Simulating the Permeation of Toxic Chemicals through Barrier Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Permeation System
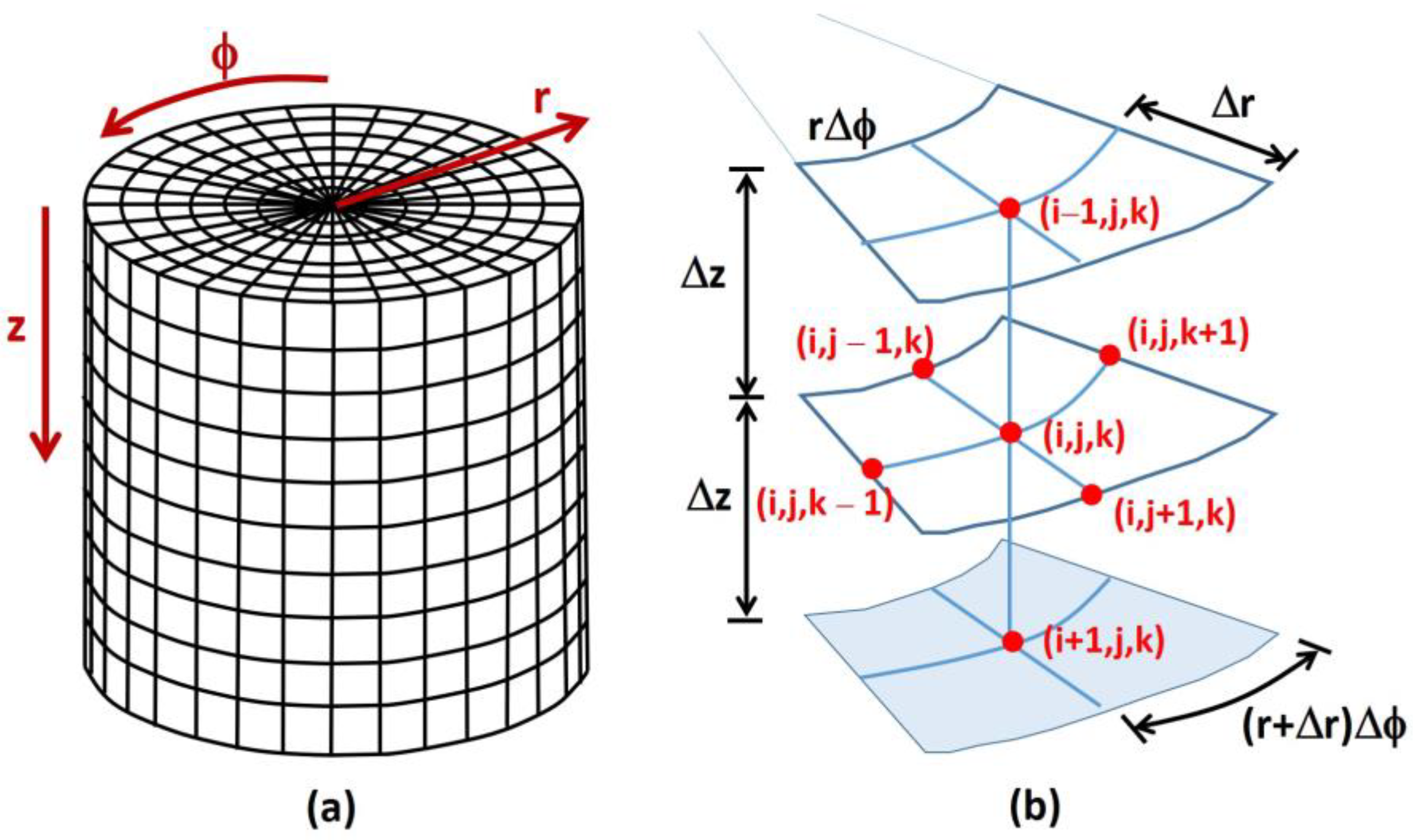
2.2. Numerical Simulations
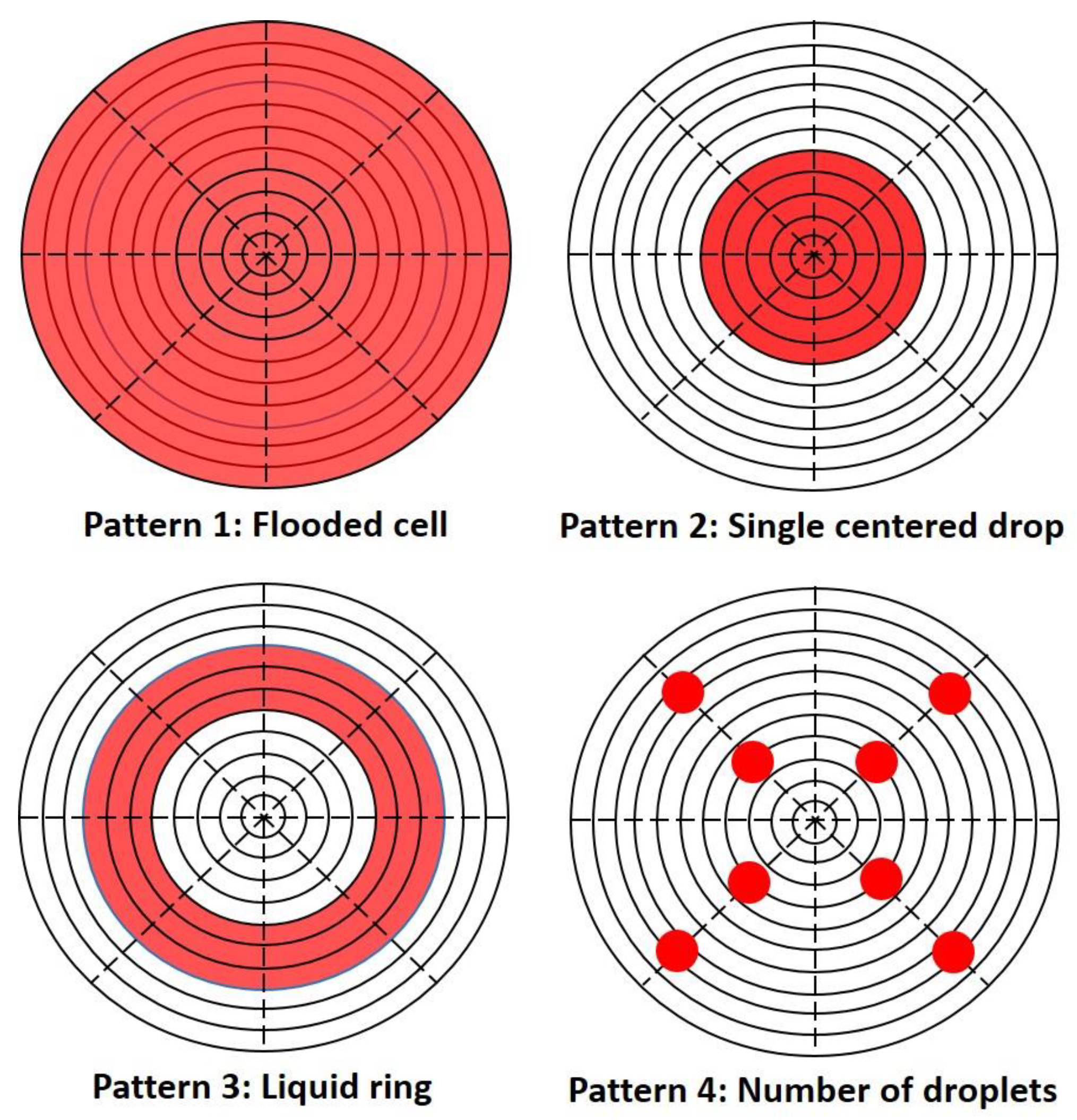
2.3. Different Liquid Patterns and Surface Area Coverage
3. Results and Discussion
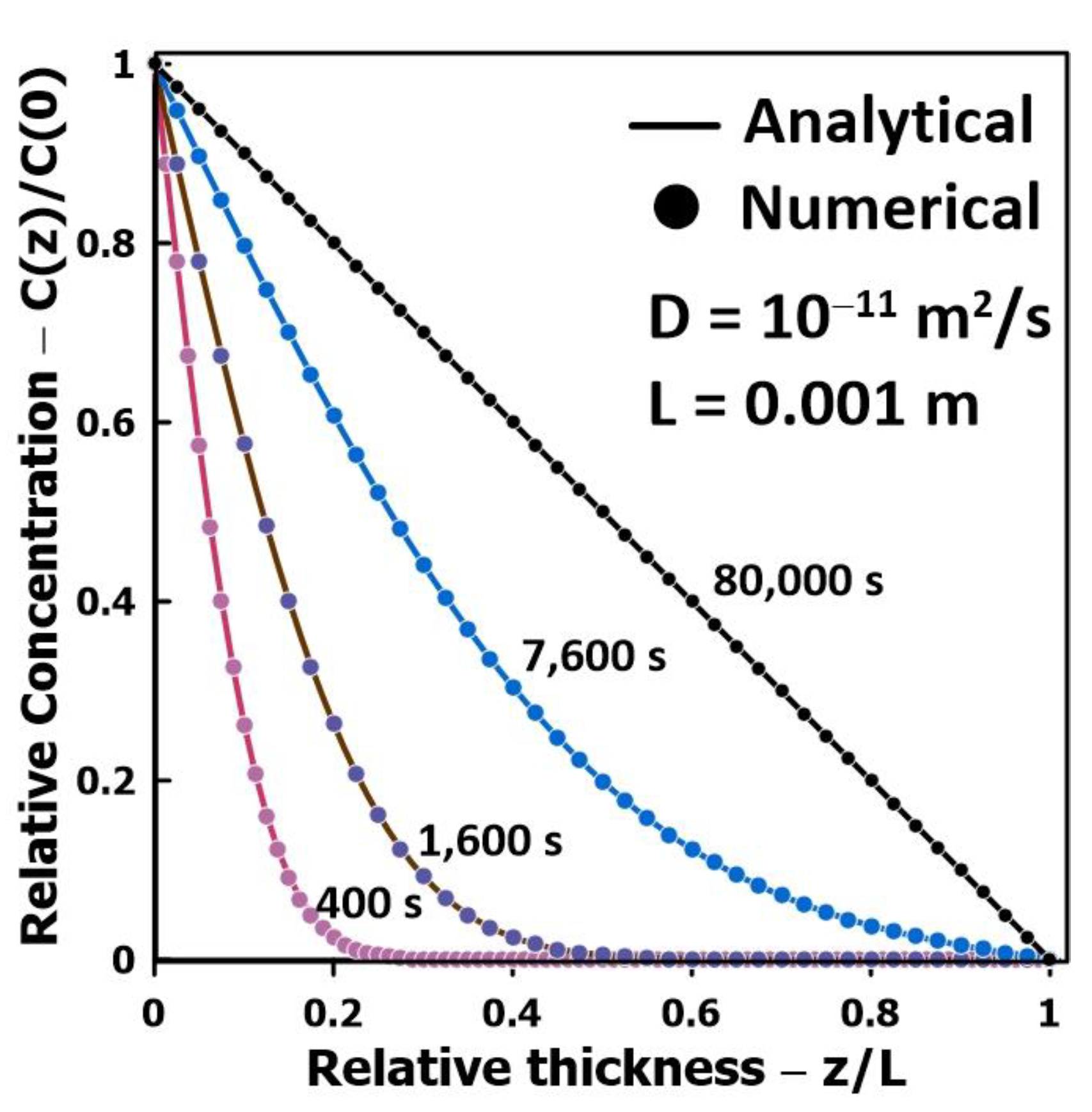
3.1. Validation of the Numerical Simulations
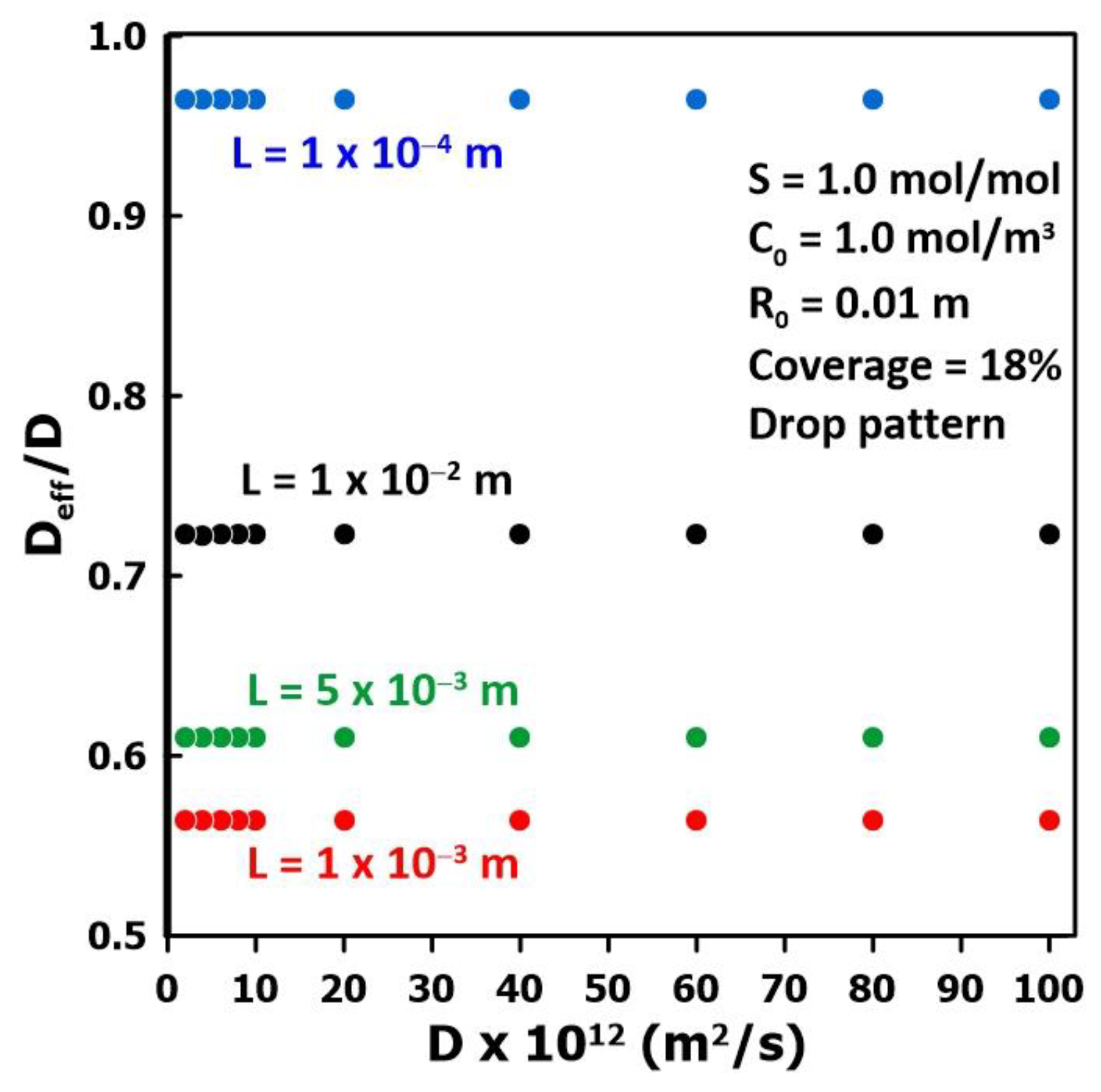
3.2. The Impact of the Membrane Diffusivity
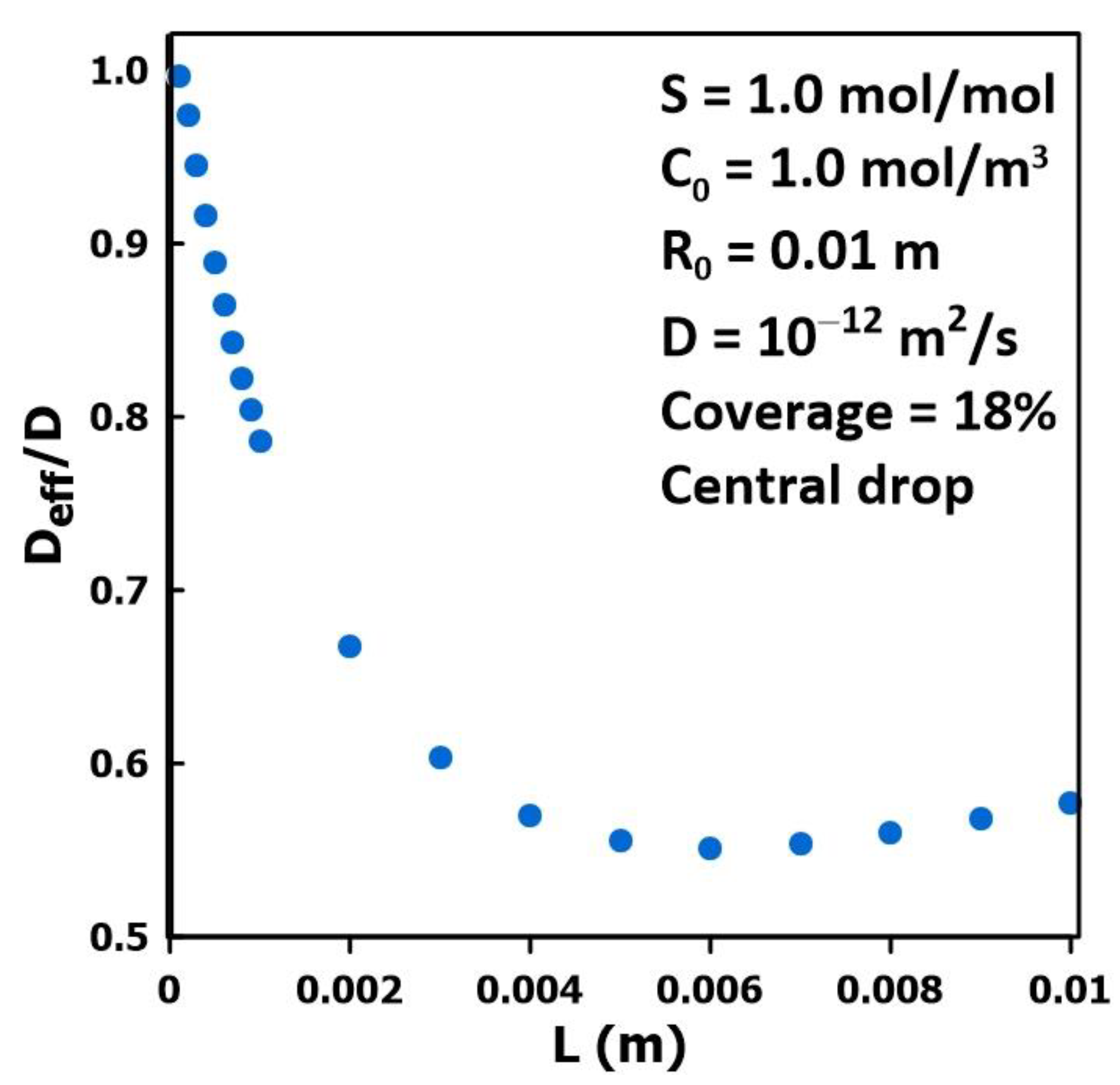
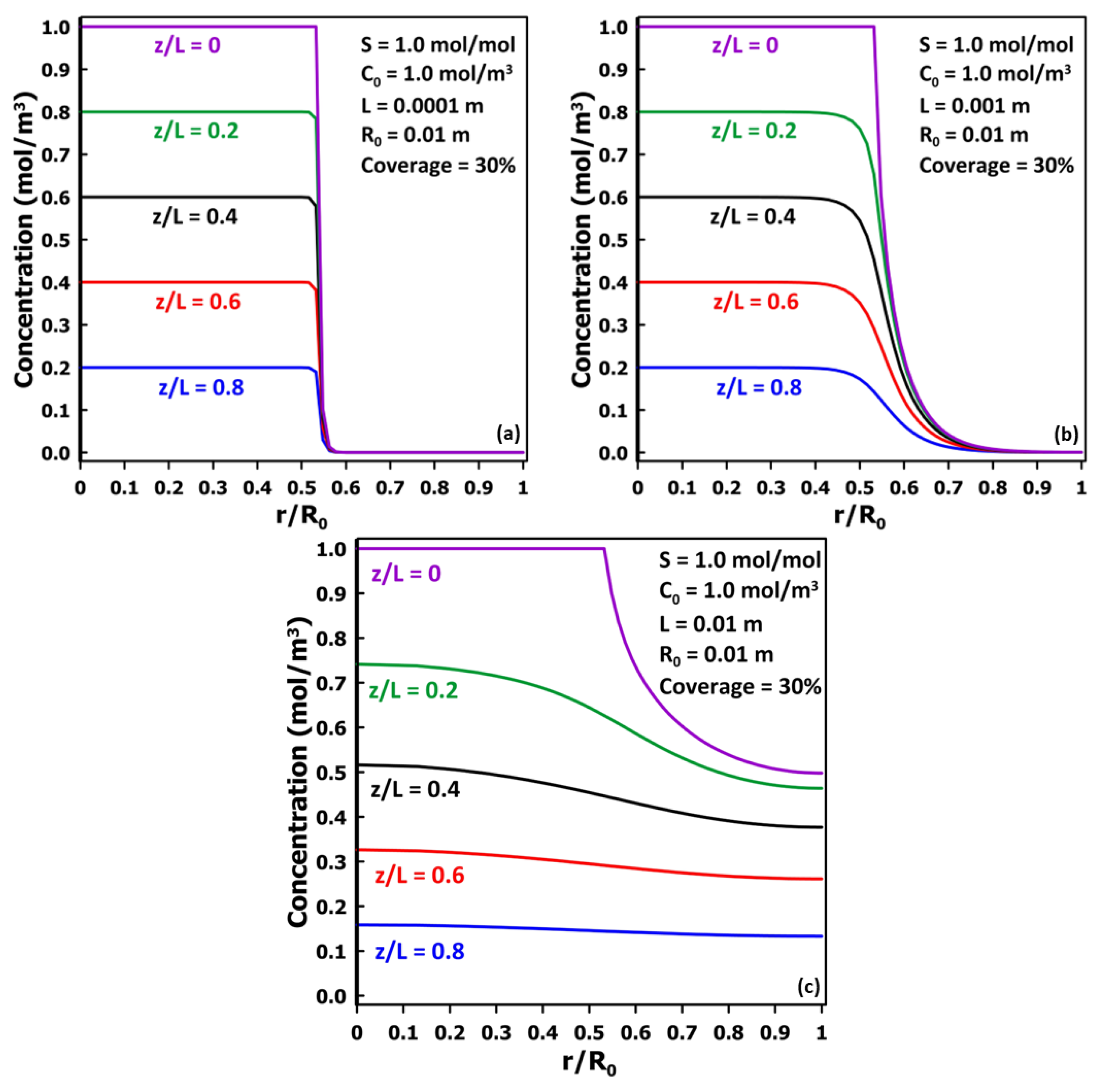
3.3. The Impact of Membrane Thickness
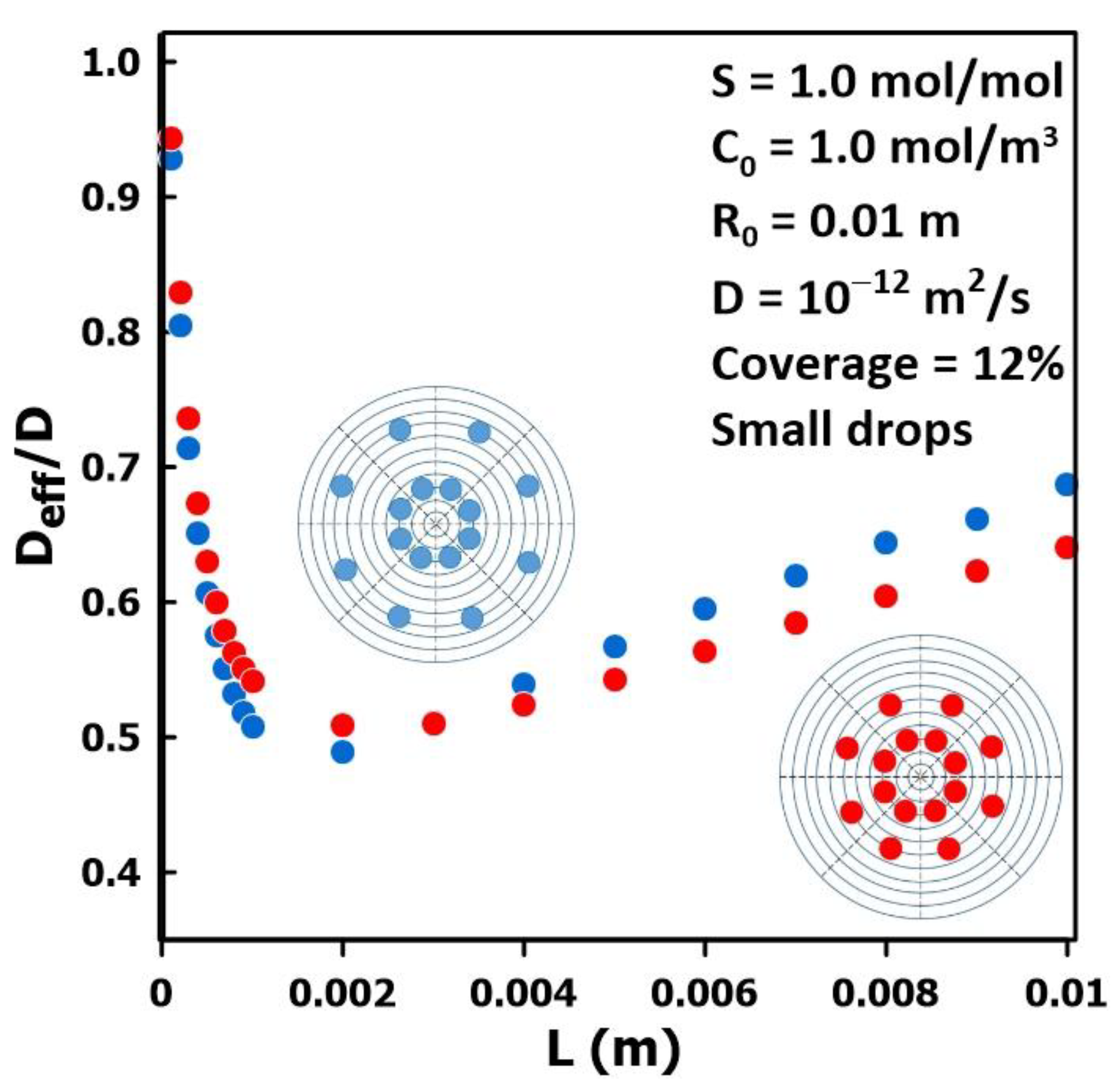
3.4. The Impact of Permeate Geometry
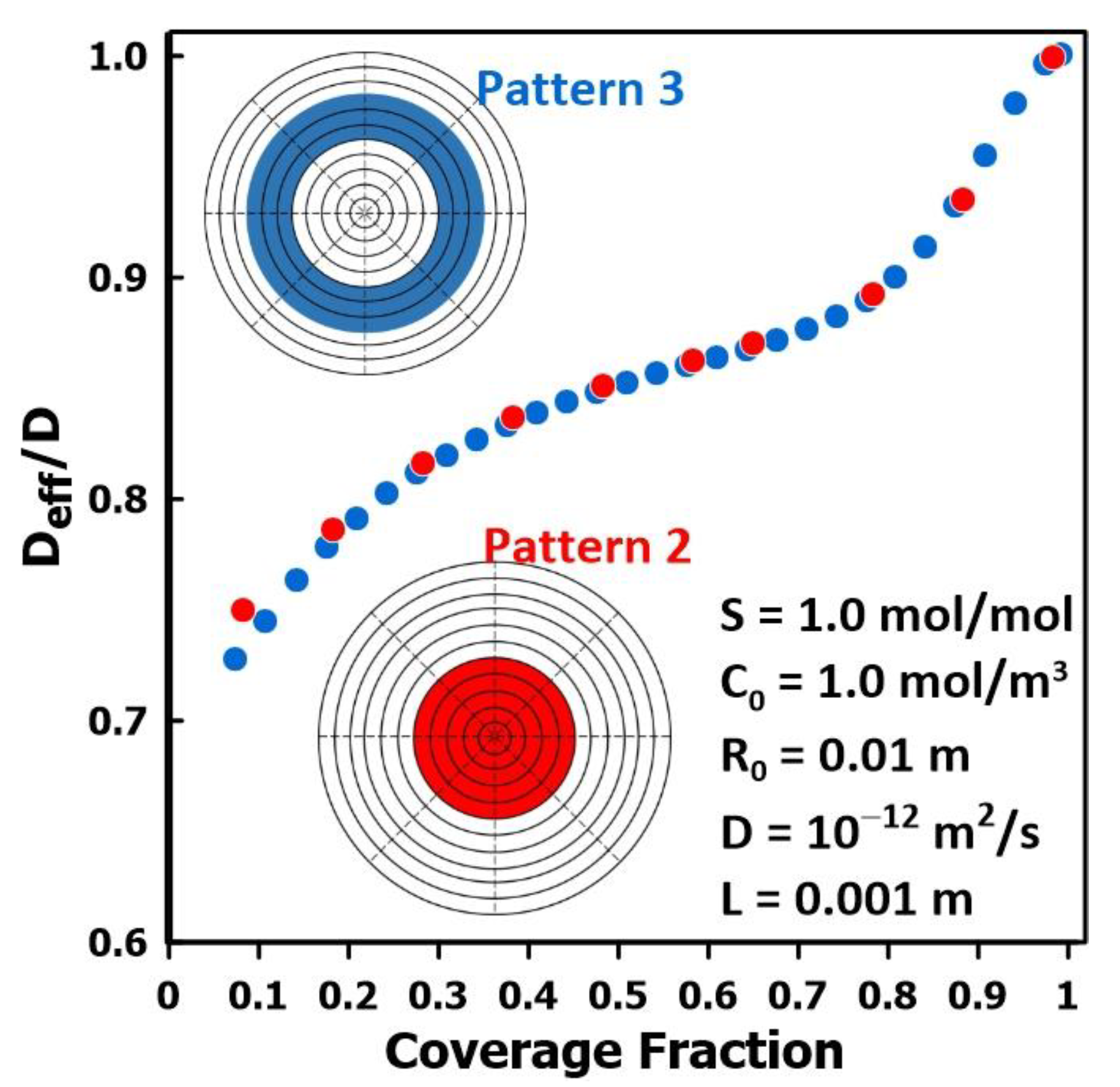
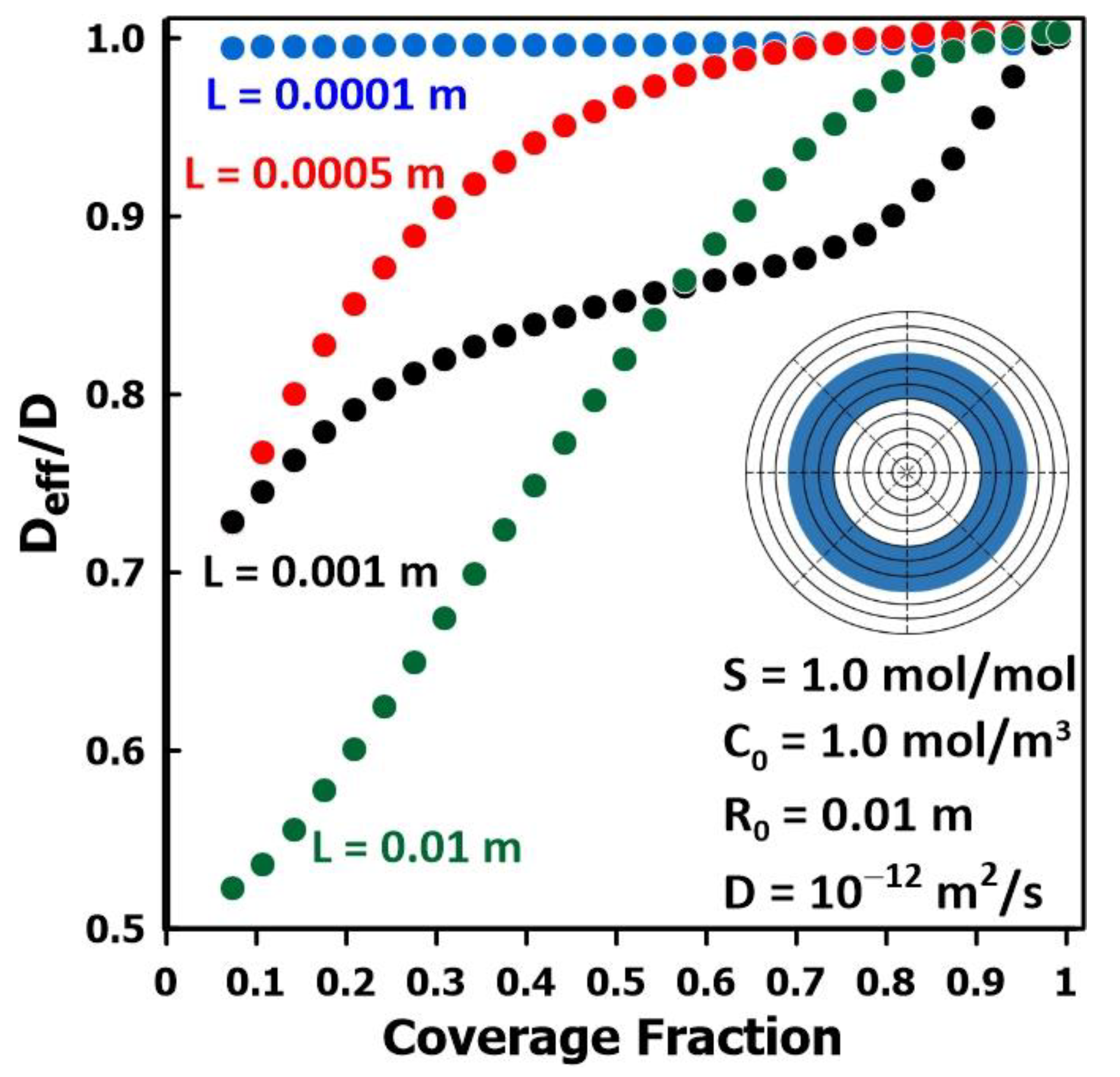
3.5. The Impact of Membrane Surface Area Covered by the Permeate
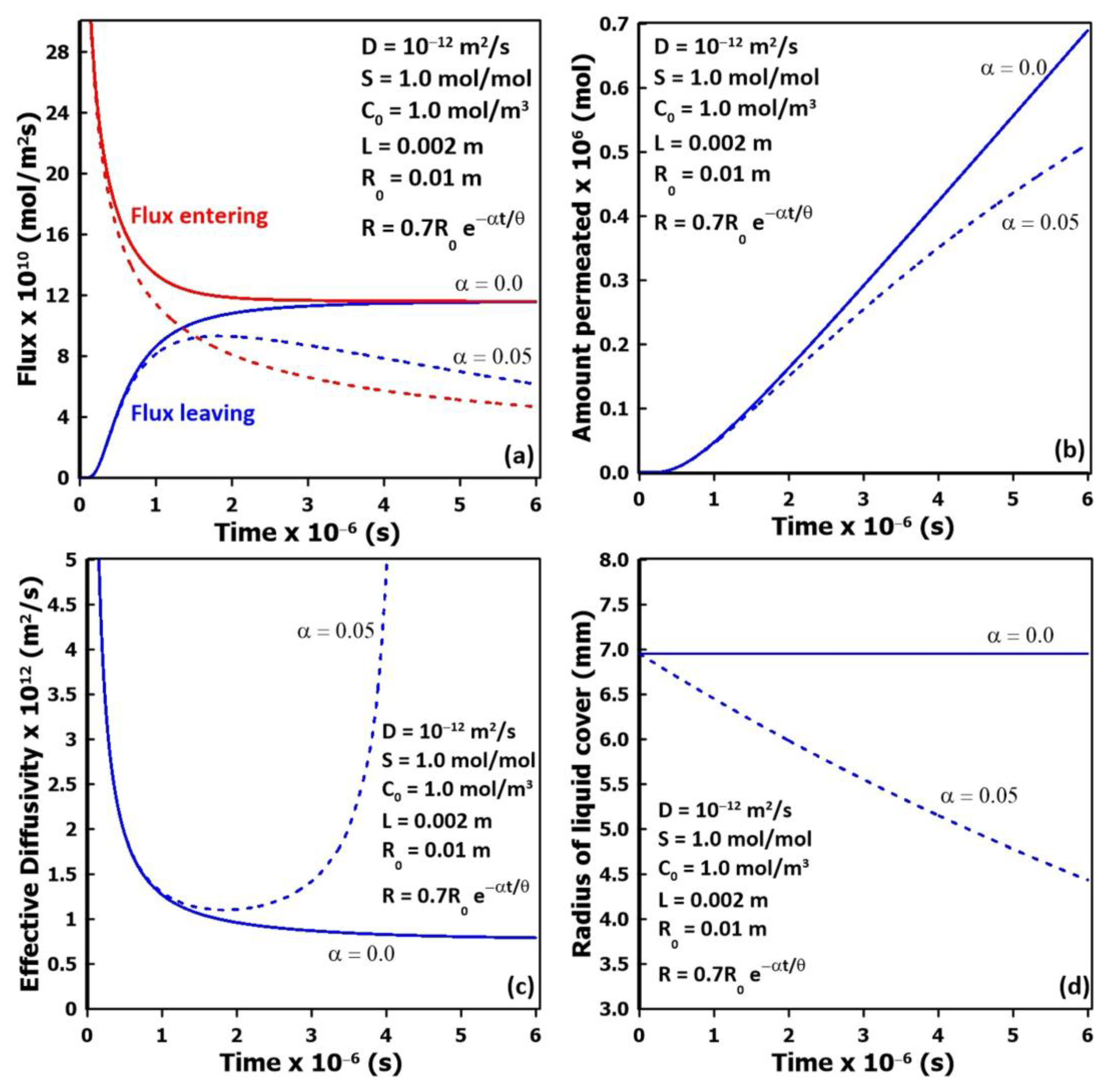
3.6. Receding Liquid Coverage
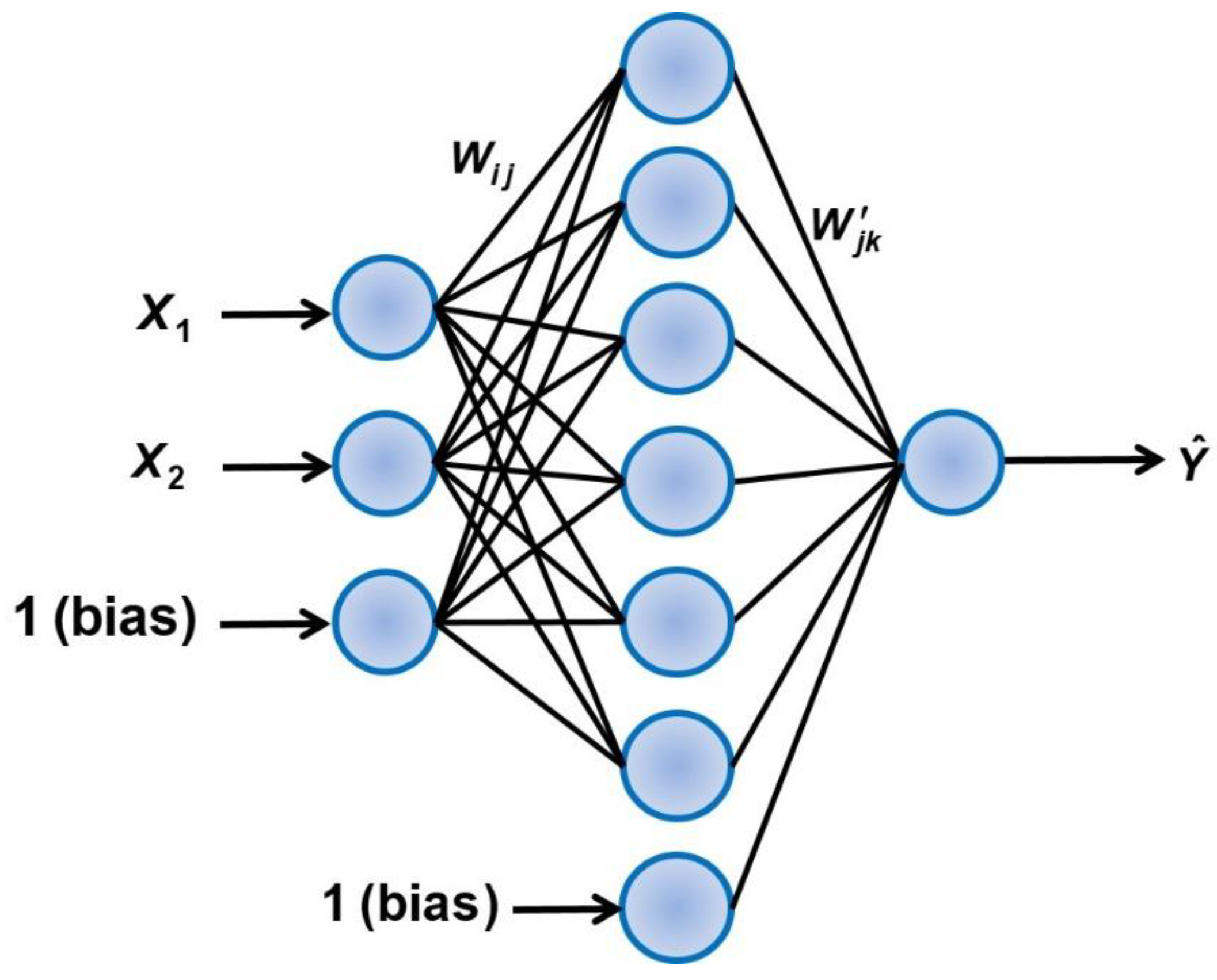
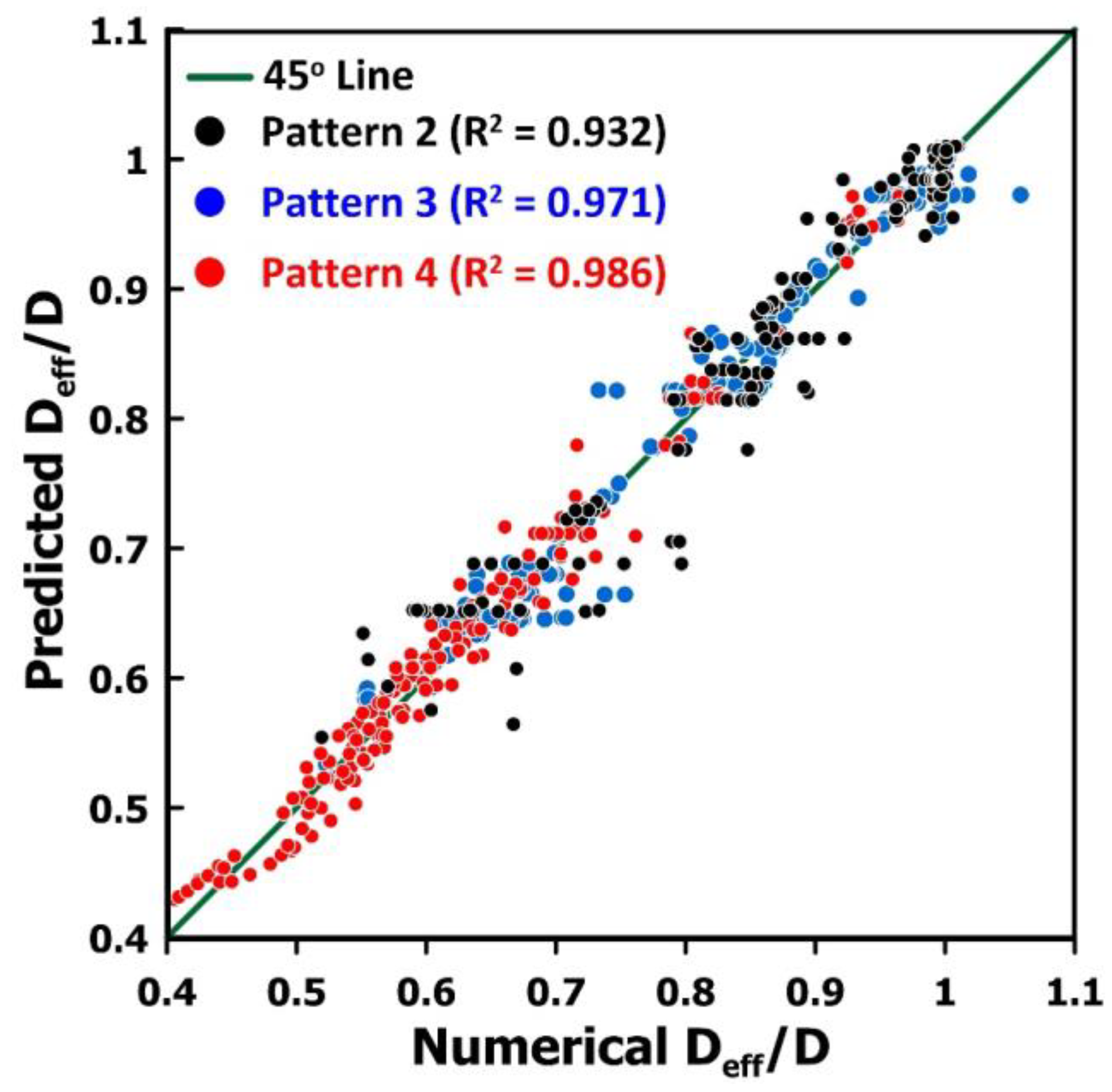
3.7. Predictive Model for the Estimation of the Diffusivity Ratio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Dickson, E.F.G. Personal Protective Equipment for Chemical, Biological, and Radiological Hazards: Design, Evaluation and Selection, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Daugherty, M.L.; Watson, A.P.; Vo-Dinh, T. Currently Available Permeability and Breakthrough Data Characterizing Chemical Warfare Agents and Their Simulants in Civilian Protective Clothing Mater. J. Hazard. Mater. 1992, 30, 243–267. [Google Scholar] [CrossRef]
- Government of Canada, C.C. for O. H. and S. CCOHS: Chemical Protective Clothing-Glove Selection. Available online: https://www.ccohs.ca/oshanswers/prevention/ppe/gloves.html (accessed on 14 June 2023).
- Paul, D.R. The Solution-Diffusion Model for Swollen Membranes. Sep. Purif. Methods 1976, 5, 33–50. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Pinnau, I.; Freeman, B. (Eds.) Materials Science of Membranes for Gas and Vapor Separation, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Banaee, S.; Hee, S.S.Q. Glove Permeation of Chemicals: The State of the Art of Current Practice, Part 1: Basics and the Permeation Standards. J. Occup. Environ. Hyg. 2019, 16, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Test Operations Procedure (TOP) 08-2-501A, Permeation Testing of Materials with Chemical Agents or Simulants (Swatch Testing); Final TOP 08-2-501A; U.S. Army Dugway Proving Ground: Dugway, UT 84022-5000, 2013. Available online: https://apps.dtic.mil/sti/pdfs/ADA586844.pdf (accessed on 19 March 2023).
- Frisch, H.L. The Time Lag in Diffusion. J. Phys. Chem. 1957, 61, 93–95. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Review of Time Lag Permeation Technique as a Method for Characterisation of Porous Media and Membranes. Adsorption 1997, 3, 283–312. [Google Scholar] [CrossRef]
- Rivin, D.; Lindsay, R.S.; Shuely, W.J.; Rodriguez, A. Liquid Permeation Through Nonporous Barrier Materials. J. Membr. Sci. 2005, 246, 39–47. [Google Scholar] [CrossRef]
- Burganos, V.N. Modeling and Simulation of Membrane Structure and Transport Properties. In Fundamentals of Transport Phenomena in Membranes; Elsevier B V: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Mellstrom, G.A. Comparison of Chemical Permeation Data Obtained with ASTM and ISO Permeation Test Cells—I. The ASTM Standard Test Procedure. Ann. Occup. Hyg. 1991, 35, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.; Bergeron, S.; Bonin, H.W. On Finite-Difference Solutions of the Heat Equation in Spherical Coordinates. Numer. Heat Transf. 1987, 12, 457–474. [Google Scholar] [CrossRef]
- Wu, H.; Al-Qasas, N.; Kruczek, B.; Thibault, J. Simulation of Time-lag Permeation Experiments Using Finite Differences. J. Fluid Flow Heat Mass Transf. 2015, 2, 14–25. [Google Scholar] [CrossRef]
- Jones, M.R. Personal Protective Equipment (PPE): Practical and Theoretical Considerations. In Chemical Warfare Agents: Biomedical and Psychological Effects, Medical Countermeasures, and Emergency Response; Lukey, B.J., Romano, J.A., Jr., Salem, H., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Ouvry. CBRN Butyl Gloves OG07®. Ouvry-CBRN Protective System. Available online: https://ouvry.com/en/produit/cbrn-butyl-gloves/ (accessed on 9 May 2023).
- Phalen, R.N.; Wong, W.K. Chemical Resistance of Disposable Nitrile Gloves Exposed to Simulated Movement. J. Occup. Environ. Hyg. 2012, 9, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Kimberly Clark Professional. KleenGuardTM G10 Flex Nitrile Gloves (54333)-M Packaging, 100 Gloves/Box, 10 Boxes/Case, 1000 Gloves/Case. KC Professional. Available online: https://www.kcprofessional.com/en-ca/products/safety-and-personal-protection-equipment/hand-protection-and-gloves/general-purpose-gloves/54331/54333 (accessed on 9 May 2023).
- Lee, J.; Kim, E.-Y.; Chang, B.-J.; Han, M.; Lee, P.-S.; Moon, S.-Y. Mixed-matrix membrane reactors for the destruction of toxic chemicals. J. Membr. Sci. 2020, 605, 118112. [Google Scholar] [CrossRef]
- Motamedhashemi, M.M.Y.; Monji, M.; Egolfopoulos, F.; Tsotsis, T. A hybrid catalytic membrane reactor for destruction of a chemical warfare simulant. J. Membr. Sci. 2015, 473, 1–7. [Google Scholar] [CrossRef]
- Luo, H.B.; Lin, F.-R.; Liu, Z.-Y.; Kong, Y.-R.; Idrees, K.B.; Liu, Y.; Zou, Y.; Farha, O.K.; Ren, X.M. MOF−Polymer Mixed Matrix Membranes as Chemical Protective Layers for Solid-Phase Detoxification of Toxic Organophosphates. ACS Appl. Mater. Interfaces 2023, 15, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Cybenko, G. Approximation by Superpositions of a Sigmoidal Function. Math. Control Signals Syst. 1989, 2, 303–314. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicket, A.; Lau, V.; Thibault, J. Simulating the Permeation of Toxic Chemicals through Barrier Materials. Membranes 2024, 14, 183. https://doi.org/10.3390/membranes14090183
Bicket A, Lau V, Thibault J. Simulating the Permeation of Toxic Chemicals through Barrier Materials. Membranes. 2024; 14(9):183. https://doi.org/10.3390/membranes14090183
Chicago/Turabian StyleBicket, Alex, Vivian Lau, and Jules Thibault. 2024. "Simulating the Permeation of Toxic Chemicals through Barrier Materials" Membranes 14, no. 9: 183. https://doi.org/10.3390/membranes14090183
APA StyleBicket, A., Lau, V., & Thibault, J. (2024). Simulating the Permeation of Toxic Chemicals through Barrier Materials. Membranes, 14(9), 183. https://doi.org/10.3390/membranes14090183






