Stability and Performance of Commercial Membranes in High-Temperature Organic Flow Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
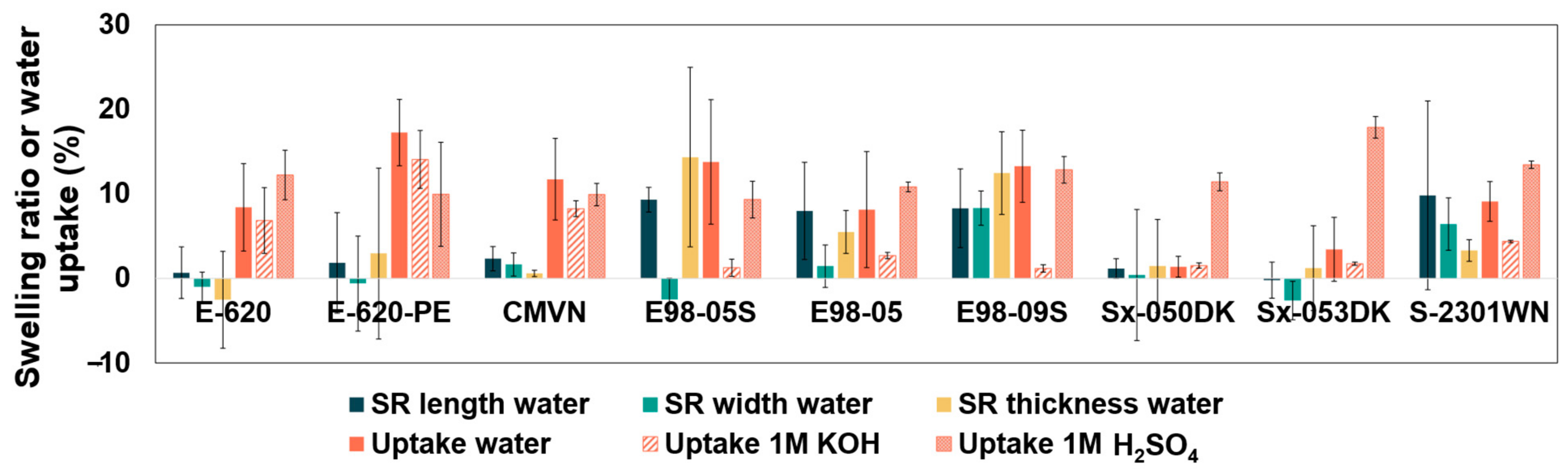
3.1. Water Uptake and Swelling Ratio
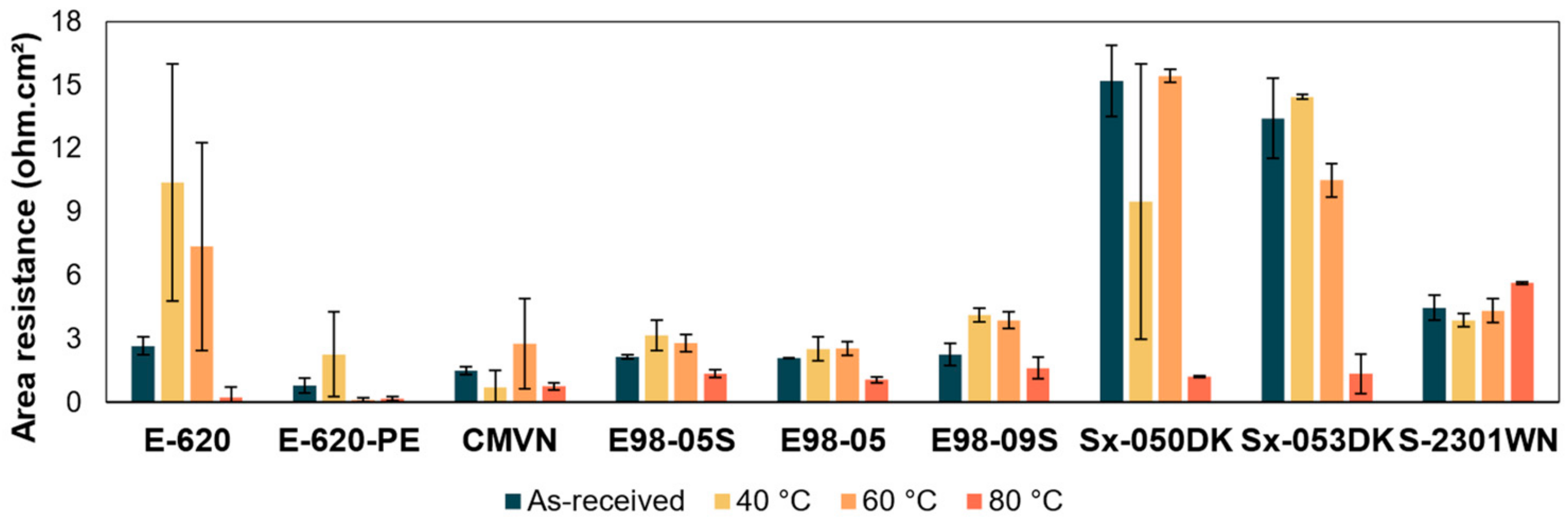
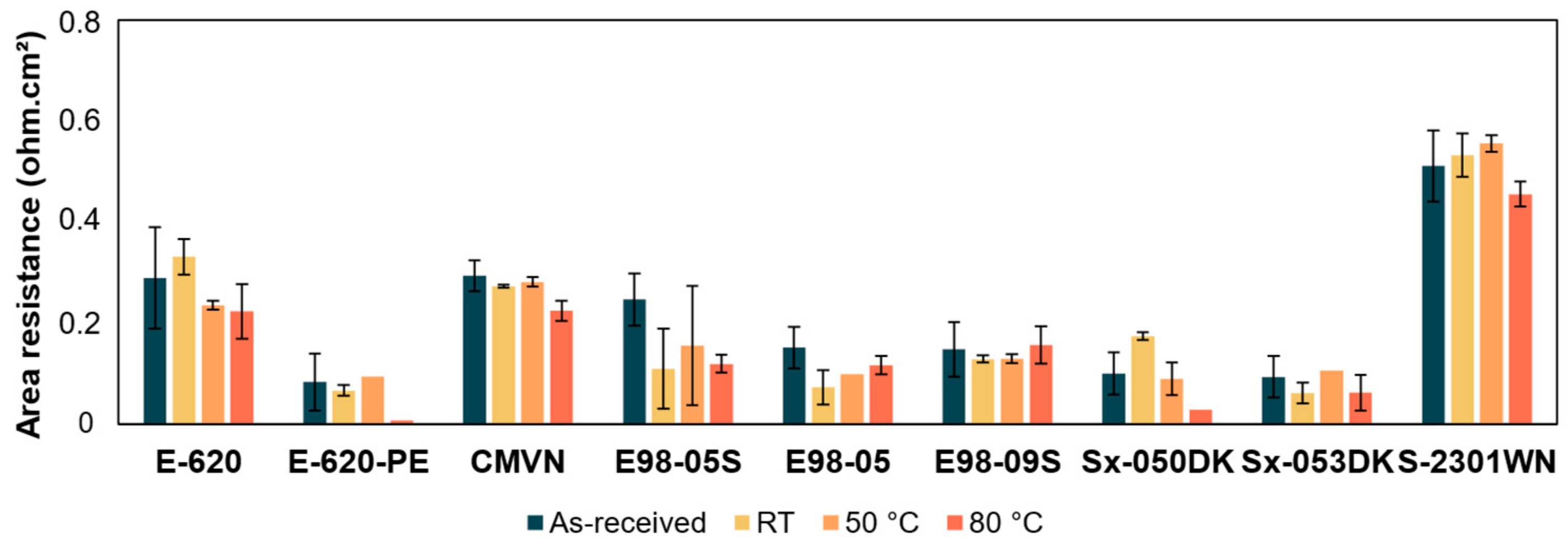
3.2. Area Resistance
3.3. Diffusion
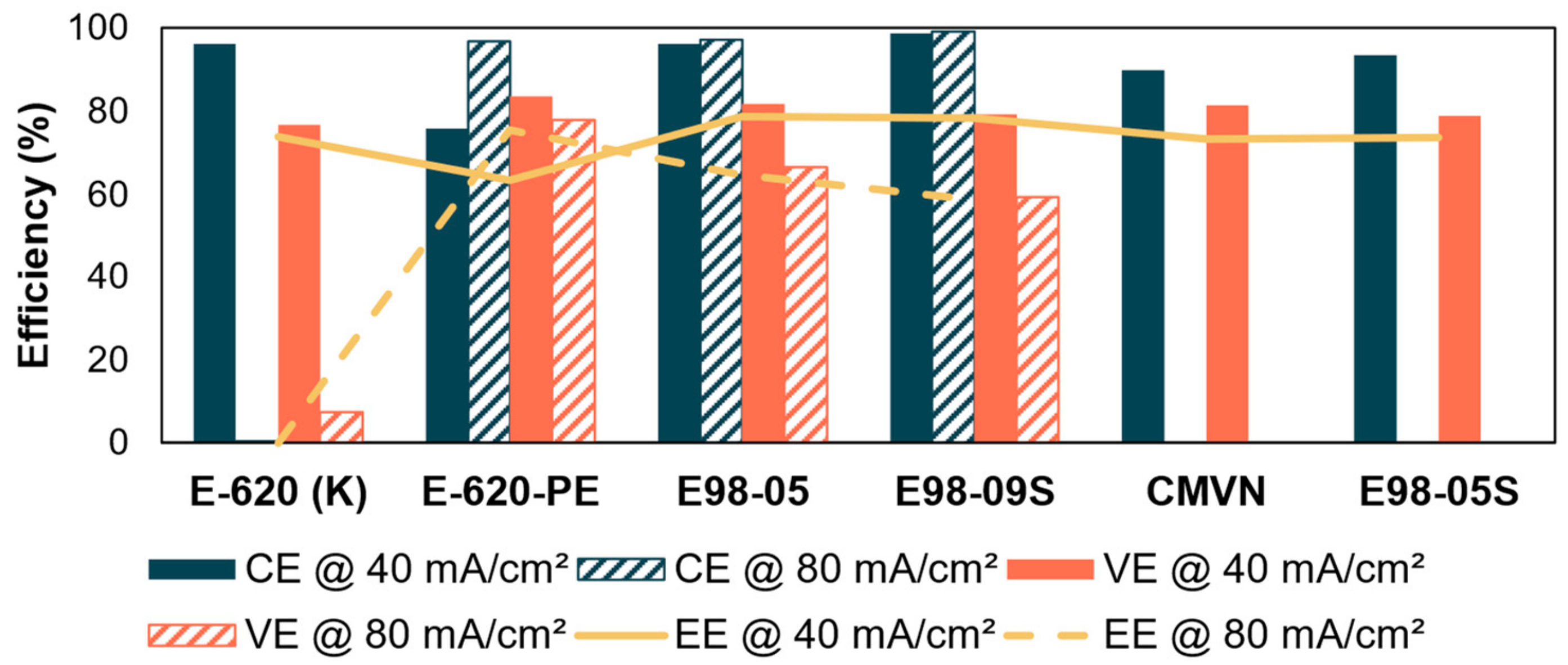
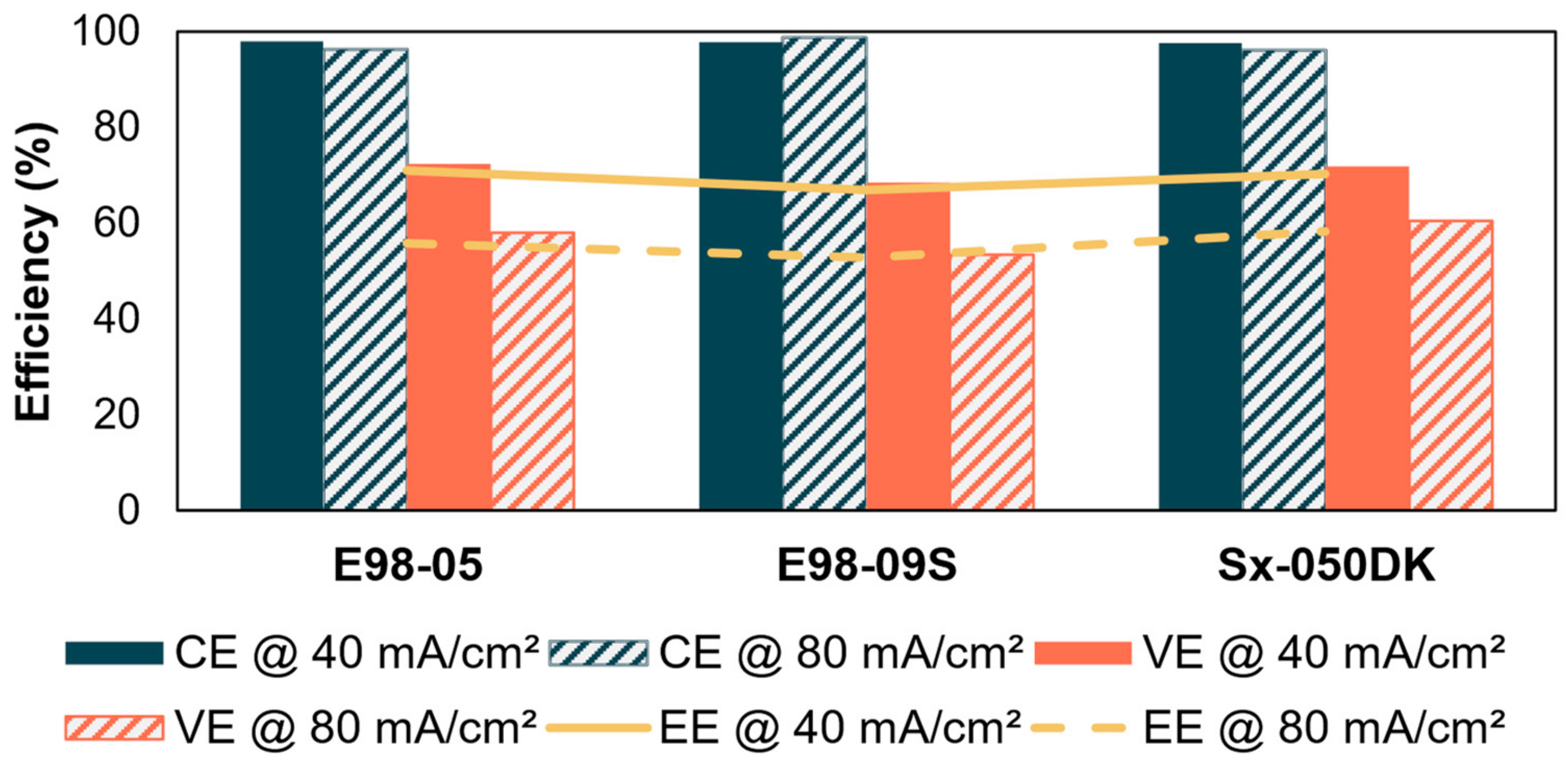
3.4. Battery Performance
3.4.1. Alkaline Battery Performance
3.4.2. Acid Battery Peformance
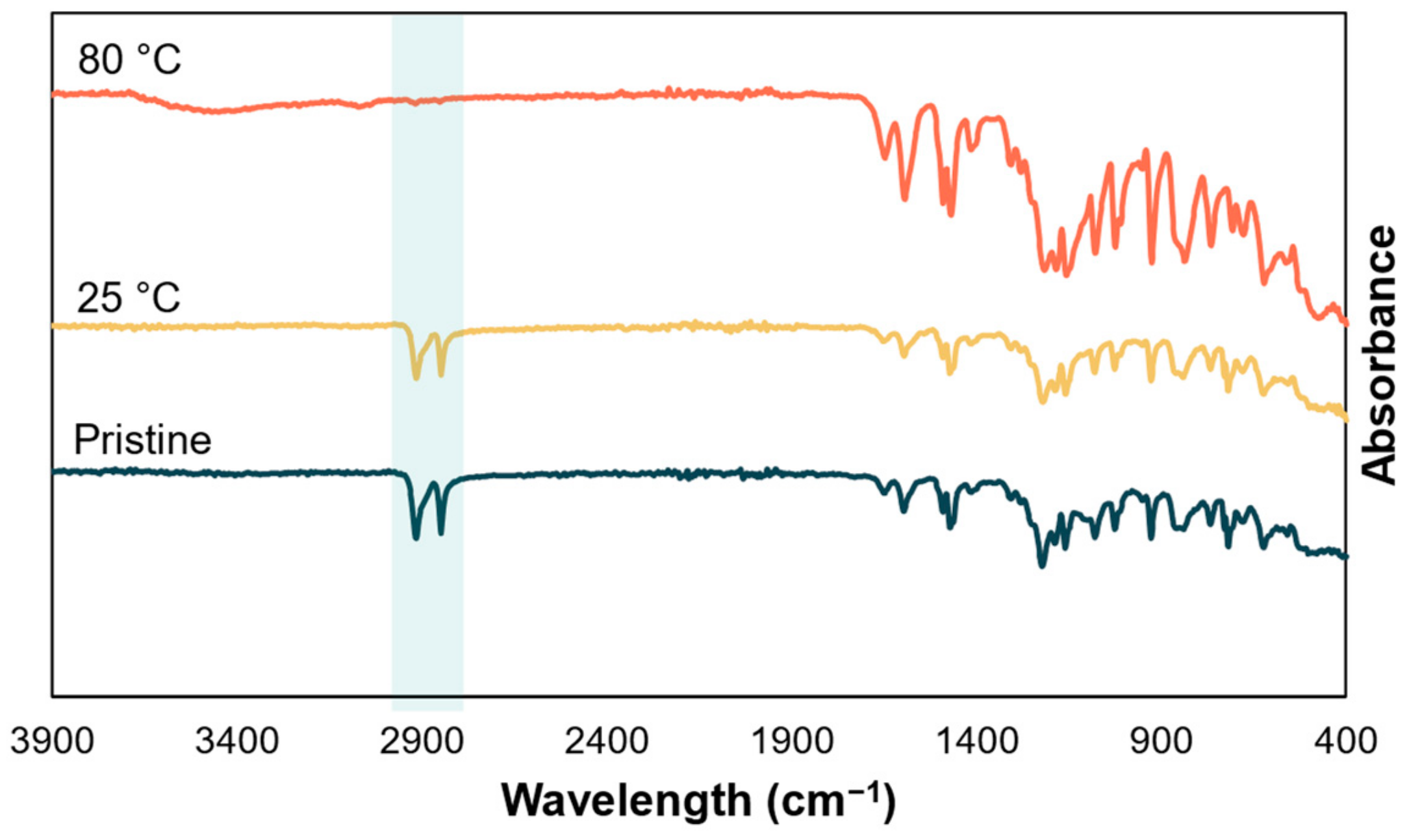
3.5. Stability to Extreme pH at High Temperature
3.5.1. Alkaline Stability
3.5.2. Acid Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.Y.; Zhang, H. Investigation of Nafion Series Membranes on the Performance of Iron-Chromium Redox Flow Battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Permatasari, A.; Lee, W.; Kwon, Y. Performance Improvement by Novel Activation Process Effect of Aqueous Organic Redox Flow Battery Using Tiron and Anthraquinone-2,7-Disulfonic Acid Redox Couple. Chem. Eng. J. 2020, 383, 123085. [Google Scholar] [CrossRef]
- Yang, B.; Hoober-Burkhardt, L.; Wang, F.; Surya Prakash, G.K.; Narayanan, S.R. An Inexpensive Aqueous Flow Battery for Large-Scale Electrical Energy Storage Based on Water-Soluble Organic Redox Couples. J. Electrochem. Soc. 2014, 161, A1371–A1380. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A Metal-Free Organic-Inorganic Aqueous Flow Battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M. Review—Highlights of UNSW All-Vanadium Redox Battery Development: 1983 to Present. J. Electrochem. Soc. 2022, 169, 070513. [Google Scholar] [CrossRef]
- Jethwa, R.B.; Hey, D.; Kerber, R.N.; Bond, A.D.; Wright, D.S.; Grey, C.P. Exploring the Landscape of Heterocyclic Quinones for Redox Flow Batteries. ACS Appl. Energy Mater. 2023, 7, 414–426. [Google Scholar] [CrossRef]
- Peng, K.; Li, Y.; Tang, G.; Liu, Y.; Yang, Z.; Xu, T. Solvation Regulation to Mitigate the Decomposition of 2, 6-Dihydroxyanthraquinone in Aqueous Organic Redox Flow Batteries. Energy Environ. Sci. 2022, 16, 430–437. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489. [Google Scholar] [CrossRef]
- Chen, H.; Cong, G.; Lu, Y.C. Recent Progress in Organic Redox Flow Batteries: Active Materials, Electrolytes and Membranes. J. Energy Chem. 2018, 27, 1304–1325. [Google Scholar] [CrossRef]
- Borchers, P.S.; Strumpf, M.; Friebe, C.; Nischang, I.; Hager, M.D.; Elbert, J.; Schubert, U.S. Aqueous Redox Flow Battery Suitable for High Temperature Applications Based on a Tailor-Made Ferrocene Copolymer. Adv. Energy Mater. 2020, 10, 2001825. [Google Scholar] [CrossRef]
- Karrech, A. Large-Scale All-Climate Vanadium Batteries. Appl. Energy 2024, 355, 122324. [Google Scholar] [CrossRef]
- Rohland, P.; Schreyer, K.; Hager, M.D.; Schubert, U.S. Anthraquinone-2.6-Dilsulfamidic Acid: An Anolyte with Low Decomposition Rates at Elevated Temperatures. RSC Adv. 2021, 11, 38759–38764. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, J. A High-Temperature Tolerance Solution for Positive Electrolyte of Vanadium Redox Flow Batteries. J. Electroanal. Chem. 2017, 801, 92–97. [Google Scholar] [CrossRef]
- Vrána, J.; Charvát, J.; Mazúr, P.; Bělský, P.; Dundálek, J.; Pocedič, J.; Kosek, J. Commercial Perfluorosulfonic Acid Membranes for Vanadium Redox Flow Battery: Effect of Ion-Exchange Capacity and Membrane Internal Structure. J. Memb. Sci. 2018, 552, 202–212. [Google Scholar] [CrossRef]
- Hwang, G.J.; Kim, S.W.; In, D.M.; Lee, D.Y.; Ryu, C.H. Application of the Commercial Ion Exchange Membranes in the All-Vanadium Redox Flow Battery. J. Ind. Eng. Chem. 2018, 60, 360–365. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Zhu, X.B. Performance of a Vanadium Redox Flow Battery with a VANADion Membrane. Appl. Energy 2016, 180, 353–359. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, Y.H.; Ryu, C.H.; Hwang, G.J. Characteristics of the All-Vanadium Redox Flow Battery Using Anion Exchange Membrane. J. Taiwan Inst. Chem. Eng. 2014, 45, 2920–2925. [Google Scholar] [CrossRef]
- Maghsoudy, S.; Rahimi, M.; Dehkordi, A.M. Investigation on Various Types of Ion-Exchange Membranes in Vanadium Redox Flow Batteries: Experiment and Modeling. J. Energy Storage 2022, 54, 105347. [Google Scholar] [CrossRef]
- Zhao, N.; Riley, H.; Song, C.; Jiang, Z.; Tsay, K.; Neagu, R.; Shi, Z. Ex-Situ Evaluation of Commercial Polymer Membranes for Vanadium Redox Flow Batteries (VRFBs). Polymers 2021, 13, 926. [Google Scholar] [CrossRef]
- Shanahan, B.; Britton, B.; Belletti, A.; Vierrath, S.; Breitwieser, M. Performance and Stability Comparison of AemionTM and Aemion+TM Membranes for Vanadium Redox Flow Batteries. RSC Adv. 2021, 11, 13077–13084. [Google Scholar] [CrossRef]
- Small, L.J.; Pratt, H.D.; Anderson, T.M. Crossover in Membranes for Aqueous Soluble Organic Redox Flow Batteries. J. Electrochem. Soc. 2019, 166, A2536–A2542. [Google Scholar] [CrossRef]
- Mohammadi, T.; Chieng, S.C.; Skyllas Kazacos, M. Water Transport Study across Ion Exchange Membranes in the Vanadium Redox Flow Battery. J. Memb. Sci. 1997, 133, 151–159. [Google Scholar] [CrossRef]
- Hu, B.; Seefeldt, C.; Debruler, C.; Liu, T.L. Boosting the Energy Efficiency and Power Performance of Neutral Aqueous Organic Redox Flow Batteries. J. Mater. Chem. A 2017, 5, 22137–22145. [Google Scholar] [CrossRef]
- Kim, S.; Thomsen, E.; Xia, G.; Nie, Z.; Bao, J.; Recknagle, K.; Wang, W.; Viswanathan, V.; Luo, Q.; Wei, X.; et al. 1 KW/1 KWh Advanced Vanadium Redox Flow Battery Utilizing Mixed Acid Electrolytes. J. Power Sources 2013, 237, 300–309. [Google Scholar] [CrossRef]
- Berling, S.; Patil, N.; García-Quismondo, E.; Palma, J.; Ponce, C.; Le, D. A Mediated Vanadium Flow Battery: Lignin as Redox-Targeting Active Material in the Vanadium Catholyte. J. Energy Storage 2023, 68, 107620. [Google Scholar] [CrossRef]
- Abbas, S.; Hwang, J.; Kim, H.; Chae, S.A.; Kim, J.W.; Mehboob, S.; Ahn, A.; Han, O.H.; Ha, H.Y. Enzyme-Inspired Formulation of the Electrolyte for Stable and E Ffi Cient Vanadium Redox Flow Batteries at High Temperatures. ACS Appl. Mater. Interfaces 2019, 11, 26842–26853. [Google Scholar] [CrossRef]
- Chang, D.-R.; Kim, Y.; Jung, S. Comprehensive Study of the Performance of Alkaline Organic Redox Flow Batteries as Large-Scale Energy Storage Systems. Int. J. Energy Res. 2019, 43, 4449–4458. [Google Scholar] [CrossRef]
- Xu, J.; Pang, S.; Wang, P.; Ji, Y. Ultrastable Aqueous Phenazine Flow Batteries with High Capacity Operated at Elevated Temperatures Ultrastable Aqueous Phenazine Flow Batteries with High Capacity Operated at Elevated Temperatures. Joule 2021, 5, 2437–2449. [Google Scholar] [CrossRef]
- Lee, W.; Park, G.; Chang, D.; Kwon, Y. The Effects of Temperature and Membrane Thickness on the Performance of Aqueous Alkaline Redox Flow Batteries Using Napthoquinone and Ferrocyanide as Redox Couple. Korean J. Chem. Eng. 2020, 37, 2326–2333. [Google Scholar] [CrossRef]
- Xing, X.; Huo, Y.; Wang, X.; Zhao, Y. A Benzophenone-Based Anolyte for High Energy Density All-Organic Redox Flow Battery. Int. J. Hydrog. Energy 2017, 42, 17488–17494. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, L.Y.; Ji, Y.N.; Leung, P.K.; Su, H.N.; Qiao, F.; Yang, W.W.; Shah, A.A.; Li, H.M. A Deep Eutectic Solvent (DES) Electrolyte-Based Vanadium-Iron Redox Flow Battery Enabling Higher Specific Capacity and Improved Thermal Stability. Electrochim. Acta 2019, 293, 426–431. [Google Scholar] [CrossRef]
- Song, W.; Peng, K.; Xu, W.; Liu, X.; Zhang, H.; Liang, X.; Ye, B.; Zhang, H.; Yang, Z.; Wu, L.; et al. Upscaled Production of an Ultramicroporous Anion-Exchange Membrane Enables Long-Term Operation in Electrochemical Energy Devices. Nat. Commun. 2023, 14, 2732. [Google Scholar] [CrossRef] [PubMed]
- Kwabi, D.G.; Lin, K.; Ji, Y.; Kerr, E.F.; Goulet, M.A.; De Porcellinis, D.; Tabor, D.P.; Pollack, D.A.; Aspuru-Guzik, A.; Gordon, R.G.; et al. Alkaline Quinone Flow Battery with Long Lifetime at PH 12. Joule 2018, 2, 1894–1906. [Google Scholar] [CrossRef]
- Redox Flow A-Cell—Redox Flow Battery Test Cell. Available online: https://redox-flow.com/shop/redox-flow-battery-test-cell/ (accessed on 29 April 2023).
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Dai, Q.; Zhao, Z.; Shi, M.; Deng, C.; Zhang, H.; Li, X. Ion Conductive Membranes for Flow Batteries: Design and Ions Transport Mechanism. J. Memb. Sci. 2021, 632, 119355. [Google Scholar] [CrossRef]
- Hu, L.; Gao, L.; Di, M.; Jiang, X.; Wu, X.; Yan, X.; Li, X.; He, G. Ion/Molecule-Selective Transport Nanochannels of Membranes for Redox Flow Batteries. Energy Storage Mater. 2021, 34, 648–668. [Google Scholar] [CrossRef]
- Fischer, P.; Mazúr, P.; Krakowiak, J. Family Tree for Aqueous Organic Redox Couples for Redox Flow Battery Electrolytes: A Conceptual Review. Molecules 2022, 27, 560. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, H.; Xu, W.; Yuan, Z.; Li, X. Mechanism and Transfer Behavior of Ions in Na Fi on Membranes under Alkaline Media. J. Memb. Sci. 2018, 566, 8–14. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Y.; Cheng, J.; Cao, G.; Yang, Y. A Study of Tiron in Aqueous Solutions for Redox Flow Battery Application. Electrochim. Acta 2010, 55, 715–720. [Google Scholar] [CrossRef]
- Yang, B.; Hoober-burkhardt, L.; Krishnamoorthy, S.; Murali, A.; Prakash, G.K.S.; Narayanan, S.R. High-Performance Aqueous Organic Flow Battery with Quinone-Based Redox Couples at Both Electrodes. J. Electrochem. Soc. 2016, 163, A1442–A1449. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, T.; Skyllas-kazacos, M. Membrane Stability Studies for Vanadium Redox Cell Applications. J. Appl. Electrochem. 2004, 03430, 137–145. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, L.; Wang, C.; Li, N. Side-Chain-Type Anion Exchange Membranes for Vanadium Flow Battery: Properties and Degradation Mechanism. J. Mater. Chem. A 2018, 6, 22778–22789. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.; Hu, J.; Xu, W.; Cao, J.; Zhang, H. Degradation Mechanism of Sulfonated Poly (Ether Ether Ketone) (SPEEK) Ion Exchange Membranes under Vanadium Flow Battery Medium. Phys. Chem. Chem. Phys. 2014, 16, 19841–19847. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. V5+ Degradation of Sulfonated Radel Membranes for Vanadium Redox Flow Batteries. Phys. Chem. Chem. Phys. 2013, 15, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Lulay, F.; Weidlich, C.; Valtiner, M.; Pichler, C.M. Membrane Degradation in Redox Flow Batteries. Green Chem. Lett. Rev. 2023, 16, 2274529. [Google Scholar] [CrossRef]
- Kim, S.; Tighe, T.B.; Schwenzer, B.; Yan, J.; Zhang, J.; Liu, J.; Yang, Z.; Hickner, M.A. Chemical and Mechanical Degradation of Sulfonated Poly (Sulfone) Membranes in Vanadium Redox Flow Batteries. J. Appl. Electrochem. 2011, 41, 1201–1213. [Google Scholar] [CrossRef]
- Gao, M.; Salla, M.; Zhang, F.; Zhi, Y.; Wang, Q. Membrane Fouling in Aqueous Redox Flow Batteries. J. Power Sources 2022, 527, 231180. [Google Scholar] [CrossRef]
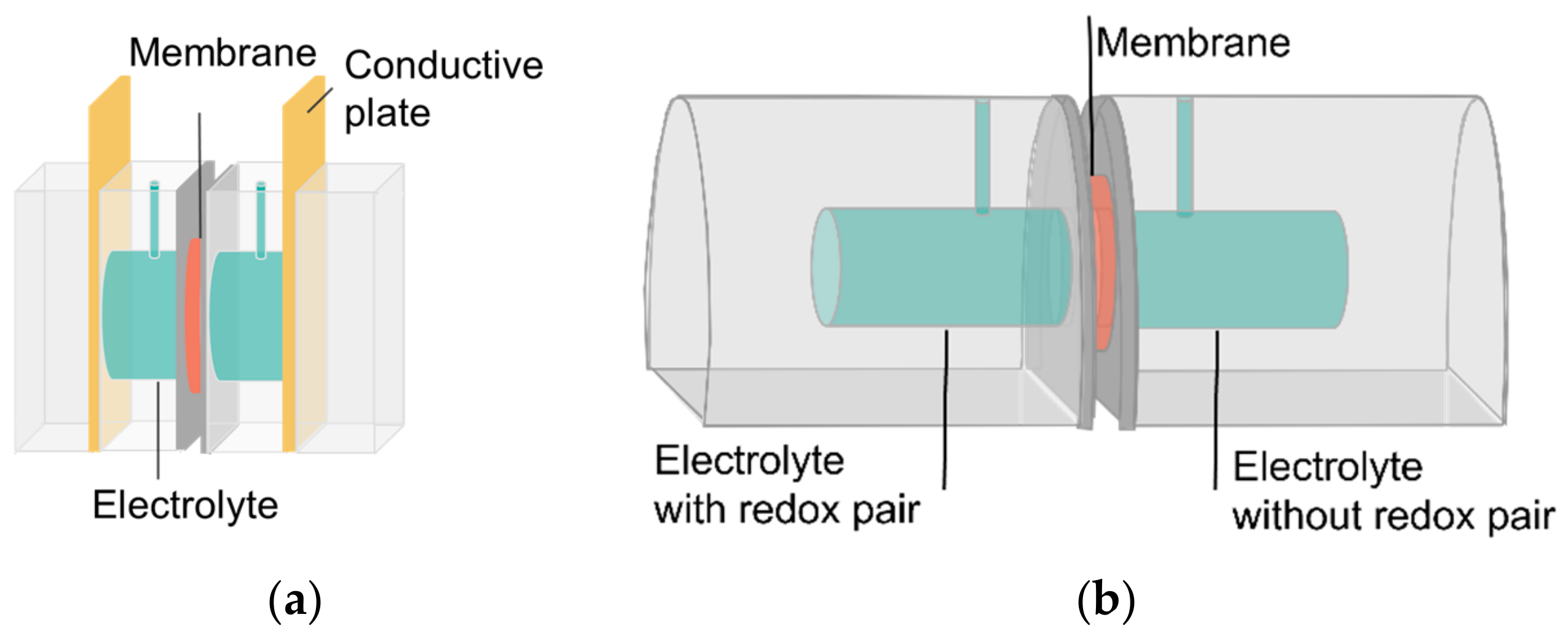



| Membrane | Company, Series | Type | Counter ion | Chemistry | Thickness (µm) |
|---|---|---|---|---|---|
| E-620 | Fumatech, fumasep | CEM | K+ | Sulfonated poly aryl ether ketone (SPAEK) [33] | 20 |
| E-620-PE | Fumatech, fumasep | CEM | K+ | SPAEK [33] with poly ethylene (PE) reinforcement | 20 |
| CMVN | AGC, Selemion | CEM | Na+ | Hydrocarbon with polyolefin reinforcement | 100 |
| E98-05S | Solvay, Aquivion | CEM | H+ | Perfluorinated sulfonic acid | 50 |
| E98-05 | Solvay, Aquivion | CEM | H+ | Perfluorinated sulfonic acid | 50 |
| E98-09S | Solvay, Aquivion | CEM | H+ | Perfluorinated sulfonic acid | 90 |
| Sx-050DK | AGC, FORBLUE | CEM | K+ | Perfluorinated sulfonic acid | 50 |
| Sx-053DK | AGC, FORBLUE | CEM | K+ | Perfluorinated sulfonic acid | 50 |
| S-2301WN | AGC, FORBLUE | CEM | Na+ | Perfluorinated sulfonic acid with poly tetra fluoroethylene (PTFE) fiber reinforcement | 330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Cauter, C.J.; Li, Y.; Van Herck, S.; Vankelecom, I.F.J. Stability and Performance of Commercial Membranes in High-Temperature Organic Flow Batteries. Membranes 2024, 14, 177. https://doi.org/10.3390/membranes14080177
Van Cauter CJ, Li Y, Van Herck S, Vankelecom IFJ. Stability and Performance of Commercial Membranes in High-Temperature Organic Flow Batteries. Membranes. 2024; 14(8):177. https://doi.org/10.3390/membranes14080177
Chicago/Turabian StyleVan Cauter, Chiari J., Yun Li, Sander Van Herck, and Ivo F. J. Vankelecom. 2024. "Stability and Performance of Commercial Membranes in High-Temperature Organic Flow Batteries" Membranes 14, no. 8: 177. https://doi.org/10.3390/membranes14080177





