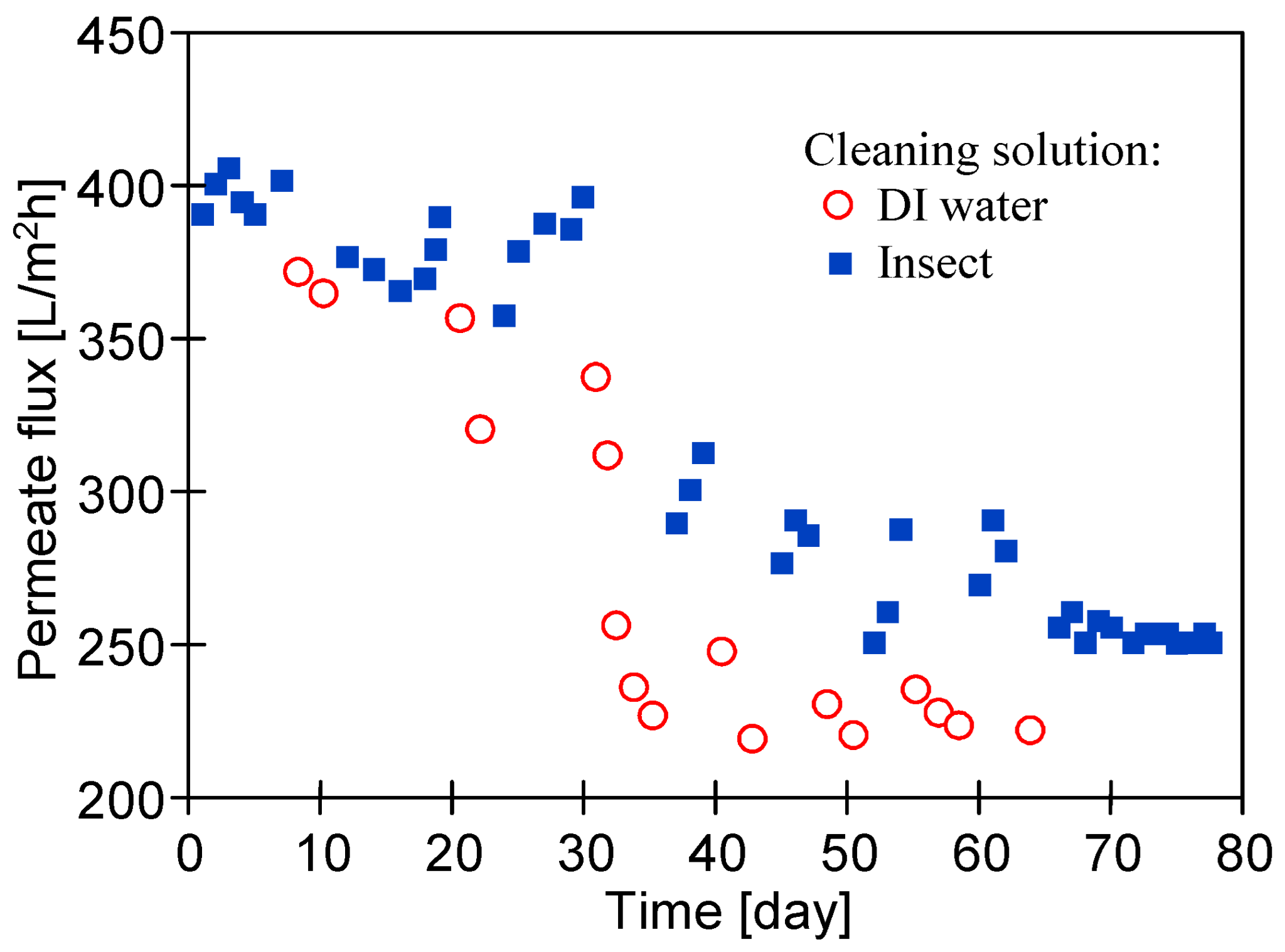
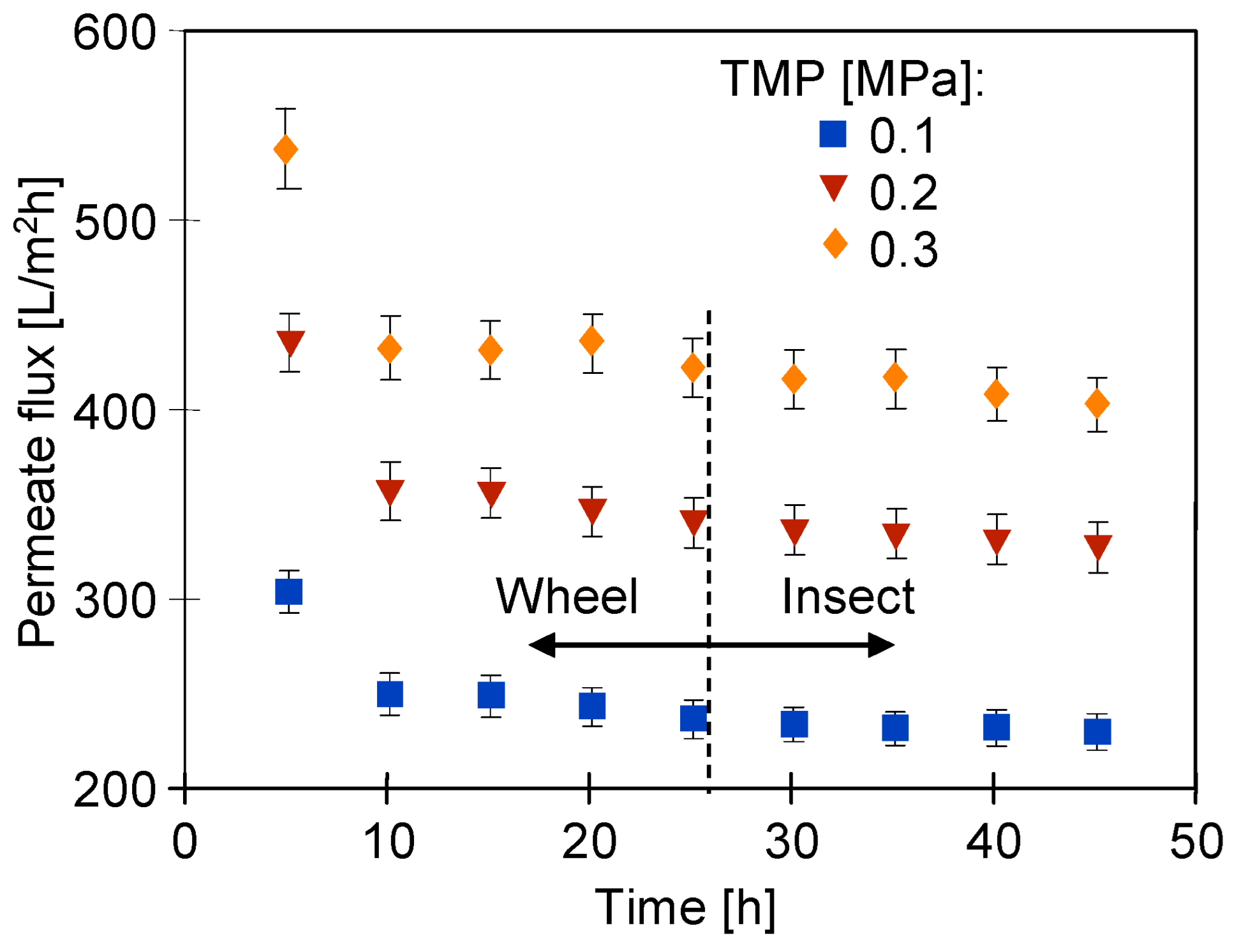
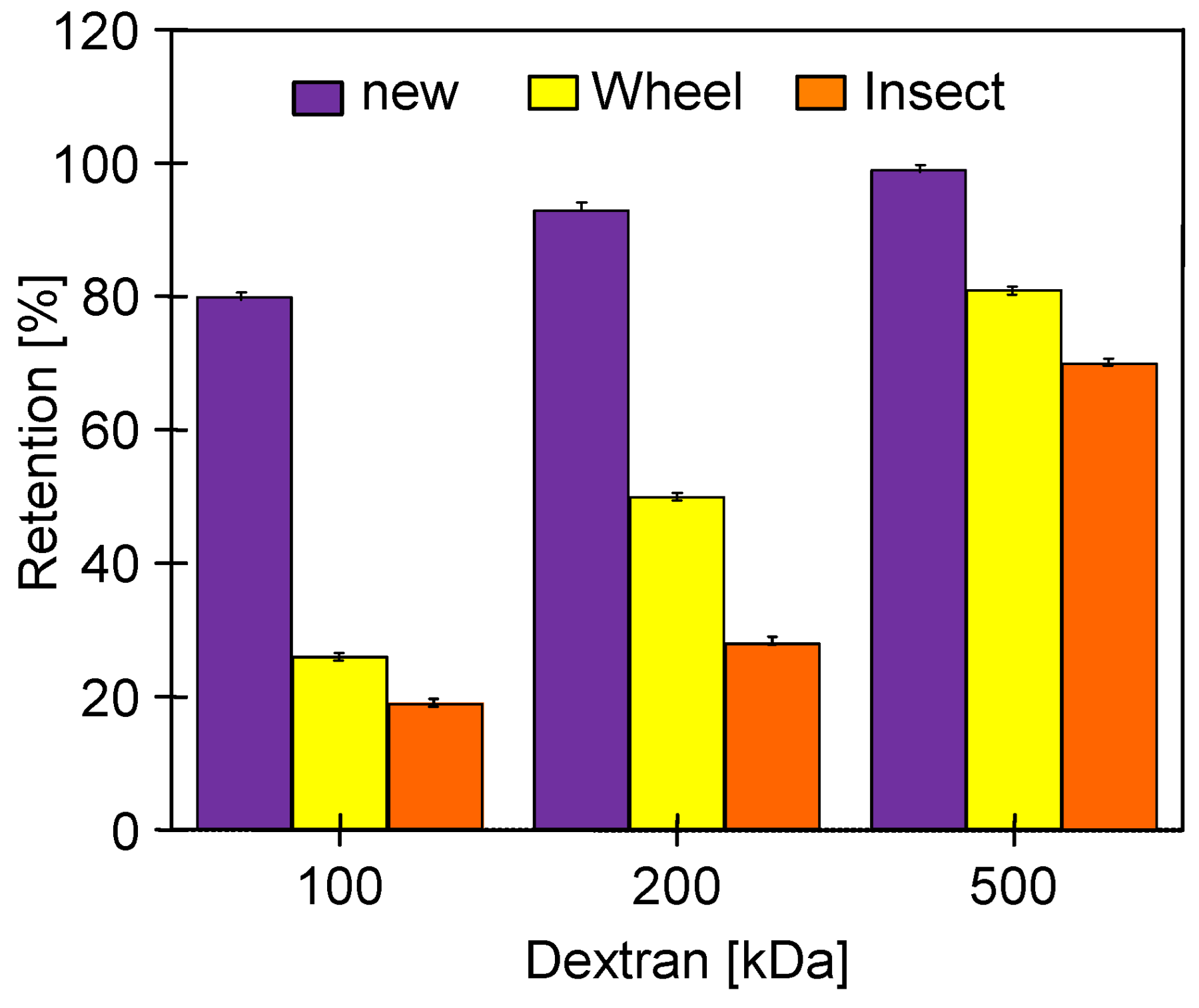









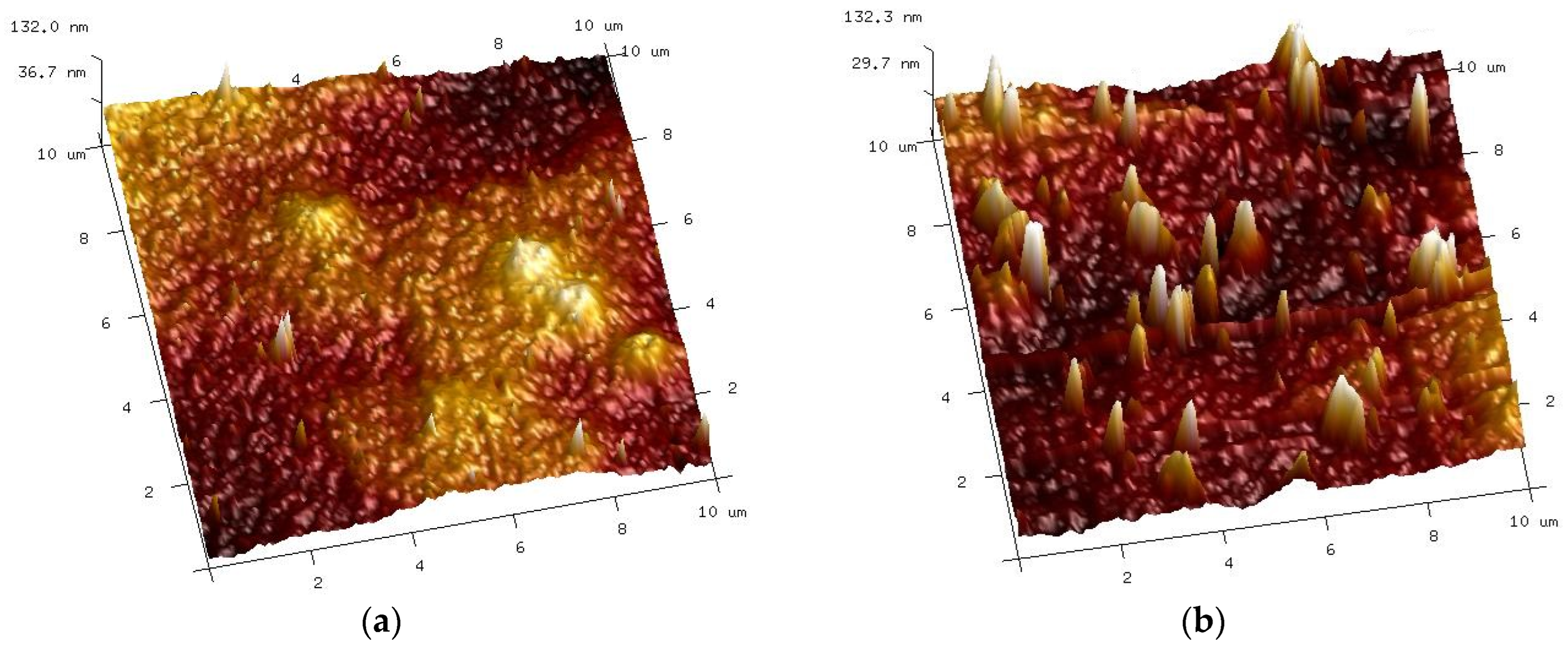
| Sample | Rq [nm] | Ra [nm] | Rmax [nm] |
|---|---|---|---|
| Pristine | 21.5 ± 2.5 | 17.1 ± 3.6 | 179 ± 31.5 |
| Washed | 54.3 ± 18.8 | 39.4 ± 12.5 | 380 ± 93.8 |
| Insect | |||
| Soaked | 27.9 ± 7.5 | 22.2 ± 3.2 | 295 ± 45.5 |
| Filtered | 15.8 ± 5.4 | 12.1 ± 2.2 | 230 ± 36.3 |
| Wheel | |||
| Soaked | 20.3 ± 2.6 | 15.9 ± 1.8 | 203 ± 27.6 |
| Filtered | 25.6 ± 5.4 | 16.7 ± 3.2 | 320 ± 61.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryta, M.; Woźniak, P.; Mozia, S. Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater. Membranes 2024, 14, 122. https://doi.org/10.3390/membranes14060122
Gryta M, Woźniak P, Mozia S. Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater. Membranes. 2024; 14(6):122. https://doi.org/10.3390/membranes14060122
Chicago/Turabian StyleGryta, Marek, Piotr Woźniak, and Sylwia Mozia. 2024. "Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater" Membranes 14, no. 6: 122. https://doi.org/10.3390/membranes14060122
APA StyleGryta, M., Woźniak, P., & Mozia, S. (2024). Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater. Membranes, 14(6), 122. https://doi.org/10.3390/membranes14060122








