The Utilization of Chicken Egg White Waste-Modified Nanofiber Membrane for Anionic Dye Removal in Batch and Flow Systems: Comprehensive Investigations into Equilibrium, Kinetics, and Breakthrough Curve
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CEW-Modified Nanofiber Membrane
2.3. Operating Parameters on CEW Immobilization and AO7 Capture
2.4. Kinetic and Equilibrium Isotherm Studies
2.5. Desorption Studies
2.6. Removal of AO7 Dye in Flow Process
2.7. Breakthrough Parameter Analysis
2.8. Breakthrough Curve Modeling
2.8.1. Thomas Model
2.8.2. BDST Model
2.9. Data Analysis
3. Results and Discussion
3.1. Nanofiber Membrane Properties
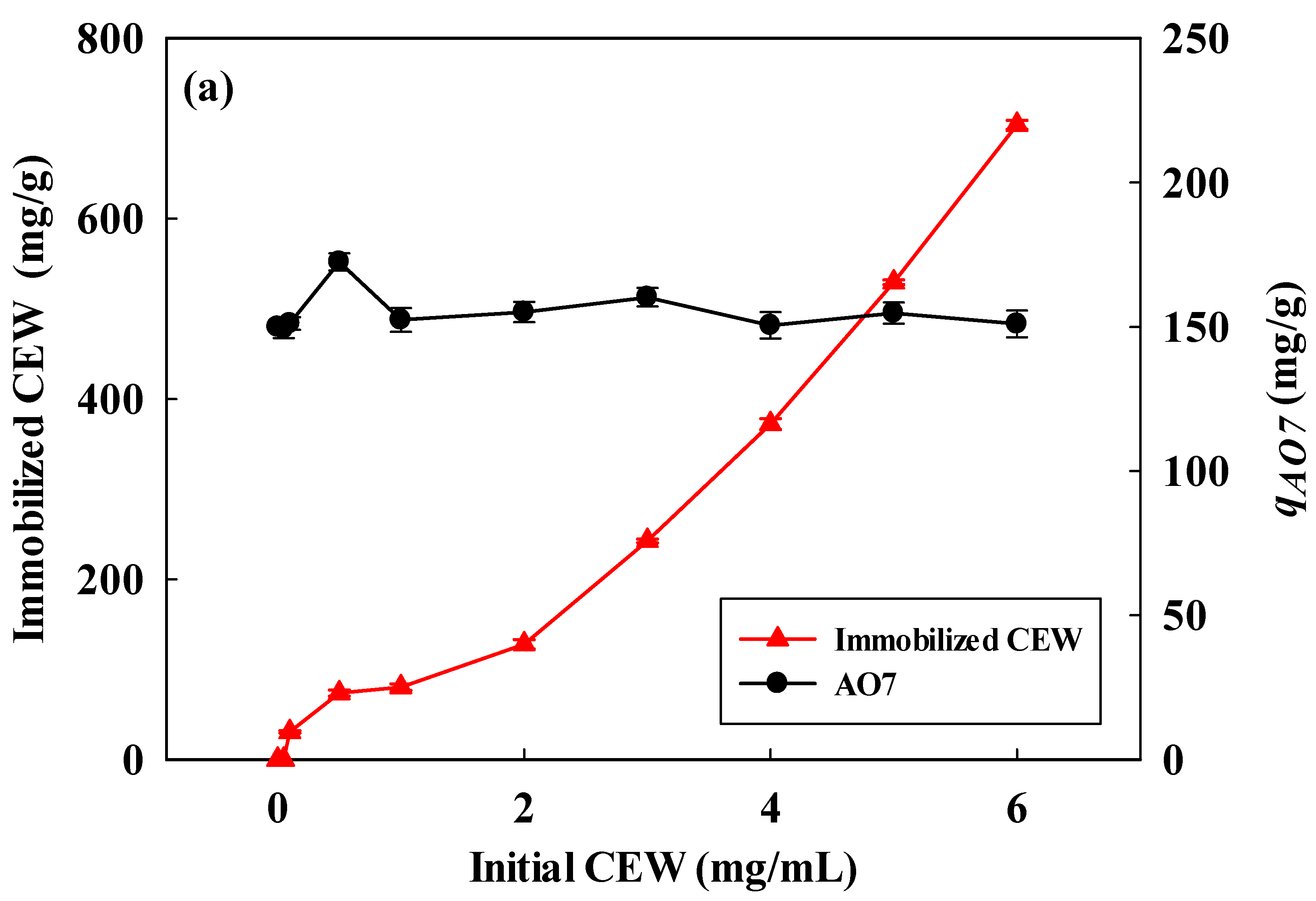
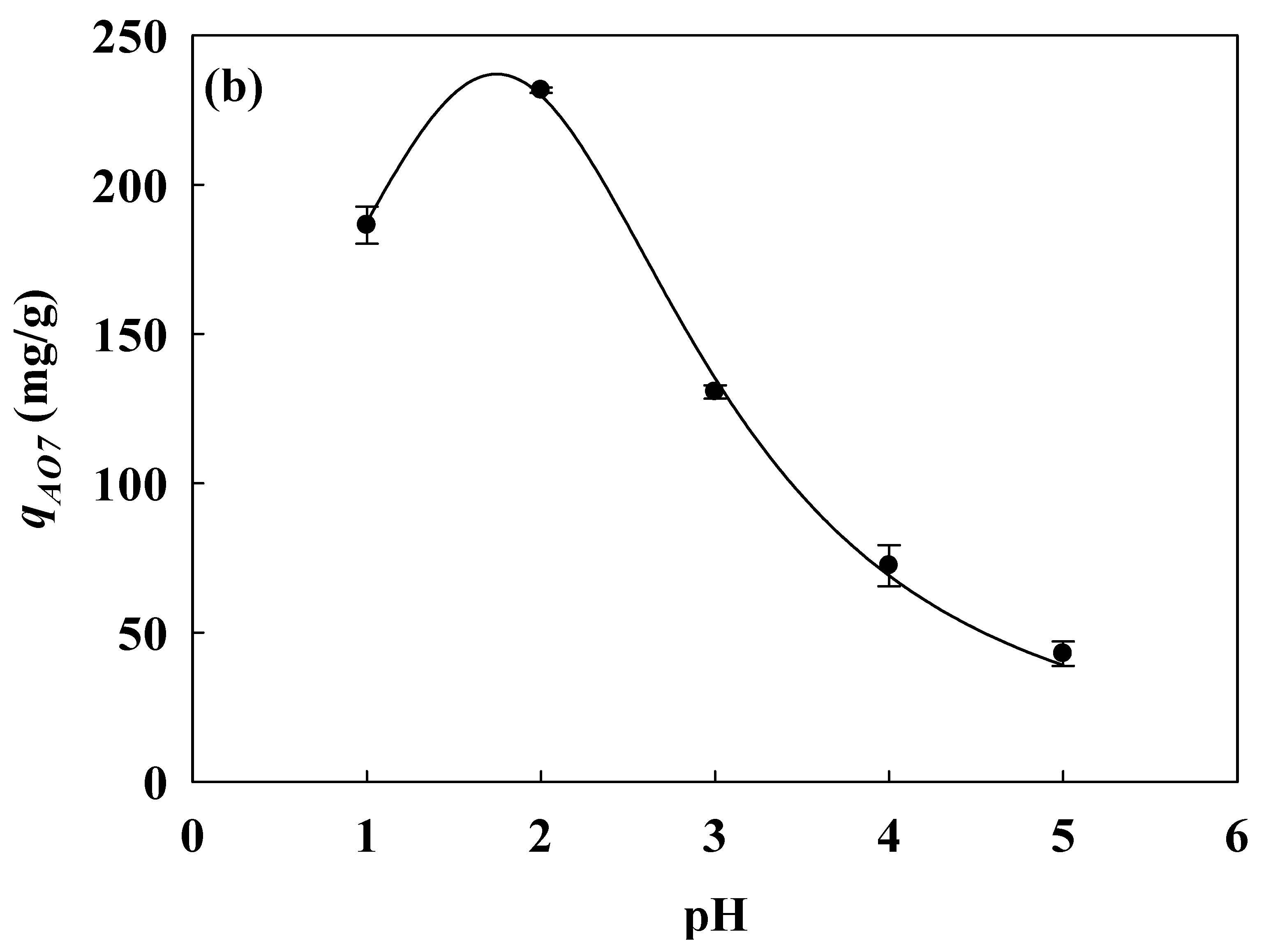
3.2. Optimization of CEW Immobilized onto Acidic Nanofibers
Coupling Concentration and Adsorption pH for CEW
3.3. Kinetic Studies
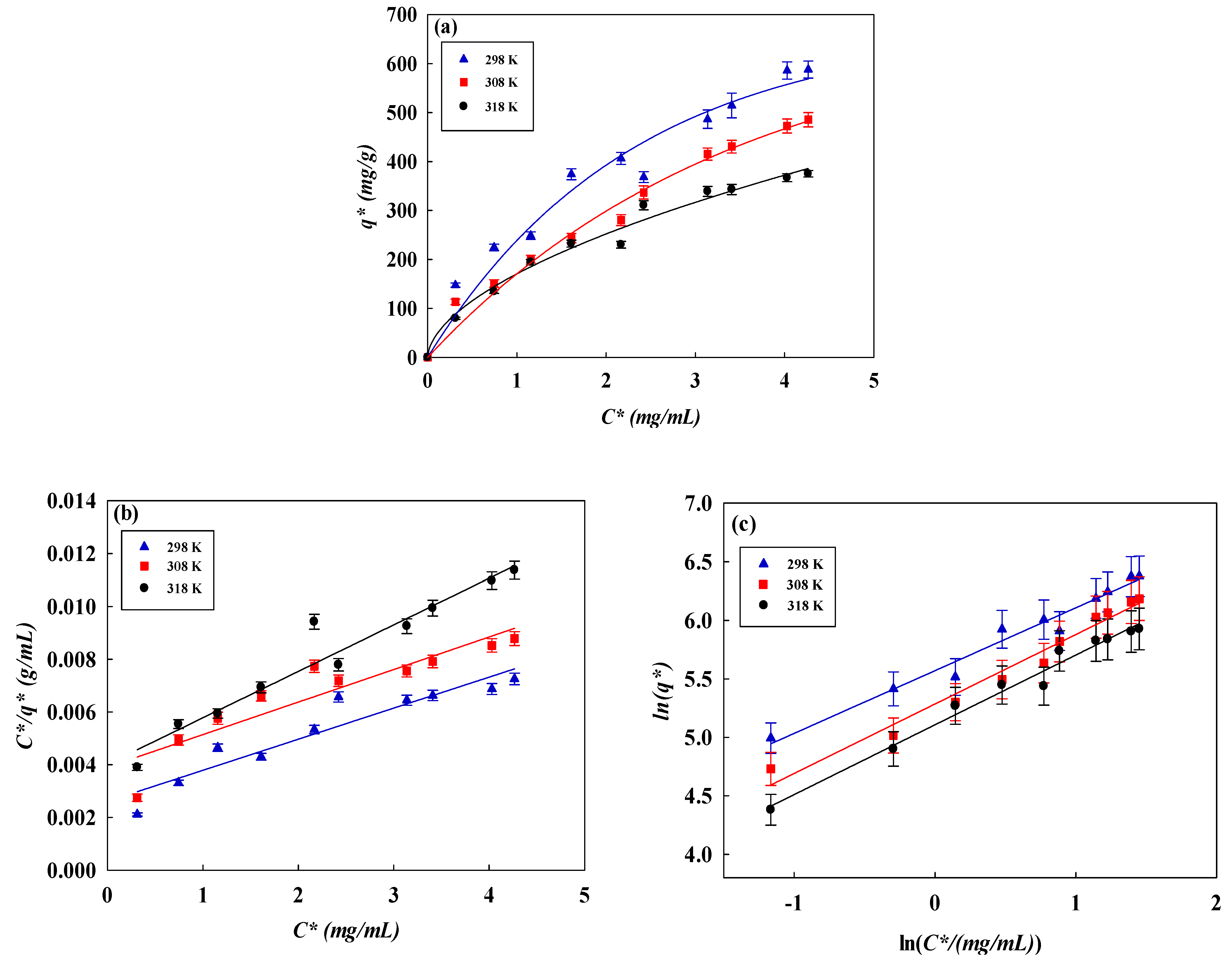
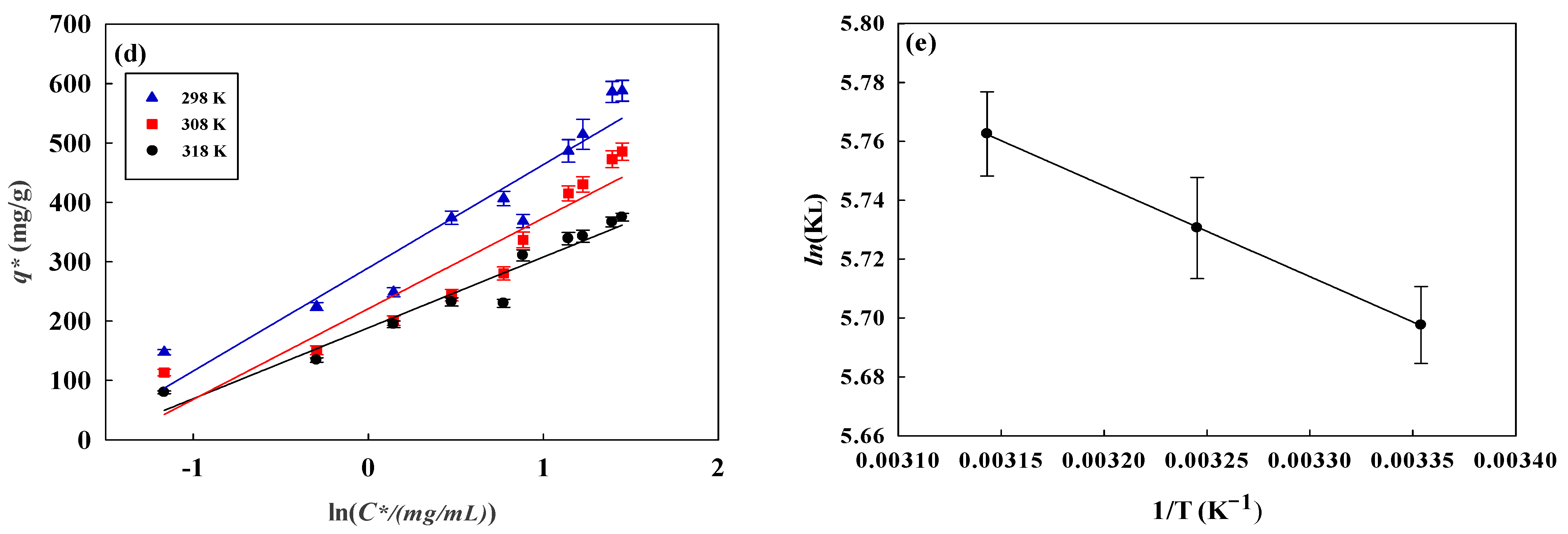
3.4. Isotherm Studies
3.5. Thermodynamic Parameters
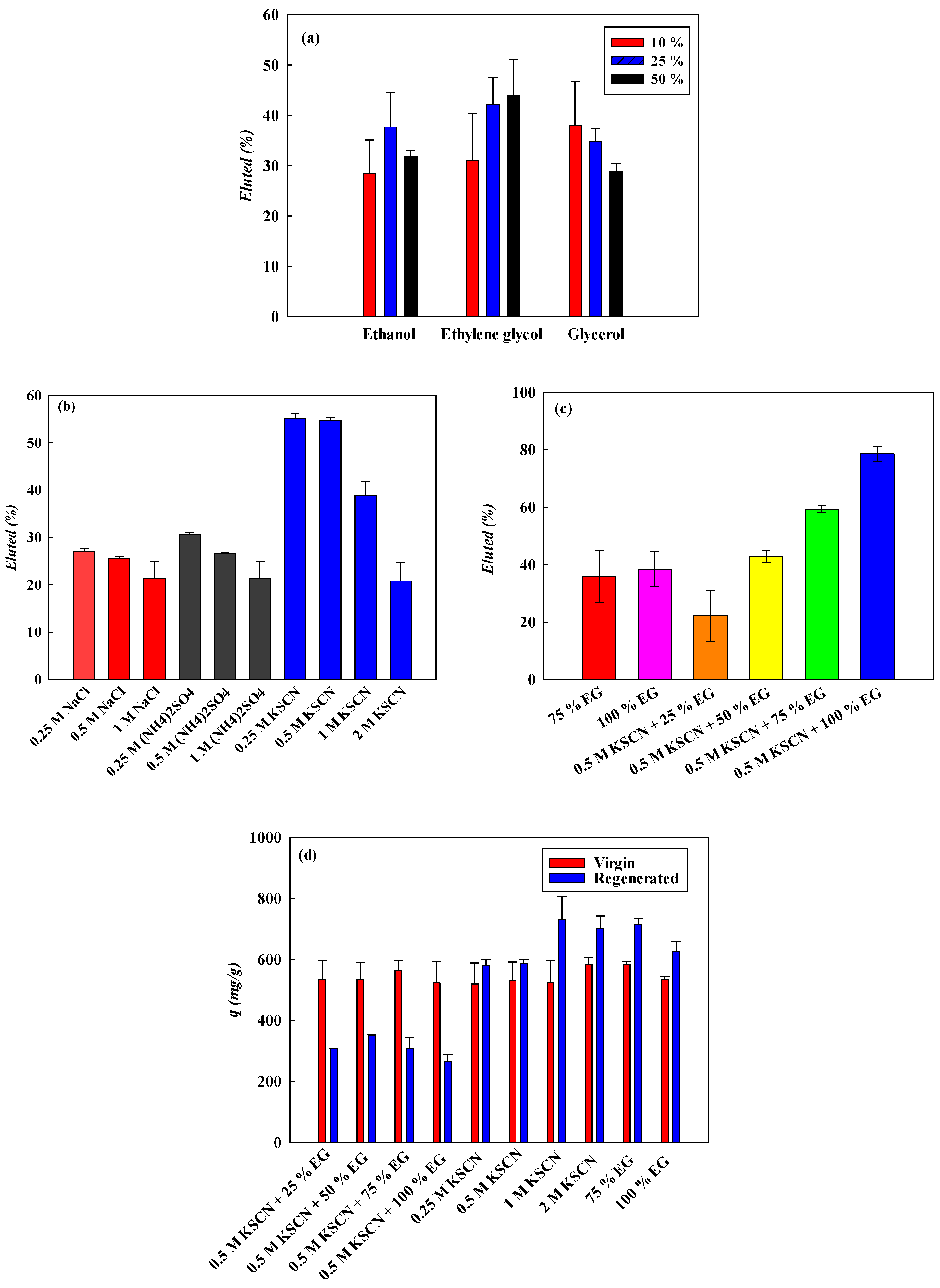
3.6. Desorption Studies
3.6.1. Effect of Organic Solvents
3.6.2. Effect of Liquid Ionic Strength and Salts
3.6.3. Effect of Composite Eluents
3.6.4. Regeneration
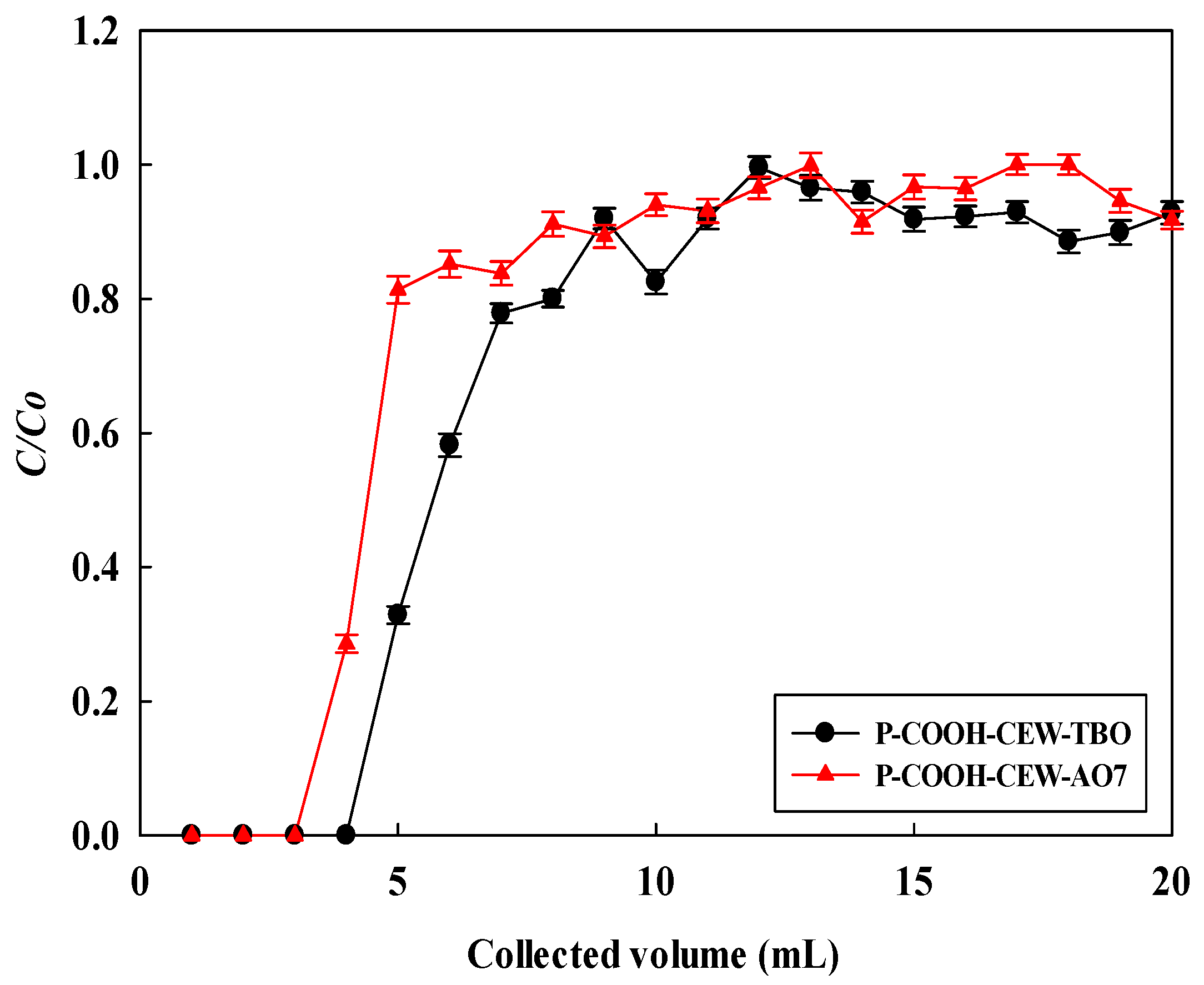
3.7. Removal of Dyes in Flow Process
3.8. Breakthrough Curve Modeling
3.9. Remarks on the Comparison with Other Adsorbers for AO7 Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Arrhenius constant |
| AM | Membrane effective area (cm2) |
| AO7 | Acid orange 7 dye |
| BV | Membrane bed volume |
| BDST | Bed depth service time |
| b | Temkin constant (J/mol) |
| C0 | Initial AO7 dye concentration (mg/mL) |
| C* | Equilibrium AO7 dye concentration in aqueous solution (mg/mL) |
| CEW | Chicken egg white |
| Ea | Activation energy for AO7 dye adsorption |
| F | Flow rate (mL/min) |
| HMTZ | Length of the mass transfer zone |
| J | Permeation flux (mL/cm2·min) |
| k1 | Pseudo-first-order kinetic constant (1/min) |
| k2 | Pseudo-second-order kinetic constant (g/mg·min) |
| kBDST | BDST kinetic rate constant (mL/(mg·min). |
| ki | Intra-particle diffusion rate constant |
| I | A constant with its value proportional to the boundary layer (mg/g) |
| KL | Langmuir constant related to adsorption intensity (mL/mg) |
| KF | Freundlich constant related to adsorption intensity (mg/g) |
| KT | Temkin constant related to adsorption intensity (mL/mg) |
| Thomas model constant (mL/min·mg) | |
| MAER | Membrane adsorber exhaustion rate |
| MBU | Membrane bed utilization (%) |
| n | Freundlich empirical constant and related to adsorption intensity |
| P | Productivity (mg/g·min) |
| Qo | Binding capacity of the membrane bed per unit bed volume (mg/mL) |
| q | Removal capacity for AO7 dye in batch mode (mg/g) |
| q* | Equilibrium removal capacity for AO7 dye in batch mode (mg/g) |
| qmax | Maximum removal capacity for AO7 dye in batch mode (mg/g) |
| qt | Removal capacity for AO7 dye at any given time t in batch mode (mg/g) |
| R | Gas constant (8.314 J/mol/K) |
| T | Absolute temperature (K) |
| TBO | Toluidine blue O dye |
| t | Adsorption time (min) |
| t10% | Time required for 10% CEW breakthrough (min) |
| t90% | Time required for 90% CEW breakthrough (min) |
| tb | Time required at 10% breakthrough (min) |
| V | Total volume of permeated solution (mL) |
| Vb | 10% breakthrough volume (mL) |
| VM | Membrane volume (mL) |
| WM | Weight of the membrane adsorber (g) |
| Z | Length of the membrane bed (cm) |
| ΔG° | Changes in the apparent free energy (J/mol) |
| ΔH° | Changes in the apparent enthalpy (J/mol) |
| ΔS° | Changes in the apparent entropy (J/mol·K) |
| τ | Residence time in the membrane (min) |
| ε | Porosity of the membrane (%) |
| α | Initial sorption rate in the Elovich model (mg/g·min) |
| Constant related to the extent of surface coverage and activation energy for chemisorption in the Elovich model (g/mg) | |
| Thomas model constant (mL/min·mg) | |
| qe | Equilibrium binding capacity in flow mode (mg/g) |
| Ct | Breakthrough concentration (mg/mL) at time t (min) |
| Z | Membrane bed height (cm) |
| Q0 | Binding capacity of the membrane bed per unit bed volume (mg/mL) |
| υ | Linear velocity (cm/min) |
References
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.; Gupta, A. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Nasar, A.; Inamuddin. Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Chang, Y.-N.; Deng, C.-H.; Zhang, J.; Liu, H.-Y.; Huang, S.-Y. Magnetic chitosan-graphene oxide composite for anti-microbial and dye removal applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, S.A.; Sundarrajan, S.; Nizar, S.A.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Huong, D.T.M.; Chai, W.S.; Show, P.L.; Lin, Y.-L.; Chiu, C.-Y.; Tsai, S.-L.; Chang, Y.-K. Removal of cationic dye waste by nanofiber membrane immobilized with waste proteins. Int. J. Biol. Macromol. 2020, 164, 3873–3884. [Google Scholar] [CrossRef]
- Pakalapati, H.; Show, P.L.; Chang, J.-H.; Liu, B.-L.; Chang, Y.-K. Removal of dye waste by weak cation-exchange nanofiber membrane immobilized with waste egg white proteins. Int. J. Biol. Macromol. 2020, 165, 2494–2507. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef]
- Phan, D.N.; Khan, M.Q.; Nguyen, N.T.; Phan, T.T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Ooi, C.W.; Show, P.L.; Hoe, B.C.; Chai, W.S.; Chiu, C.-Y.; Wang, S.S.-S.; Chang, Y.-K. Removal of Ionic Dyes by Nanofiber Membrane Functionalized with Chitosan and Egg White Proteins: Membrane Preparation and Adsorption Efficiency. Membranes 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.-L.; Thanh, D.T.H.; Show, P.-L.; How, S.-C.; Chiu, C.-Y.; Hsu, M.; Chia, S.R.; Chen, K.-H.; Chang, Y.-K. Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications. Membranes 2022, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-L.; Wang, S.S.S.; Ooi, C.W.; Thew, X.E.C.; Lai, Y.-R.; Chiu, C.-Y.; Hsu, M.; Chen, K.-H.; Chang, Y.-K. Reactive Green 19 dye-ligand immobilized on the aminated nanofiber membranes for efficient adsorption of lysozyme: Process development and optimization in batch and flow systems. Food Chem. 2023, 406, 135028. [Google Scholar] [CrossRef] [PubMed]
- Gnoumou, E.; Tran, T.T.A.; Srinophakun, P.; Liu, B.-L.; Chiu, C.-Y.; Lee, H.-C.; Wang, C.-Y.; Chen, K.-H.; Chang, Y.-K. Optimization of lysozyme-modified ion exchange nanofiber membrane for efficient capture of Escherichia coli: Antibacterial and cytotoxic studies. J. Taiwan Inst. Chem. Eng. 2024, 157, 105400. [Google Scholar] [CrossRef]
- Ruiz-Cornejo, J.C.; Sebastián, D.; Lázaro, M.J. Synthesis and applications of carbon nanofibers: A review. Rev. Chem. Eng. 2020, 36, 493–511. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, X.; Tang, Q.; Ma, M.; Jin, Y.; Sheng, L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods 2022, 11, 2434. [Google Scholar] [CrossRef]
- Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers 2021, 13, 510. [Google Scholar] [CrossRef]
- Hartati, S.; Zulfi, A.; Maulida, P.Y.D.; Yudhowijoyo, A.; Dioktyanto, M.; Saputro, K.E.; Noviyanto, A.; Rochman, N.T. Synthesis of Electrospun PAN/TiO2/Ag Nanofibers Membrane As Potential Air Filtration Media with Photocatalytic Activity. ACS Omega 2022, 7, 10516–10525. [Google Scholar] [CrossRef]
- Chen, P.; Chai, M.; Mai, Z.; Liao, M.; Xie, X.; Lu, Z.; Zhang, W.; Zhao, H.; Dong, X.; Fu, X.; et al. Electrospinning polyacrylonitrile (PAN) based nanofiberous membranes synergic with plant antibacterial agent and silver nanoparticles (AgNPs) for potential wound dressing. Mater. Today Commun. 2022, 31, 103336. [Google Scholar] [CrossRef]
- Ghosh, R. Purification of lysozyme by microporous PVDF membrane-based chromatographic process. Biochem. Eng. J. 2003, 14, 109–116. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, J.; Wang, Y.; Qiao, X.; Xiang, J.; Jin, Y. Study on CO2 adsorption capacity and kinetic mechanism of CO2 adsorbent prepared from fly ash. Energy 2023, 263, 125764. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Arnold, F.H.; Blanch, H.W.; Wilke, C.R. Analysis of affinity separations: I: Predicting the performance of affinity adsorbers. Chem. Eng. J. 1985, 30, B9–B23. [Google Scholar] [CrossRef]
- Eslami, T.; Jakob, L.A.; Satzer, P.; Ebner, G.; Jungbauer, A.; Lingg, N. Productivity for free: Residence time gradients during loading increase dynamic binding capacity and productivity. Sep. Purif. Technol. 2022, 281, 119985. [Google Scholar] [CrossRef]
- Chen, K.-H.; Lai, Y.-R.; Hanh, N.T.D. Breakthrough curve modeling for lysozyme by ion-exchange nanofiber membrane: Linear and nonlinear analysis. J. Taiwan Inst. Chem. Eng. 2023, 105198. [Google Scholar] [CrossRef]
- Girish, C.R.; Murty, V. Adsorption of Phenol from Aqueous Solution Using Lantana camara, Forest Waste: Packed Bed Studies and Prediction of Breakthrough Curves. Environ. Process. 2015, 2, 773–796. [Google Scholar] [CrossRef]
- Khitous, M.; Moussous, S.; Selatnia, A.; Kherat, M. Biosorption of Cd(II) by Pleurotus mutilus biomass in fixed-bed column: Experimental and breakthrough curves analysis. Desalin. Water Treat. 2016, 57, 16559–16570. [Google Scholar] [CrossRef]
- Tariq, M.; Farooq, U.; Athar, M.; Salman, M.; Tariq, M. Biosorption of Cu(II) from aqueous solution onto immobilized Ficus religiosa branch powder in a fixed bed column: Breakthrough curves and mathematical modeling. Korean J. Chem. Eng. 2019, 36, 48–55. [Google Scholar] [CrossRef]
- Hu, A.; Ren, G.; Che, J.; Guo, Y.; Ye, J.; Zhou, S. Phosphate recovery with granular acid-activated neutralized red mud: Fixed-bed column performance and breakthrough curve modelling. J. Environ. Sci. 2020, 90, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.N.; Young, B.R.; Taylor, M.; Burrell, R.; Aroua, M.K.; Baroutian, S. Breakthrough analysis of continuous fixed-bed adsorption of sevoflurane using activated carbons. Chemosphere 2020, 239, 124839. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef]
- Peng, J.; Su, Y.; Chen, W.; Zhao, X.; Jiang, Z.; Dong, Y.; Zhang, Y.; Liu, J.; Xingzhong, C. Polyamide nanofiltration membrane with high separation performance prepared by EDC/NHS mediated interfacial polymerization. J. Membr. Sci. 2013, 427, 92–100. [Google Scholar] [CrossRef]
- Chang, C.; Niu, F.; Su, Y.; Qiu, Y.; Gu, L.; Yang, Y. Characteristics and emulsifying properties of acid and acid-heat induced egg white protein. Food Hydrocoll. 2016, 54, 342–350. [Google Scholar] [CrossRef]
- Aksu, Z. Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: Effect of temperature. Sep. Purif. Technol. 2001, 21, 285–294. [Google Scholar] [CrossRef]
- Lin, W.; Murphy, C.J. A Demonstration of Le Chatelier’s Principle on the Nanoscale. ACS Cent. Sci. 2017, 3, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Barboza, M.; Almeida, R.M.R.G.; Hokka, C.O. Influence of temperature on the kinetics of adsorption and desorption of clavulanic acid by ionic exchange. Biochem. Eng. J. 2003, 14, 19–26. [Google Scholar] [CrossRef]
- Yue, X.; Huang, J.; Jiang, F.; Lin, H.; Chen, Y. Synthesis and characterization of cellulose-based adsorbent for removal of anionic and cationic dyes. J. Eng. Fiber. Fabr. 2019, 14, 1558925019828194. [Google Scholar] [CrossRef]
- Haynie, D.T. Biological Thermodynamics; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Homans, S.W. Dynamics and Thermodynamics of Ligand-Protein Interactions. In Bioactive Conformation I; Peters, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 51–82. [Google Scholar]
- Padmesh, T.V.N.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Application of Azolla rongpong on biosorption of acid red 88, acid green 3, acid orange 7 and acid blue 15 from synthetic solutions. Chem. Eng. J. 2006, 122, 55–63. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of Acid Orange 7 and Remazol Black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
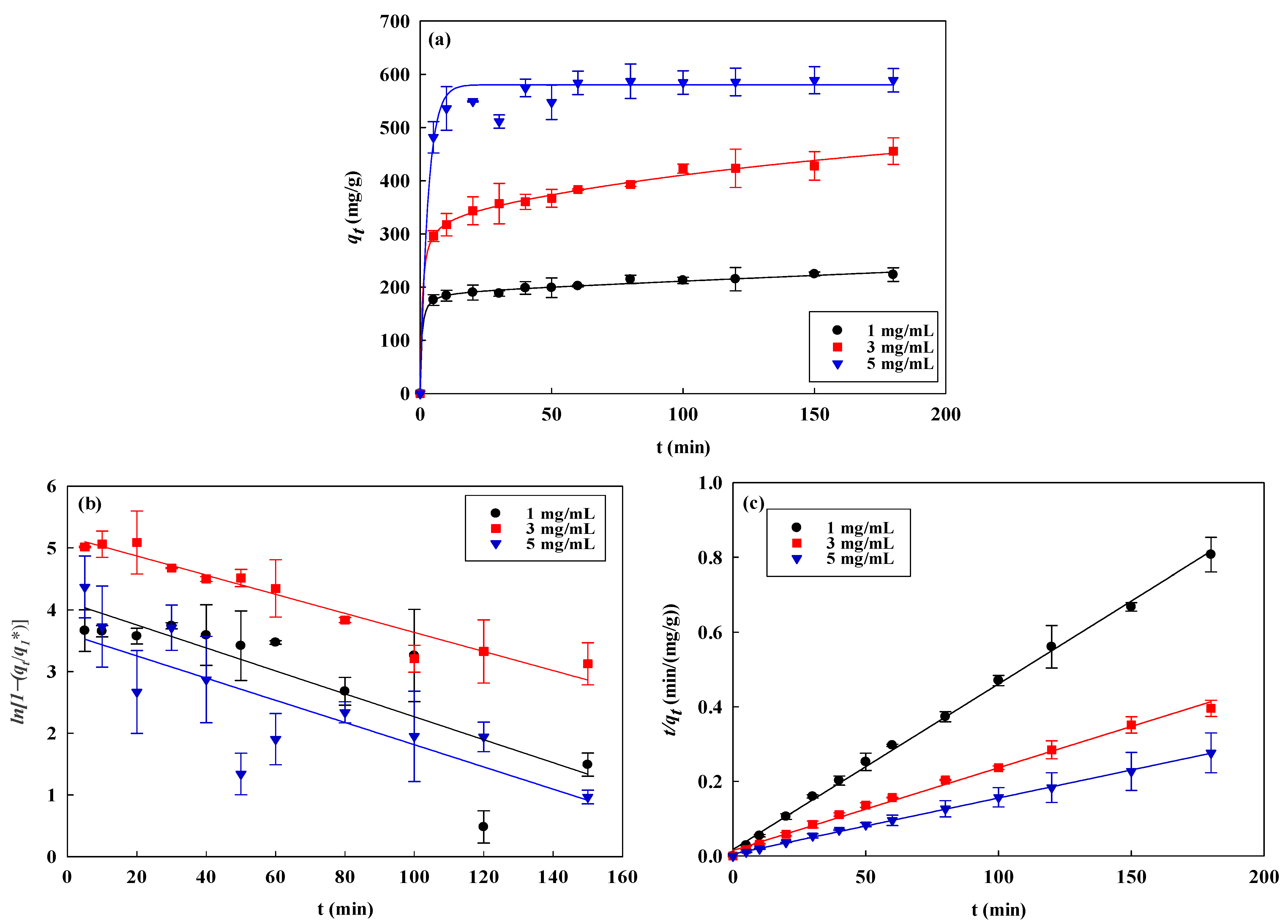
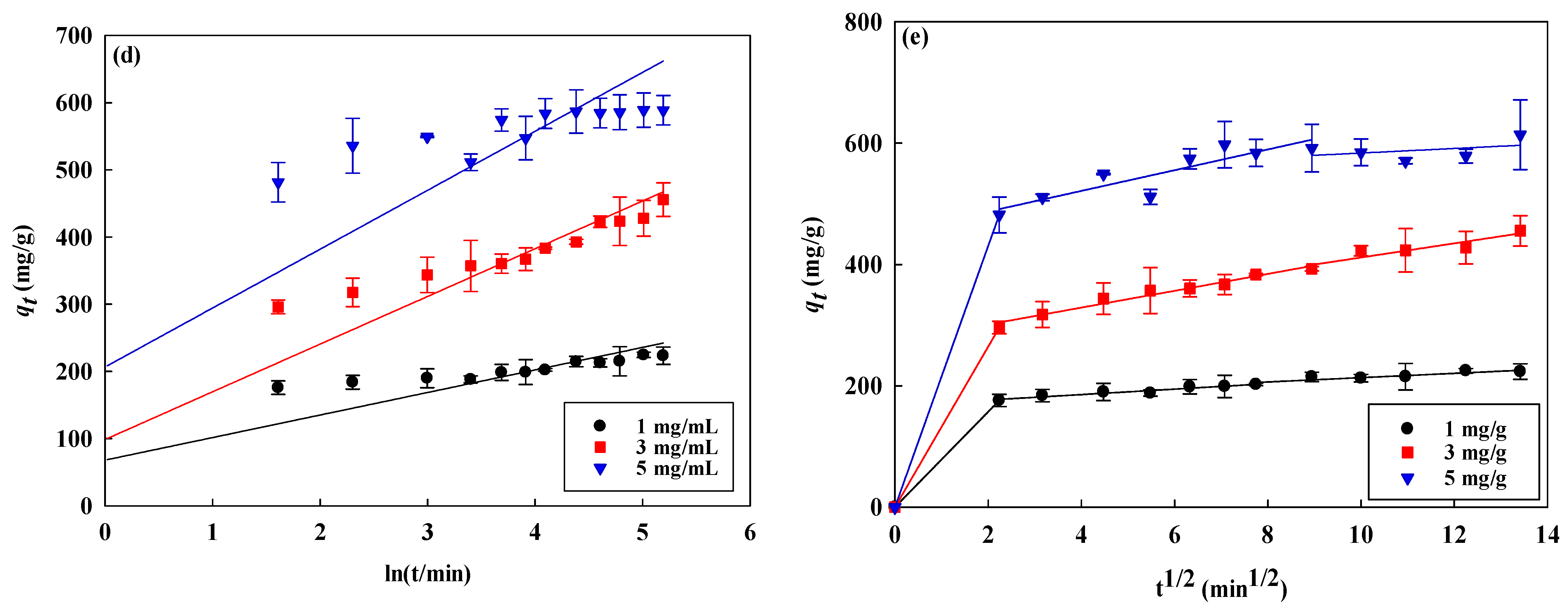
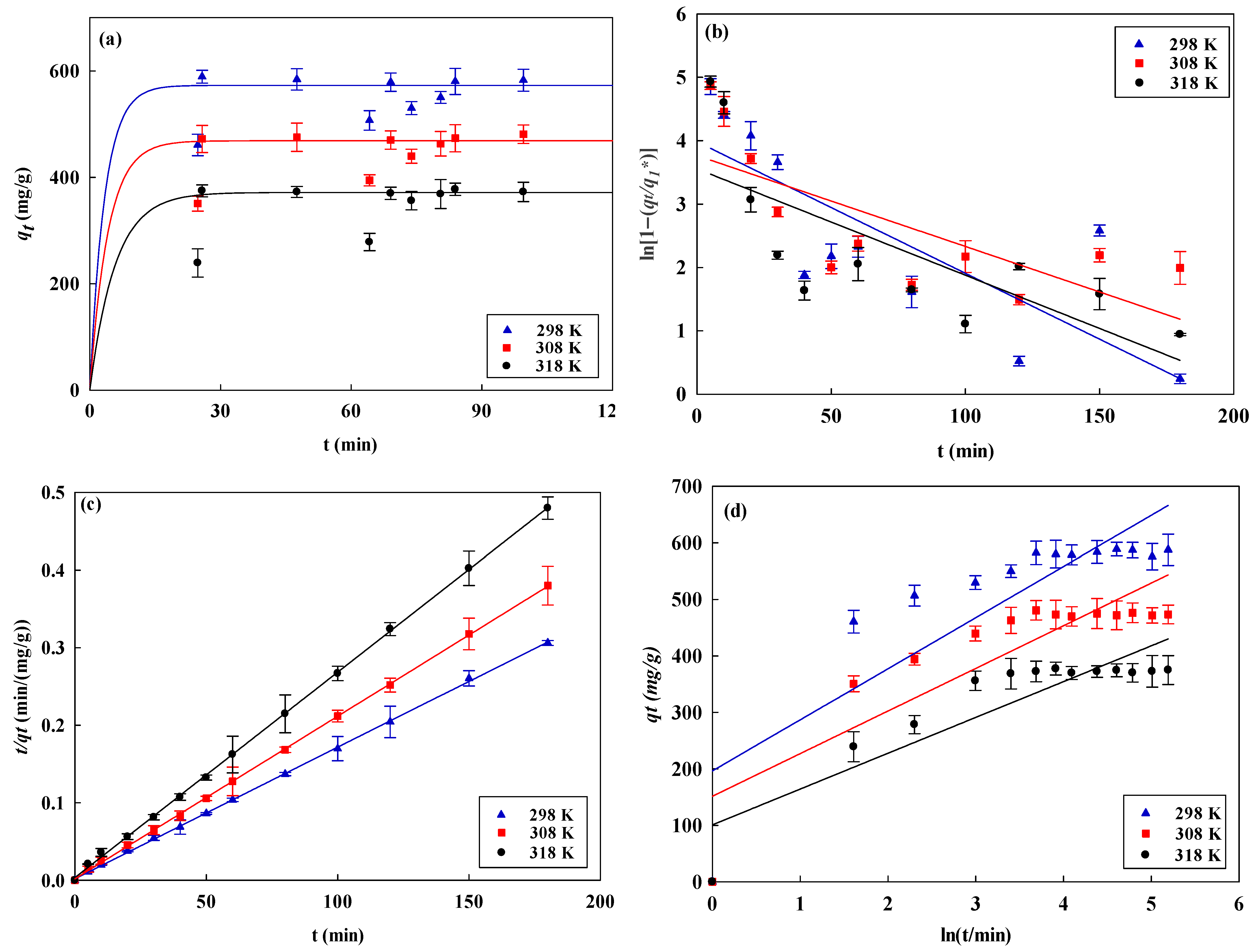

| Kinetic Models | AO7 Dye (mg/mL) | Temperature (K) | ||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 298 | 308 | 318 | |
| q*,exp (mg/g) | 232 ± 10 | 438 ± 15 | 589 ± 12 | 589 ± 12 | 481 ± 17 | 378 ± 12 |
| Pseudo-first order | ||||||
| k1 | 1.86 × 10−2 | 1.54 × 10−2 | 1.19 × 10−2 | 1.19 × 10−2 | 1.42 × 10−2 | 1.18 × 10−1 |
| R2 | 0.6410 | 0.8644 | 0.6366 | 0.6366 | 0.5475 | 0.5584 |
| Pseudo-second order | ||||||
| k2 (g/mg·min) | 6.02 × 10−5 | 1.10 × 10−5 | 1.41 × 10−4 | 1.41 × 10−4 | 1.38 × 10−4 | 1.30 × 10−4 |
| q*,cal (mg/g) | 225.2 | 451.5 | 589.3 | 589.3 | 477.5 | 377.5 |
| R2 | 0.9939 | 0.9901 | 0.9996 | 0.9996 | 0.9998 | 0.9996 |
| Elovich | ||||||
| α | 255.21 | 285.22 | 924.62 | 791.94 | 560.20 | 310.11 |
| β | 2.98 × 10−2 | 1.41 × 10−2 | 1.14 × 10−2 | 1.10 × 10−2 | 1.32 × 10−2 | 1.58 × 10−2 |
| R2 | 0.7304 | 0.8419 | 0.6728 | 0.7165 | 0.7381 | 0.7917 |
| Intra-membrane diffusion | ||||||
| ki2 | 10.57 | 23.49 | 26.42 | 27.11 | 22.56 | 19.23 |
| R2 | 0.5216 | 0.6624 | 0.4391 | 0.4622 | 0.4732 | 0.5228 |
| Temperature (K) | qexp (mg/g) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL, (mL/mg) | R2 | nF | KF (mg/g) | R2 | b (J/mol) | KT (mL/mg) | R2 | ||
| 298 | 589 ± 12 | 848.2 | 2.21 | 0.8895 | 1.86 | 262.7 | 0.9719 | 14.26 | 5.30 | 0.9076 |
| 308 | 481 ± 17 | 809.2 | 3.02 | 0.8414 | 1.68 | 197.4 | 0.9734 | 16.770 | 4.25 | 0.8907 |
| 318 | 378 ± 12 | 569.2 | 2.29 | 0.9389 | 1.68 | 165.2 | 0.9810 | 22.16 | 4.860 | 0.9466 |
| Temperature (K) | ΔG° kJ/mol | ΔH° kJ/mol | ΔS° J/(mol·K) |
|---|---|---|---|
| 298 | −1.97 | 2.56 | 15.20 |
| 308 | −2.83 | 16.53 | |
| 318 | −2.19 | 14.94 |
| Dye | Operating Conditions | Breakthrough Curve Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z (cm) | pH | Co (mg/mL) | F (mL/min) | t10% | t90% | Vb (mL) | BV | HMTZ (μm) | MAER (× 10−3) | DBC (mg/g) | EBC (mg/g) | MBU (%) | Productivity (P) (mg/min·g) | |
| AO7 | 115 | 2 | 1 | 1 | 3.45 | 7.91 | 3.45 | 81.08 | 64.84 | 4.35 | 230.0 | 318 ± 10 | 72.33 | 66.67 |
| TBO | 115 | 10 | 1 | 1 | 4.37 | 8.96 | 8.69 | 102.70 | 57.17 | 3.43 | 291.3 | 385.1 ± 9.7 | 75.65 | 66.67 |
| Dye | Operating Conditions | Thomas Model | BDST Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z (μm) | pH | Co (mg/mL) | F (mL/min) | qe (cal) | qe (exp) | R2 | E (%) | kBDST | No (cal) | No (exp) | R2 | E (%) | ||
| AO7 | 115 | 2 | 1 | 1 | 0.77 | 210.4 | 318 ± 10 | 0.7134 | 51.16 | 2.10 | 394.9 | 408.9 ± 7.5 | 0.9114 | 3.55 |
| TBO | 115 | 10 | 1 | 1 | 1.14 | 288.6 | 385.1 ± 9.7 | 0.8874 | 33.45 | 1.29 | 547.3 | 549.7 ± 8.2 | 0.8874 | 0.46 |
| Type of Adsorbent | qmax (mg/g) | qmax (μmol/g) | References |
|---|---|---|---|
| NM-COOH-CEW Nanofiber membrane | 589 ± 12 | 1681 | This work |
| NM-COOH-CS-CEW Nanofiber membrane | 307 | 876 | [22] |
| Magnetic graphene/chitosan | 43 | 122 | [58] |
| Bottom ash | 4 | 12 | [57] |
| Canola stalk | 25 | 72 | [51] |
| Azolla rongpong | 77 | 220 | [52] |
| Spent brewery grains | 30 | 87 | [53] |
| Untreated sugarcane bagasse | 28 | 80 | [54] |
| Beech wood sawdust | 5 | 14 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-R.; Thanh, D.T.H.; Tran, Q.T.P.; Liu, B.-L.; Srinophakun, P.; Chiu, C.-Y.; Chen, K.-H.; Chang, Y.-K. The Utilization of Chicken Egg White Waste-Modified Nanofiber Membrane for Anionic Dye Removal in Batch and Flow Systems: Comprehensive Investigations into Equilibrium, Kinetics, and Breakthrough Curve. Membranes 2024, 14, 128. https://doi.org/10.3390/membranes14060128
Chen Y-R, Thanh DTH, Tran QTP, Liu B-L, Srinophakun P, Chiu C-Y, Chen K-H, Chang Y-K. The Utilization of Chicken Egg White Waste-Modified Nanofiber Membrane for Anionic Dye Removal in Batch and Flow Systems: Comprehensive Investigations into Equilibrium, Kinetics, and Breakthrough Curve. Membranes. 2024; 14(6):128. https://doi.org/10.3390/membranes14060128
Chicago/Turabian StyleChen, Yun-Rou, Dinh Thi Hong Thanh, Quynh Thi Phuong Tran, Bing-Lan Liu, Penjit Srinophakun, Chen-Yaw Chiu, Kuei-Hsiang Chen, and Yu-Kaung Chang. 2024. "The Utilization of Chicken Egg White Waste-Modified Nanofiber Membrane for Anionic Dye Removal in Batch and Flow Systems: Comprehensive Investigations into Equilibrium, Kinetics, and Breakthrough Curve" Membranes 14, no. 6: 128. https://doi.org/10.3390/membranes14060128
APA StyleChen, Y.-R., Thanh, D. T. H., Tran, Q. T. P., Liu, B.-L., Srinophakun, P., Chiu, C.-Y., Chen, K.-H., & Chang, Y.-K. (2024). The Utilization of Chicken Egg White Waste-Modified Nanofiber Membrane for Anionic Dye Removal in Batch and Flow Systems: Comprehensive Investigations into Equilibrium, Kinetics, and Breakthrough Curve. Membranes, 14(6), 128. https://doi.org/10.3390/membranes14060128







