Utilization of Water-Insoluble Carbon Nitride-Phosphotungstic Acid Hybrids in Composite Proton Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
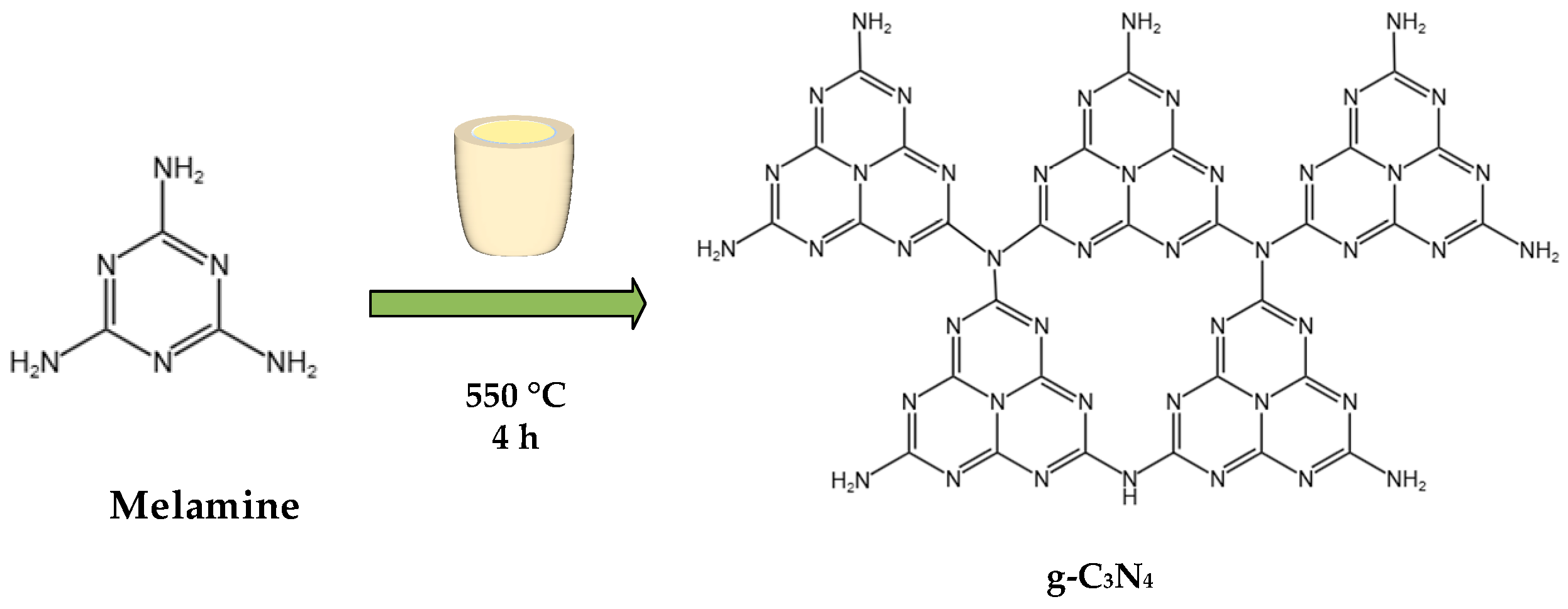
2.2. Preparation of g-C3N4
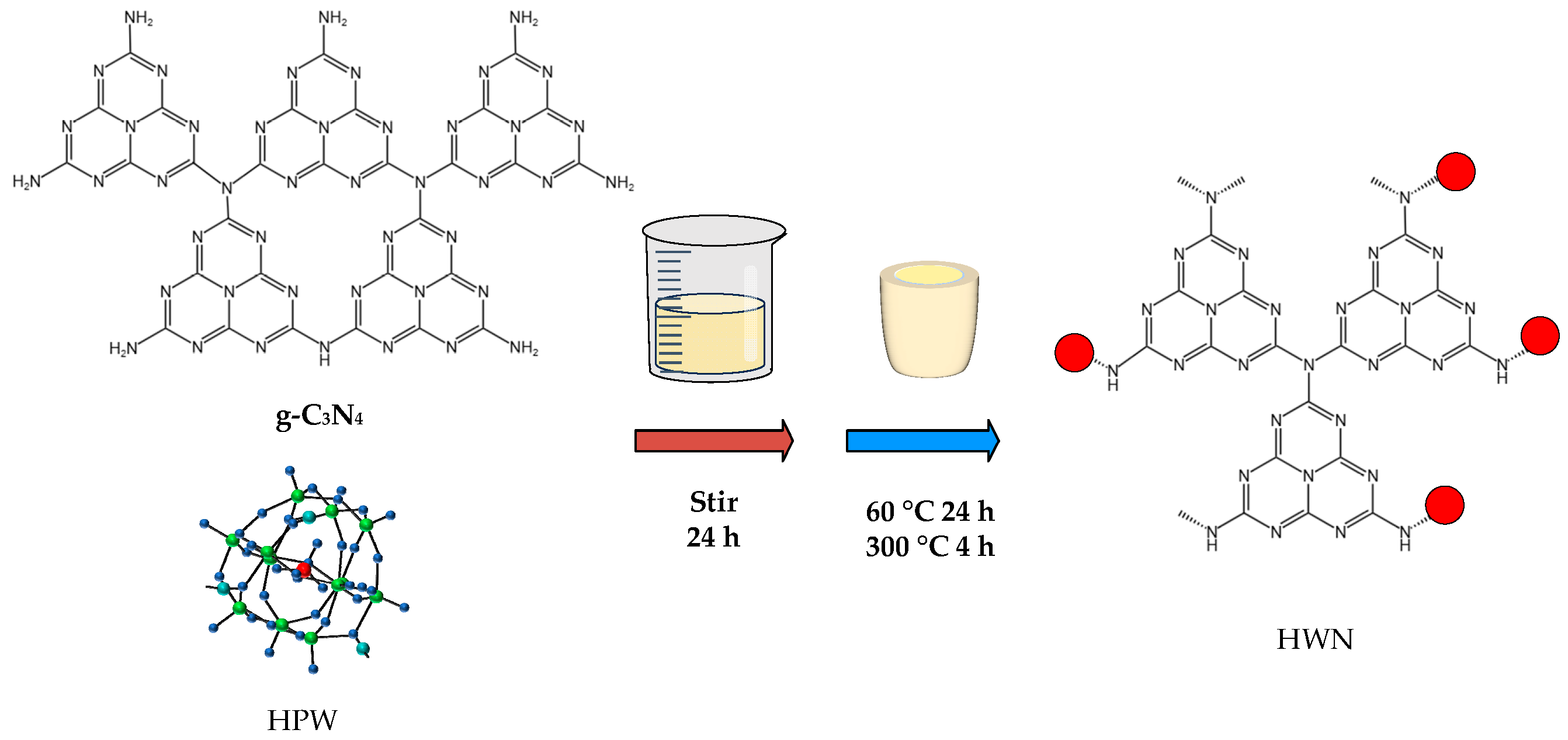
2.3. Preparation of HPW/g-C3N4
2.4. Preparation of SPEEK
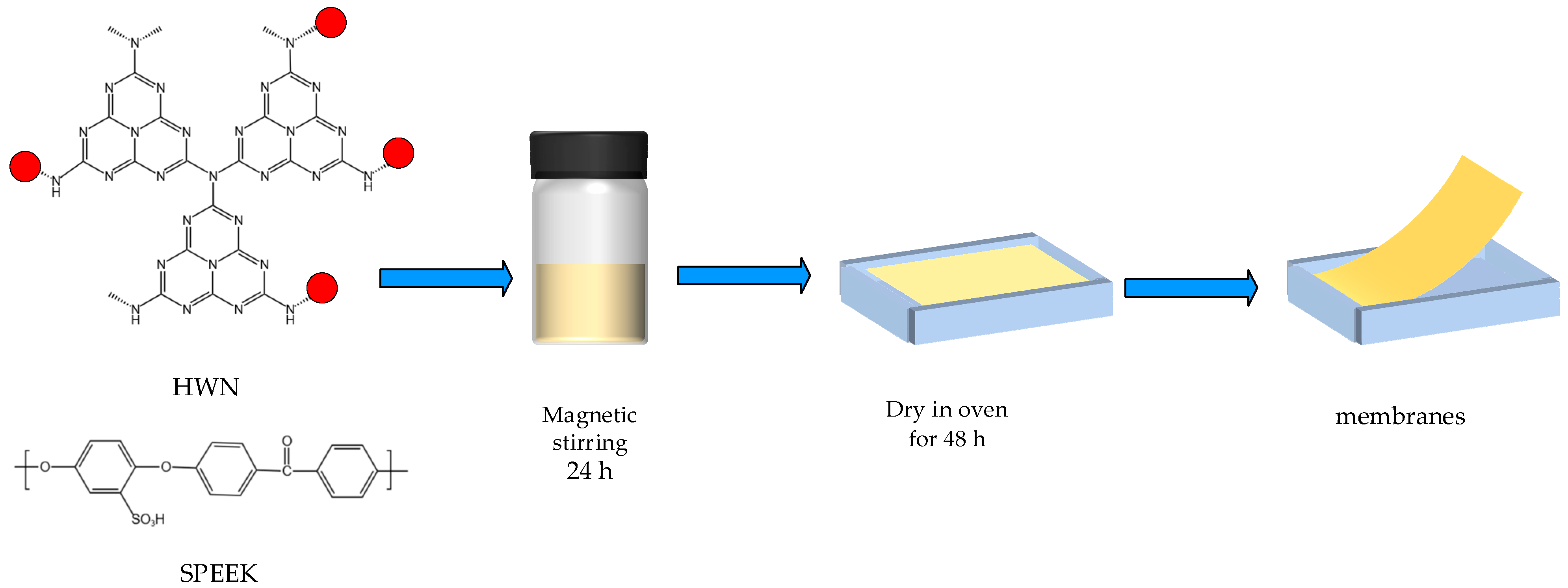
2.5. Preparation of SPEEK/HWN Composite Membranes
2.6. Characterization
2.7. Water Uptake, Swelling Ratio, Ion-Exchange Capacity (IEC) and Proton Conductivity
3. Results and Discussion
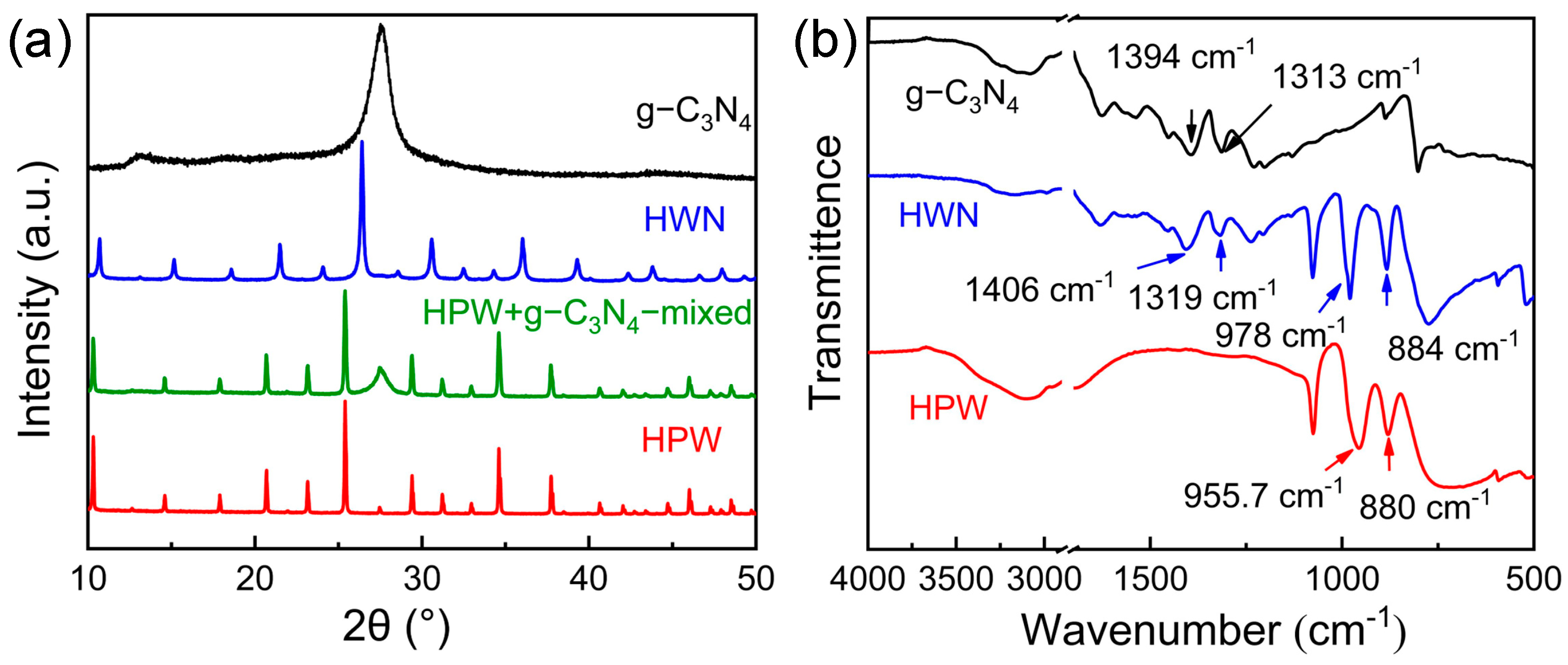
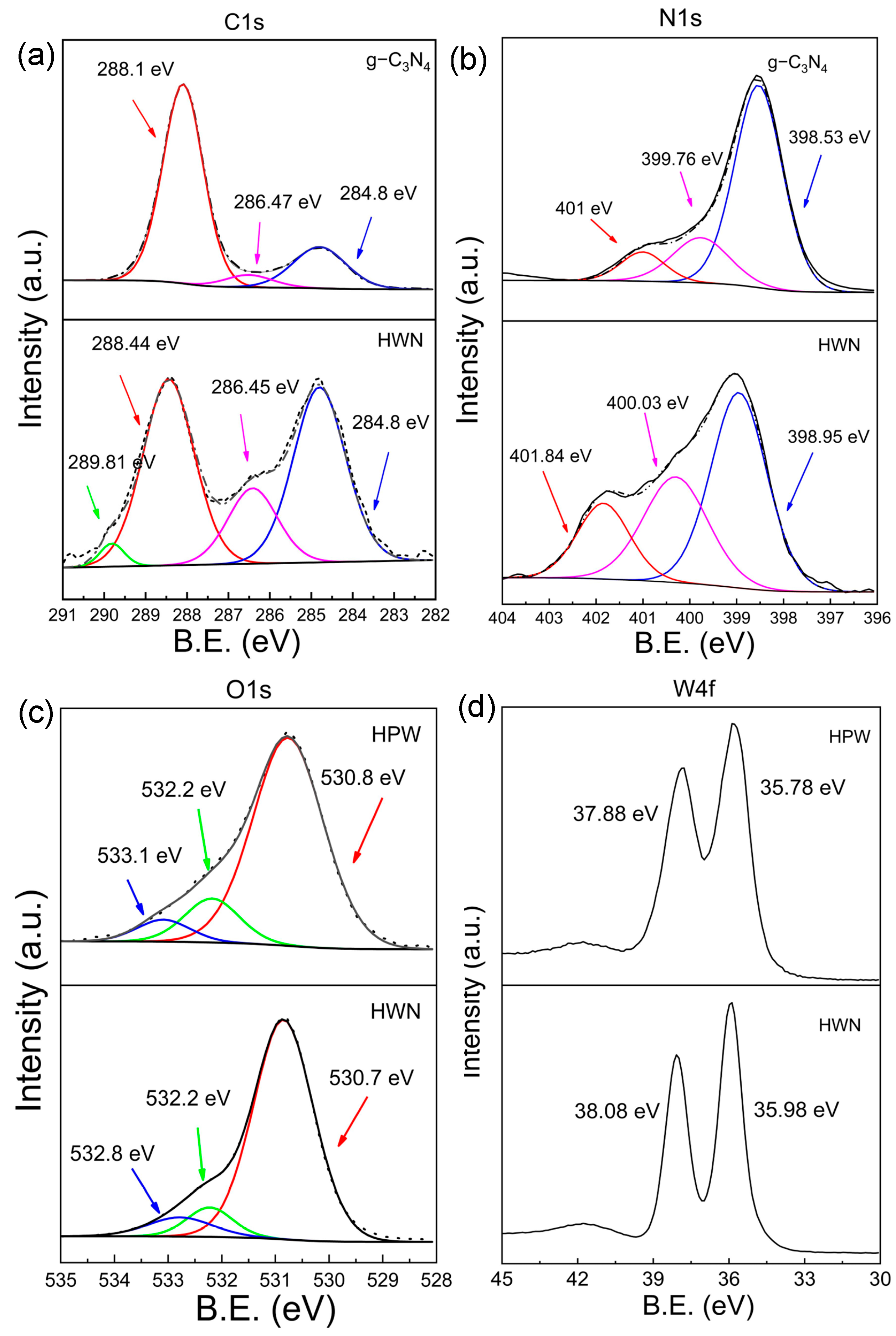
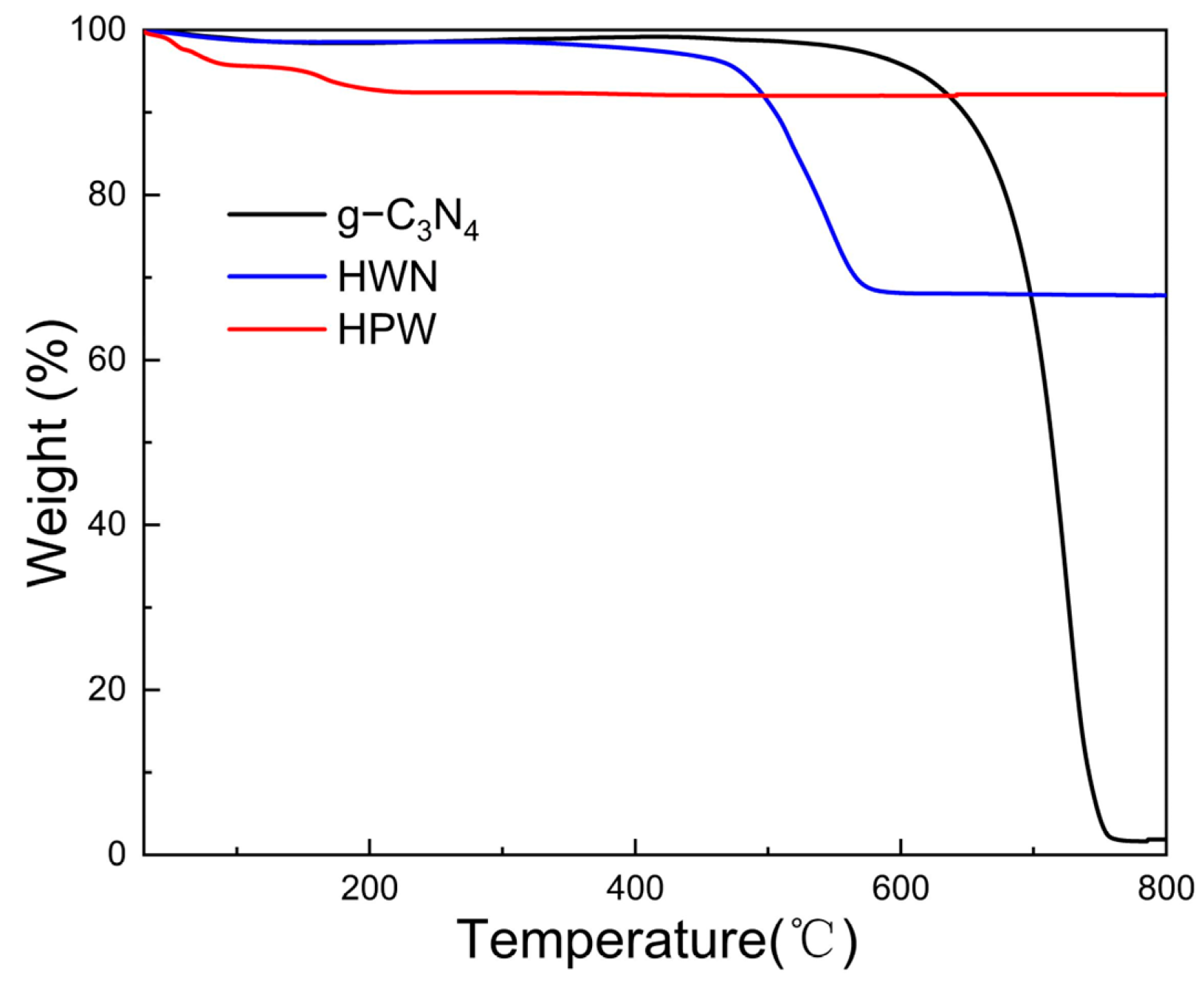
3.1. Characterization of HWN
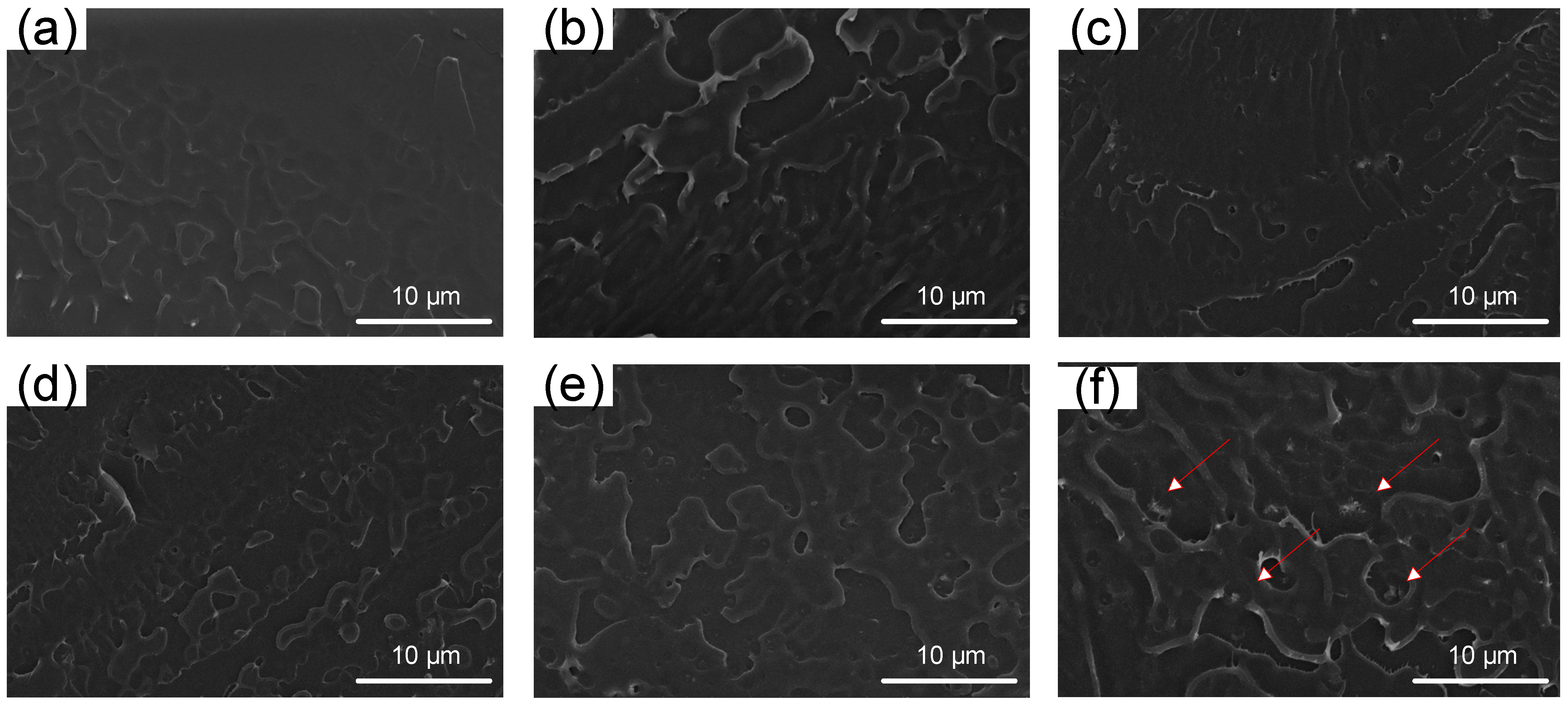
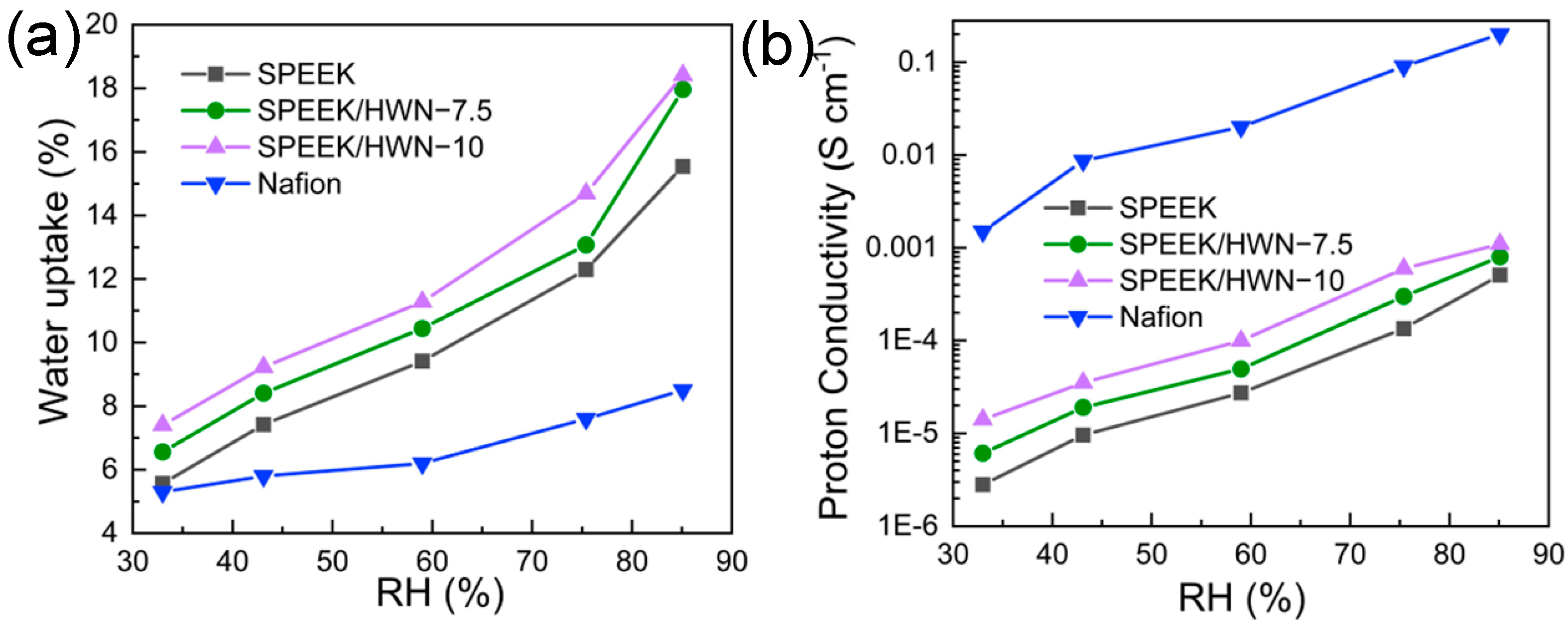
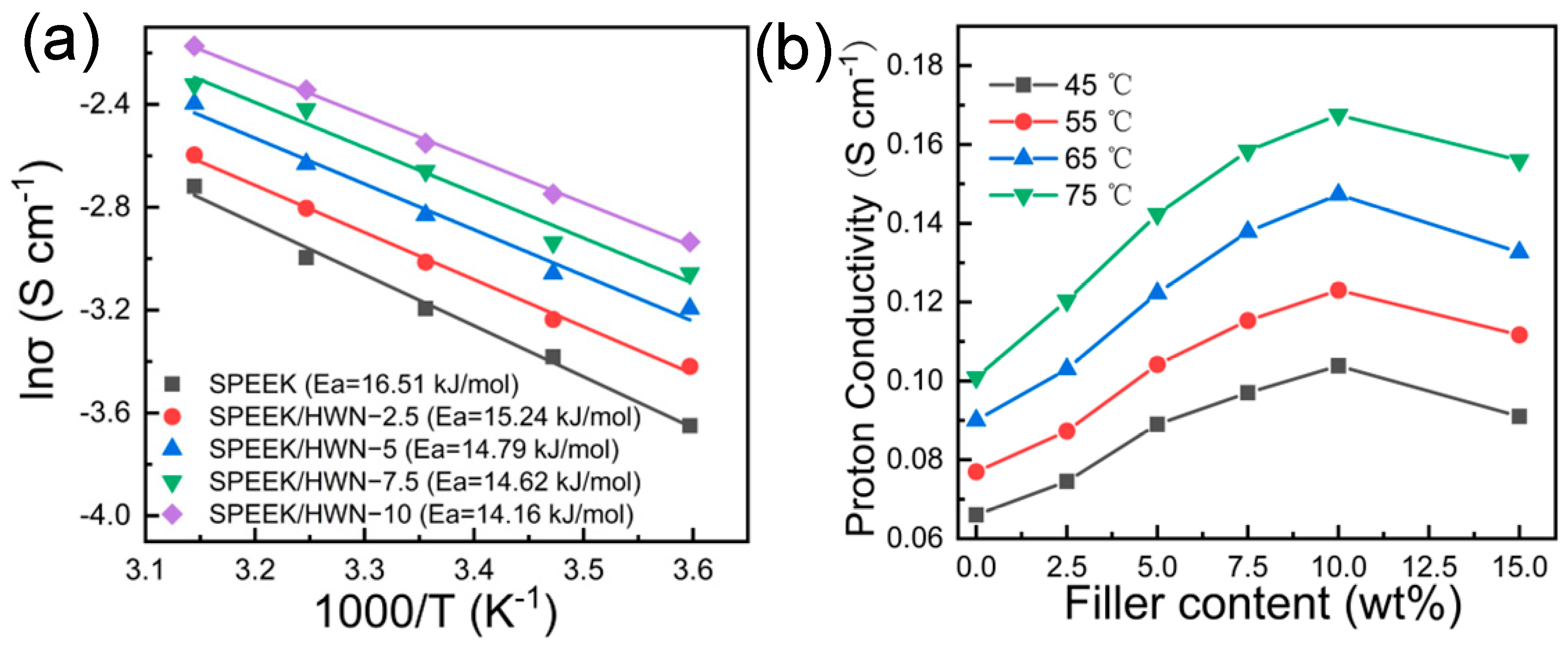
3.2. Characterization of the SPEEK/HWN Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Zhang, J.; Ning, X.; Tian, M.; Long, Y.; Ramakrishna, S. Recent advances in designing and tailoring nanofiber composite electrolyte membranes for high-performance proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 25225–25251. [Google Scholar] [CrossRef]
- Wei, P.; Sui, Y.; Huang, D.; Zhu, B.; Meng, X.; Zhou, Q. NH2-UiO66 functionalized polyimide nanofiber to anchor HPW and fabricate high-performance sandwich-structure membrane for achieving excellent durability. Chem. Eng. J. 2023, 475, 146512. [Google Scholar] [CrossRef]
- Ali, M.M.; Basem, A.; Azam, A.; Rizvi, S.J.A.; Rashid, F.L. Synthesis, characterization and optimization of sulfonated poly-ether-ether-ketone (sPEEK)/functionalized carbon nanotubes (c-CNTs) nanocomposite membranes for fuel cell application. Chin. J. Anal. Chem. 2024, 52, 100365. [Google Scholar] [CrossRef]
- Ji, J.; Li, H.; Wang, W.; Li, J.; Zhang, W.; Li, K.; Yang, T.; Jin, W.; Tang, Y.; Li, W.; et al. Silane-crosslinked polybenzimidazole with different hydroxyl content for high-temperature proton exchange membrane. J. Membr. Sci. 2024, 694, 122423. [Google Scholar] [CrossRef]
- Das, A.; Hazarika, M.; Deka, N.; Jana, T. Mixed Matrix Membranes Fabricated from Nanofillers of a Porous Organic Polymer for Applications as Proton Exchange Membranes. ACS Appl. Nano Mater. 2024, 7, 8081–8092. [Google Scholar] [CrossRef]
- Lu, Z.; Yuan, X.; Jia, X.; Lin, J.; He, S. High-performance proton exchange membrane employing water-insoluble hybrid formed by chemically bonding phosphotungstic acid with polydopamine. Clean Energy Sci. Technol. 2024, 2, 138. [Google Scholar] [CrossRef]
- Bai, E.; Zhu, H.; Sun, C.; Liu, G.; Xie, X.; Xu, C.; Wu, S. A Comparative Study of Nafion 212 and Sulfonated Poly(Ether Ether Ketone) Membranes with Different Degrees of Sulfonation on the Performance of Iron-Chromium Redox Flow Battery. Membranes 2023, 13, 820. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Park, B.-H.; Han, M.-K.; Yoo, D.J. Enhanced performance and durability of composite membranes containing anatase titanium oxide for fuel cells operating under low relative humidity. Int. J. Energy Res. 2022, 46, 4835–4851. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compa, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Dixit, P.; Majhi, J.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in perfluorosulfonic acid-based proton exchange membranes for fuel cell applications: A review. Chem. Eng. J. Adv. 2022, 12, 100372. [Google Scholar] [CrossRef]
- Qian, P.; Zhou, W.; Zhang, Y.; Chao, D.; Song, M. Review and Perspectives of Sulfonated Poly(ether ether ketone) Proton Exchange Membrane for Vanadium Flow Batteries. Energy Fuels 2023, 37, 17681–17707. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Nik Zaiman, N.F.H. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Gao, H.; Dong, C.; Wang, Q.; Zhu, H.; Meng, X.; Cong, C.; Zhou, Q. Improving the proton conductivity of proton exchange membranes via incorporation of HPW-functionalized mesoporous silica nanospheres into SPEEK. Int. J. Hydrogen Energy 2018, 43, 21940–21948. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, T.; Shi, B.; Fan, C.; Liu, Y.; Yin, Z.; Gao, Z.; Zhang, Z.; Wu, H.; Jiang, Z. Porous organic polymer with high-density phosphoric acid groups as filler for hybrid proton exchange membranes. J. Membr. Sci. 2023, 666, 121147. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S.; Illathvalappil, R.; Kurungot, S.; Ramya, K. Studies on nano composites of SPEEK/ethylene glycol/cellulose nanocrystals as promising proton exchange membranes. Electrochim. Acta 2019, 293, 260–272. [Google Scholar] [CrossRef]
- Kumari, M.; Sodaye, H.S.; Bindal, R.C. Cross-linked sulfonated poly(ether ether ketone)-poly ethylene glycol/silica organic–inorganic nanocomposite membrane for fuel cell application. J. Power Sources 2018, 398, 137–148. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Jeon, J.-D.; Kwak, S.-Y. Ion-exchange composite membranes pore-filled with sulfonated poly(ether ether ketone) and Engelhard titanosilicate-10 for improved performance of vanadium redox flow batteries. J. Power Sources 2018, 383, 1–9. [Google Scholar] [CrossRef]
- da Silva, M.J.; Rodrigues, A.A.; Lopes, N.P. Keggin Heteropolyacid Salt Catalysts in Oxidation Reactions: A Review. Inorganics 2023, 11, 162. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Chen, D.; Jin, Q.; Chen, J.; Cao, Y. Preparation of phosphotungstic acid hybrid proton exchange membranes by constructing proton transport channels for direct methanol fuel cells. Polymer 2023, 265, 125589. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Li, Z.; Wu, H.; Yin, Y.; Cao, L.; He, X.; Jiang, Z. Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications. Electrochim. Acta 2017, 240, 186–194. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, G.; Zhang, N.; Li, H.; Zhang, Y.; Shao, K.; Han, M.; Lew, C.M.; Na, H. Surface modification of heteropoly acid/SPEEK membranes by polypyrrole with a sandwich structure for direct methanol fuel cells. J. Mater. Chem. 2010, 20, 9239. [Google Scholar] [CrossRef]
- Meng, X.; Song, K.; Lv, Y.; Cong, C.; Ye, H.; Dong, Y.; Zhou, Q. SPEEK proton exchange membrane with enhanced proton conductivity stability from phosphotungstic acid-encapsulated silica nanorods. Mater. Chem. Phys. 2021, 272, 125045. [Google Scholar] [CrossRef]
- Ryu, S.K.; Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Yoo, D.J. Enhancing Physicochemical Properties and Single Cell Performance of Sulfonated Poly(arylene ether) (SPAE) Membrane by Incorporation of Phosphotungstic Acid and Graphene Oxide: A Potential Electrolyte for Proton Exchange Membrane Fuel Cells. Polymers 2021, 13, 2364. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, Y.; Qiu, M.; Shi, B.; He, X.; Fan, C.; Mao, X.; Wu, H.; Jiang, Z. Enhancing Proton Conductivity of Sulfonated Poly(ether ether ketone)-Based Membranes by Incorporating Phosphotungstic-Acid-Coupled Graphene Oxide. Ind. Eng. Chem. Res. 2021, 60, 4460–4470. [Google Scholar] [CrossRef]
- Cao, Q.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Graphitic carbon nitride and polymers: A mutual combination for advanced properties. J. R. Soc. Chem. 2020, 7, 762. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous graphitic carbon nitride for solar photocatalytic applications. J. R. Soc. Chem. 2020, 5, 765. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308. [Google Scholar] [CrossRef]
- Chernyak, A.V.; Chub, A.V.; Sanginov, E.A.; Barzilovich, P.Y. An NMR study of nanostructured ammonium 12-phosphotungstate. Russ. Chem. Bull. 2013, 62, 1798–1802. [Google Scholar] [CrossRef]
- Zhai, S.; Song, H.; Jia, X.; Yanga, K.; Feng, M.; He, S.; Lin, J. Fabrication of water-insoluble phosphotungstic acid-carbon nitride nanohybrids for promoting proton transport of nanocomposite proton exchange membranes. J. Power Sources 2021, 506, 230195. [Google Scholar] [CrossRef]
- Shmygleva, L.V.; Kayumov, R.R.; Baranov, A.A.; Shilov, G.V.; Leonova, L.S. Influence of calcination temperature of acidic ammonium salts of phosphotungstic acid on their composition and properties. J. Solid State Chem. 2021, 303, 122527. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, X.; Liu, X. g-C3N4/Carbon doped ammonium phosphotungstate heterojunction with pyromellitic diimide as organic electron mediator for efficient acetamiprid photocatalytic degradation. Mater. Sci. Semicond. Process. 2023, 158, 107350. [Google Scholar] [CrossRef]
- Yu, Z.; Yue, X.; Fan, J.; Xiang, Q. Crystalline Intramolecular Ternary Carbon Nitride Homojunction for Photocatalytic Hydrogen Evolution. ACS Catal. 2022, 12, 6345–6358. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Shabanikia, A.; Enhessari, M. Fabrication BaZrO3/PBI-based nanocomposite as a new proton conducting membrane for high temperature proton exchange membrane fuel cells. J. Power Sources 2015, 276, 62–72. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Yu, X.; Clark, J.H. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B 2008, 81, 182–191. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X. Nanosheet thickness control of nitrogen-doped polyimide/ammonium phosphotungstate composite for photocatalytic mineralization of imidacloprid. J. Environ. Chem. Eng. 2024, 12, 111993. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Yan, Z.; He, Y.; Lan, J.; Hou, H. Photoreductive synthesis of nanoscale zero-valent iron rod assisted by phosphotungstic acid over graphite carbon nitride and its enhanced removal of Cr(VI) from water. Appl. Surf. Sci. 2022, 582, 152479. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zhu, S.; Luo, S.; Yan, L.; Dai, Y.; Guo, Y.; Yang, Y. Removal of Cr(VI) from water by a biochar-coupled g-C3N4 nanosheets composite and performance of a recycled photocatalyst in single and combined pollution systems. Appl. Catal. B 2019, 243, 386–396. [Google Scholar] [CrossRef]
- da Costa, N.L.; Pereira, L.G.; Resende, J.V.M.; Mendoza, C.A.D.; Ferreira, K.K.; Detoni, C.; Souza, M.M.; Gomes, F.N. Phosphotungstic acid on activated carbon: A remarkable catalyst for 5-hydroxymethylfurfural production. Mol. Catal. 2021, 500, 111334. [Google Scholar] [CrossRef]
- Li, K.; Hu, J.; Li, W.; Ma, F.; Xu, L.; Guo, Y. Design of mesostructured H3PW12O40-silica materials with controllable ordered and disordered pore geometries and their application for the synthesis of diphenolic acid. J. Mater. Chem. 2009, 19, 8628–8638. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Wu, Z.; Yuan, X.; Luo, H. Preparation of Keggin-type mono-lacunary phosphotungstic-ammonium salt and its catalytic performance in ammoximation of cyclohexanone. Chem. Eng. J. 2015, 280, 670–676. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, X.; Xun, S.; He, M.; Zhu, L.; Wu, L.; Yuan, M.; Zhu, W.; Li, H. Synthesis of carbon nitride supported amphiphilic phosphotungstic acid based ionic liquid for deep oxidative desulfurization of fuels. J. Mol. Liq. 2020, 308, 113059. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Li, C.; Liu, R.; Bi, S.; Zhang, P.; Zhou, Y.; Mai, Y. Ordered Bicontinuous Mesoporous Polymeric Semiconductor Photocatalyst. ACS Nano 2020, 14, 13652–13662. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, L.; Zeng, Z.; Luo, S.; Luo, X.; Liu, X.; Guo, H.; Guo, Y. Fabrication of H3PW12O40-doped carbon nitride nanotubes by one-step hydrothermal treatment strategy and their efficient visible-light photocatalytic activity toward representative aqueous persistent organic pollutants degradation. Appl. Catal. B 2014, 156–157, 141–152. [Google Scholar] [CrossRef]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Inorg. Chim. Acta 2013, 399, 85–94. [Google Scholar] [CrossRef]
- Liu, J.; Xie, S.; Geng, Z.; Huang, K.; Fan, L.; Zhou, W.; Qiu, L.; Gao, D.; Ji, L.; Duan, L.; et al. Carbon Nitride Supramolecular Hybrid Material Enabled High-Efficiency Photocatalytic Water Treatments. Nano Lett. 2016, 16, 6568–6575. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Huang, J.; Yang, J.; Liu, X. Positive effects of phosphotungstic acid on the in-situ solid-state polymerization and visible light photocatalytic activity of polyimide-based photocatalyst. Appl. Catal. B 2018, 226, 487–498. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Zhang, J.; Wu, C.; Zhang, J.; Xiang, Y.; Lu, S. Simultaneously enhancing ionic conduction and mechanical strength of poly (ether sulfones)-poly(vinyl pyrrolidone) membrane by introducing graphitic carbon nitride nanosheets for high temperature proton exchange membrane fuel cell application. J. Membr. Sci. 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, N.; Hu, Z.; Chen, S. Sulfonated graphitic carbon nitride nanosheets as proton conductor for constructing long-range ionic channels proton exchange membrane. J. Membr. Sci. 2020, 601, 117908. [Google Scholar] [CrossRef]
- Ajaikumar, S.; Pandurangan, A. HPW and supported HPW catalyzed condensation of aromatic aldehydes with aniline: Synthesis of DATPM derivatives. J. Mol. Catal. A Chem. 2008, 286, 21–30. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Pei, T.; Zhang, R.; Liu, Y. Anchoring HPW by amino-modified MIL-101(Cr) to improve the properties of SPEEK in proton exchange membranes. J. Appl. Polym. Sci. 2023, 140, e53978. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Zhang, H.; Zhang, T.; Zhang, B.; Cao, S.; Liu, J. Polydopamine-modified graphene oxide nanocomposite membrane for proton exchange membrane fuel cell under anhydrous conditions. J. Mater. Chem. A 2014, 2, 9548–9558. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Ni, C.; Wang, L.; Liu, B.; Liu, J.; Zhang, M.; Men, Y.; Sun, Z.; Xie, H.; et al. Effect of aminated nanocrystal cellulose on proton conductivity and dimensional stability of proton exchange membranes. Appl. Surf. Sci. 2019, 466, 691–702. [Google Scholar] [CrossRef]
- Oh, K.-H.; Lee, D.; Choo, M.-J.; Park, K.H.; Jeon, S.; Hong, S.H.; Park, J.-K.; Choi, J.W. Enhanced Durability of Polymer Electrolyte Membrane Fuel Cells by Functionalized 2D Boron Nitride Nanoflakes. ACS Appl. Mater. Interfaces 2014, 6, 7751–7758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Yu, W.; Shi, J.; Shi, H. Sulfonated poly(ether ether ketone)-based hybrid membranes containing polydopamine-decorated multiwalled carbon nanotubes with acid-base pairs for all vanadium redox flow battery. J. Membr. Sci. 2018, 564, 916–925. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Shao, P.; Burns, C.M.; Feng, X. Sulfonation of poly(ether ether ketone)(PEEK): Kinetic study and characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Rao, Z.; Zhang, A.; Liu, X.; Zhu, D.; Wang, G.; Lan, M.; Wang, Z.; Jiang, L.; Tang, B.; Liu, H. Construction of Surface Sulfonic and Amino Acid-Base Pair Modified Graphene Oxide for Effectively Promoting the Selectivity of the Proton Exchange Membrane. ACS Appl. Polym. Mater. 2023, 5, 7539–7547. [Google Scholar] [CrossRef]
- Wu, J.; Wang, F.; Fan, X.; Chu, J.; Cheng, F.; Hu, F.; Liu, H.; Zhang, Q.; Xu, Z.; Gong, C. Phosphoric acid-doped Gemini quaternary ammonium-grafted SPEEK membranes with superhigh proton conductivity and mechanical strength for direct methanol fuel cells. J. Membr. Sci. 2023, 672, 121431. [Google Scholar] [CrossRef]



| Membrane | IEC (mmol g−1) | Proton Conductivity (S cm−1) | Water Uptake (%) | Swelling Ratio (%) |
|---|---|---|---|---|
| SPEEK | 1.680 | 0.043 | 30.2 | 24.8 |
| SPEEK/HWN-10 | 1.616 | 0.066 | 40.5 | 28.3 |
| Nafion212 | 0.890 | 0.100 | 22.0 | 16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Lu, Z.; Jia, X.; Yang, Z.; Wang, J.; Wang, X.; Lin, J.; He, S. Utilization of Water-Insoluble Carbon Nitride-Phosphotungstic Acid Hybrids in Composite Proton Exchange Membranes. Membranes 2024, 14, 195. https://doi.org/10.3390/membranes14090195
Yuan X, Lu Z, Jia X, Yang Z, Wang J, Wang X, Lin J, He S. Utilization of Water-Insoluble Carbon Nitride-Phosphotungstic Acid Hybrids in Composite Proton Exchange Membranes. Membranes. 2024; 14(9):195. https://doi.org/10.3390/membranes14090195
Chicago/Turabian StyleYuan, Xiancan, Zhongrui Lu, Xiaoyang Jia, Zhuoran Yang, Jian Wang, Xiong Wang, Jun Lin, and Shaojian He. 2024. "Utilization of Water-Insoluble Carbon Nitride-Phosphotungstic Acid Hybrids in Composite Proton Exchange Membranes" Membranes 14, no. 9: 195. https://doi.org/10.3390/membranes14090195







