Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
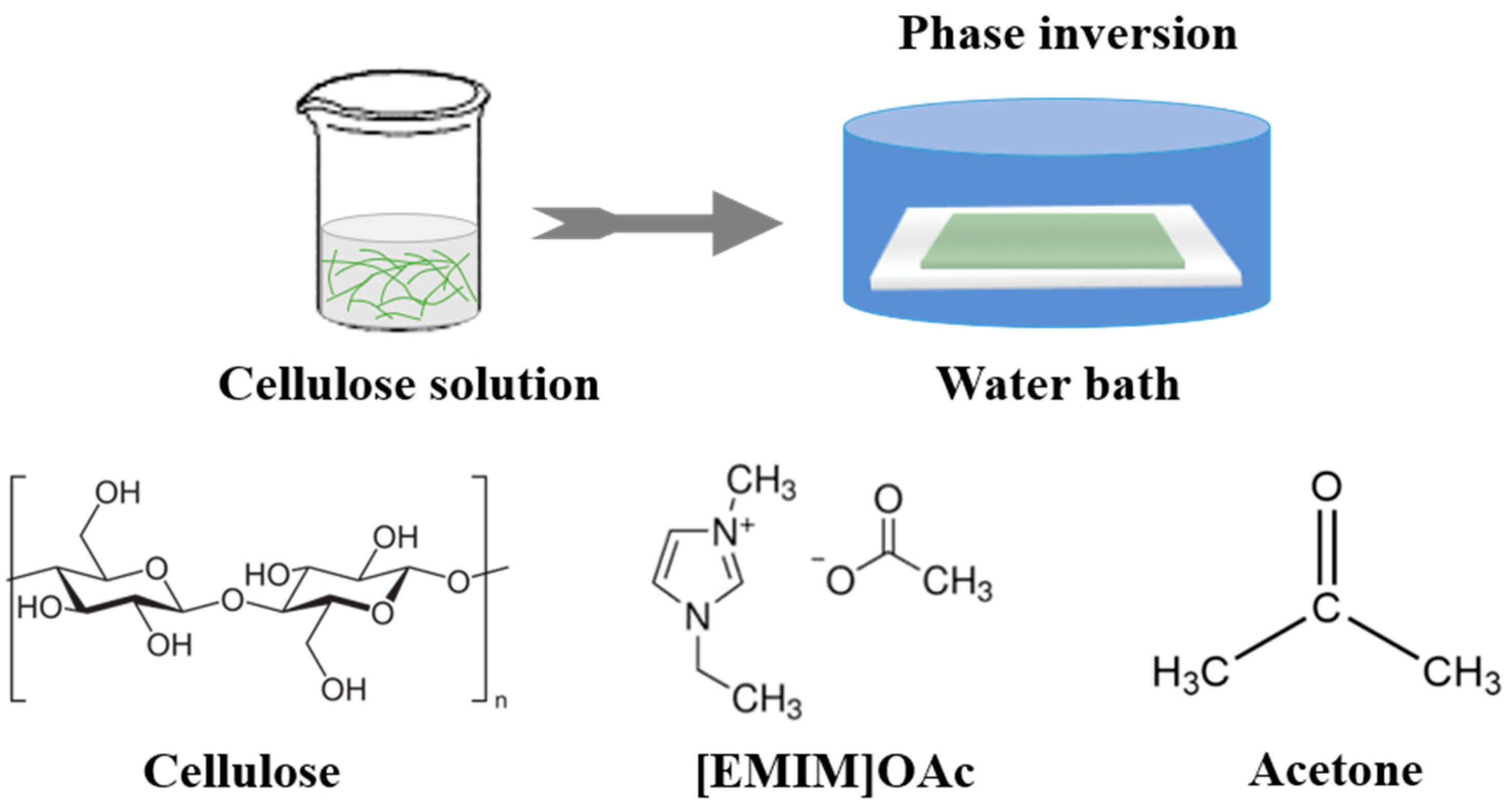
2.2. Preparation of the Casting Solution
2.3. Membrane Fabrication
2.4. Characterization
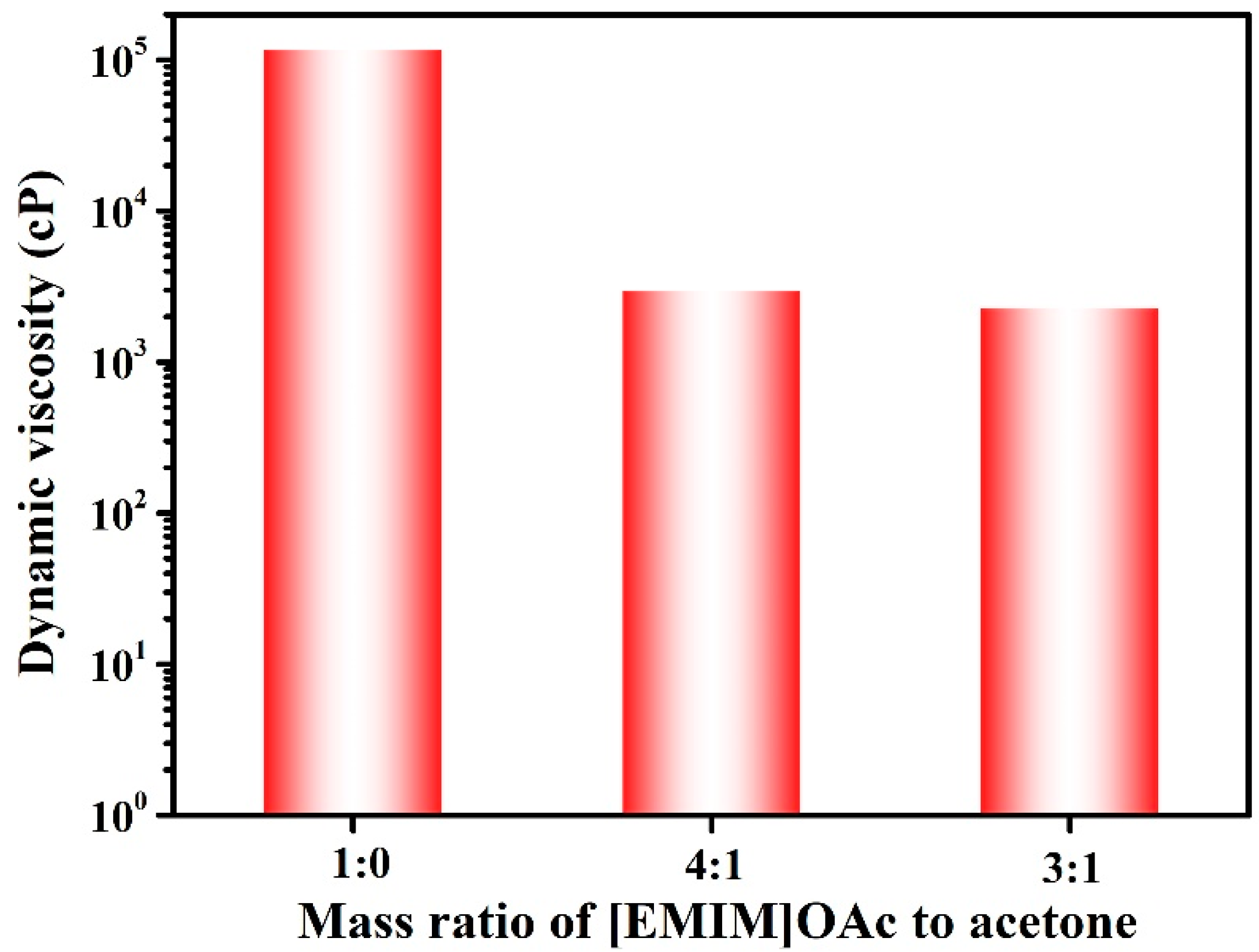
2.4.1. Viscosity Measurements
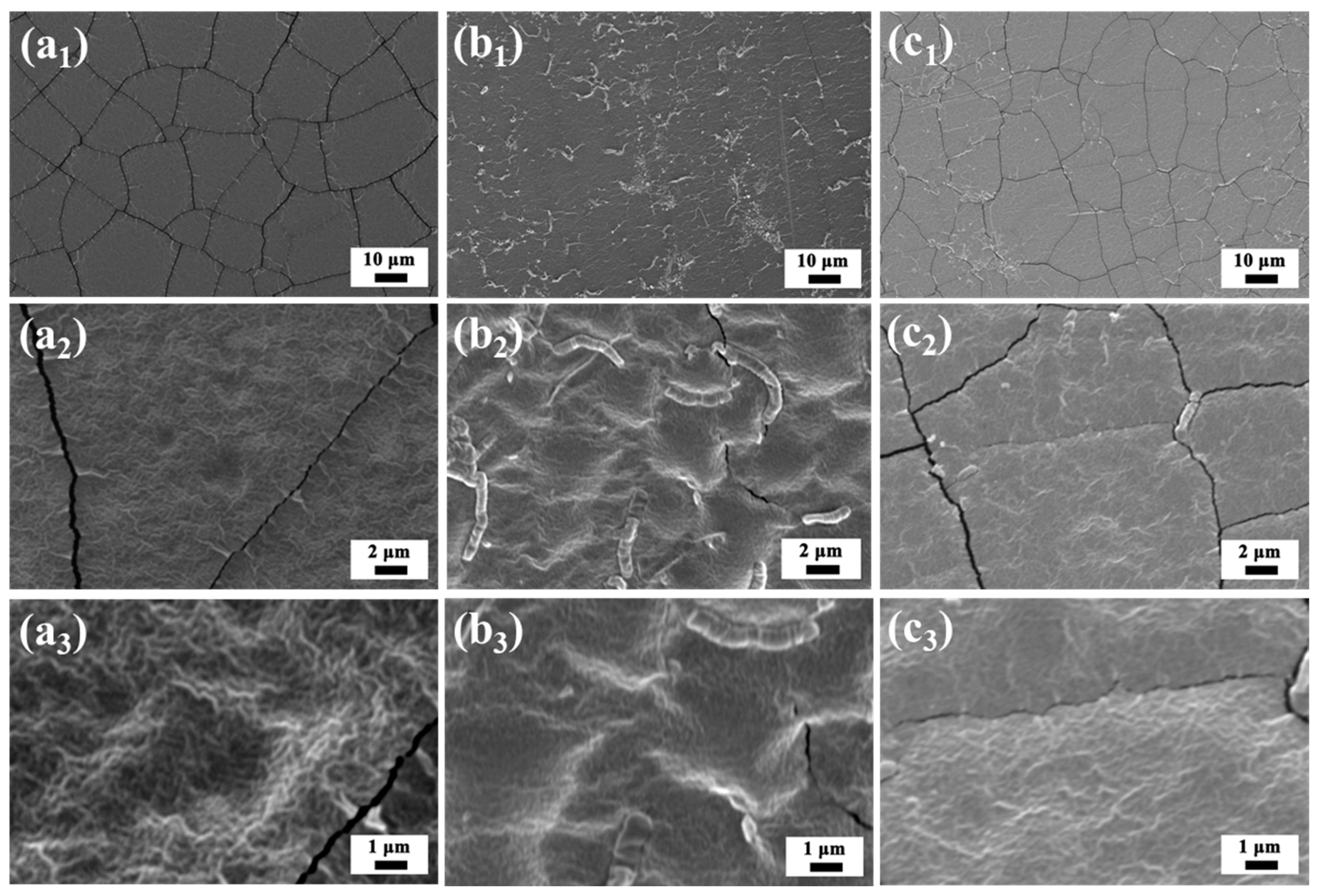
2.4.2. Characterization of Membrane Structure
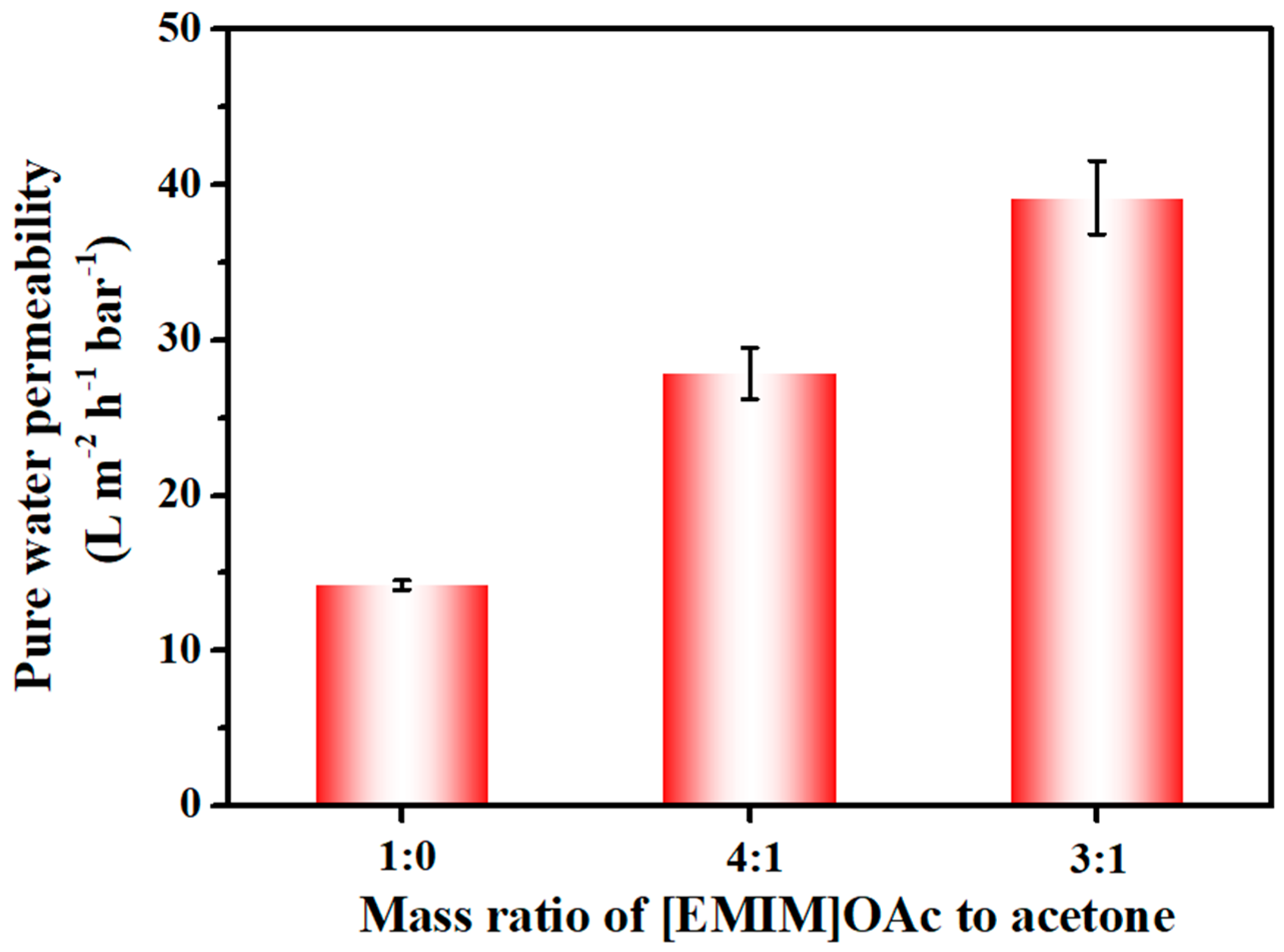
2.4.3. Pure Water Permeability Test and Pore Size Measurements
2.4.4. Oil-in-Water Emulsion Retention Test
2.4.5. Fouling during Oil-in-Water Emulsion Filtration Test
3. Results and Discussion
3.1. Characterization of the Casting Solutions
3.2. Characterization of Fabricated Membranes
3.3. Filtration Performance of the Cellulose Membrane
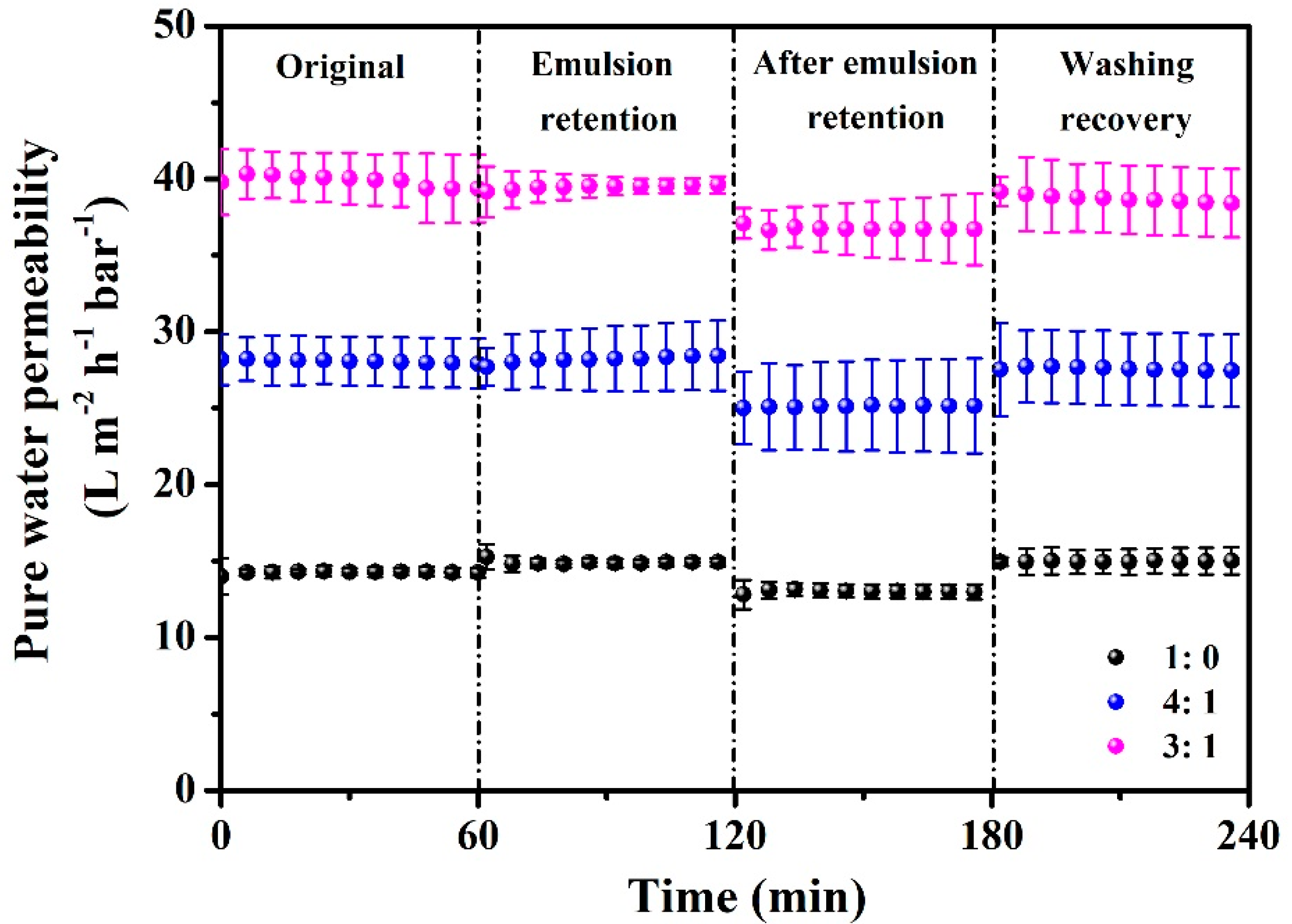
3.4. Fouling Behavior Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, S.; Gao, R. Antibacterial cellulose composite membranes prepared in ionic liquid via phase inversion method. Chem. Res. Chin. Univ. 2017, 33, 678–683. [Google Scholar] [CrossRef]
- Nevstrueva, D.; Pihlajamäki, A.; Mänttäri, M. Effect of a TiO2 additive on the morphology and permeability of cellulose ultrafiltration membranes prepared via immersion precipitation with ionic liquid as a solvent. Cellulose 2015, 22, 3865–3876. [Google Scholar] [CrossRef]
- Bai, L.; Ding, A.; Li, G.; Liang, H. Application of cellulose nanocrystals in water treatment membranes: A review. Chemosphere 2022, 308, 136426. [Google Scholar] [CrossRef]
- Du, H.; Parit, M.; Wu, M.; Che, X.; Wang, Y.; Zhang, M.; Wang, R.; Zhang, X.; Jiang, Z.; Li, B. Sustainable valorization of paper mill sludge into cellulose nanofibrils and cellulose nanopaper. J. Hazard. Mater. 2020, 400, 123106. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Guo, Y.; Hu, J.; Lin, S.; Tu, Y.; Chen, L.; Ni, Y.; Huang, L. Recent advances on cellulose-based nanofiltration membranes and their applications in drinking water purification: A review. J. Clean. Prod. 2022, 333, 130171. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Chen, G.; Hong, F.F.; Yuan, J.; Li, L.; Fang, M.; Wei, W.; Wang, X.; Wei, Y. Super solvent of cellulose with extra high solubility for tunable cellulose structure with versatile application. Carbohydr. Polym. 2022, 296, 119917. [Google Scholar] [CrossRef]
- Mi, S.; Yao, Z.; Liu, F.; Li, Y.; Wang, J.; Na, H.; Zhu, J. Homogeneous cyanoethylation of cellulose with acrylonitrile in a CO2 switchable solvent. Green Chem. 2022, 24, 8677–8684. [Google Scholar] [CrossRef]
- Wei, J.; Gao, H.; Li, Y.; Nie, Y. Research on the degradation behaviors of wood pulp cellulose in ionic liquids. J. Mol. Liq. 2022, 356, 119071. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Ma, X.; Huang, L.; Ni, Y.; Chen, L.; Ouyang, X.; Li, J. Conductive regenerated cellulose film and its electronic devices—A review. Carbohydr. Polym. 2020, 250, 116969. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Wang, W.; Li, Y.; Liu, Y.; Lu, C.; Zhang, Z. Rheological transitions and in-situ IR characterizations of cellulose/LiCl·DMAc solution as a function of temperature. Cellulose 2018, 25, 4955–4968. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, J.; Li, M.; Ao, C.; Wang, Q.; Zhao, J.; Zhang, W.; Lu, C. Facile fabrication of three-dimensional nanofibrous foams of cellulose@g-C3N4@Cu2O with superior visible-light photocatalytic performance. Carbohydr. Polym. 2023, 303, 120455. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Liebert, T.; Heinze, T. Acylation of Cellulose with N,N′-Carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol. Rapid Commun. 2004, 25, 916–920. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2021, 9, 11847–11854. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Xia, S.; Hu, J.; Chen, L.; Huang, L.; Song, Q.; Shen, X.; Zhang, W. Fabrication of bamboo cellulose-based nanofiltration membrane for water purification by cross-linking sodium alginate and carboxymethyl cellulose and its dynamics simulation. Chem. Eng. J. 2023, 473, 145403. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, L.; Guo, G.; Xie, H.; Zhang, L.; Jin, L.; Liang, S.; Hu, L.; Xu, Q.; Zheng, Q. Cellulose membranes from cellulose CO2-based reversible ionic liquid solutions. ACS Sustain. Chem. Eng. 2021, 9, 11847–11854. [Google Scholar] [CrossRef]
- Kim, D.; Le, N.L.; Nunes, S.P. The effects of a co-solvent on fabrication of cellulose acetate membranes from solutions in 1-ethyl-3-methylimidazolium acetate. J. Membr. Sci. 2016, 520, 540–549. [Google Scholar] [CrossRef]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Lee, S.; Jo, I.; Kang, J.W.; Kim, K.-S. Structural characteristics and thermal properties of regenerated cellulose, hemicellulose and lignin after being dissolved in ionic liquids. J. Ind. Eng. Chem. 2022, 107, 365–375. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Bailey, J.; Byrne, E.L.; Goodrich, P.; Kavanagh, P.; Swadźba-Kwaśny, M. Protic ionic liquids for sustainable uses. Green Chem. 2024, 26, 1092–1131. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids in green chemistry. Green Chem. 2011, 13, 225. [Google Scholar] [CrossRef]
- Szabó, L.; Milotskyi, R.; Sharma, G.; Takahashi, K. Cellulose processing in ionic liquids from a materials science perspective: Turning a versatile biopolymer into the cornerstone of our sustainable future. Green Chem. 2023, 25, 5338–5389. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–Water separation using membranes manufactured from cellulose/ionic liquid solutions. ACS Sustain. Chem. Eng. 2018, 7, 5649–5659. [Google Scholar] [CrossRef]
- Kim, D.; Nunes, S.P. Green solvents for membrane manufacture: Recent trends and perspectives. Curr. Opin. Green Sustain. Chem. 2021, 28, 100427. [Google Scholar] [CrossRef]
- Moulthrop, J.S.; Swatloski, R.P.; Moyna, G.; Rogers, R.D. High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem. Commun. 2005, 12, 1557–1559. [Google Scholar] [CrossRef]
- Qasim, U.; Rafiq, S.; Jamil, F.; Ahmed, A.; Ali, T.; Kers, J.; Khurram, M.S.; Hussain, M.; Inayat, A.; Park, Y.-K. Processing of lignocellulose in ionic liquids: A cleaner and sustainable approach. J. Clean. Prod. 2021, 323, 129189. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2007, 15, 59–66. [Google Scholar] [CrossRef]
- Usachev, S.V.; Zlenko, D.V.; Nagornova, I.V.; Koverzanova, E.V.; Mikhaleva, M.G.; Vedenkin, A.S.; Vtyurina, D.N.; Skoblin, A.A.; Nikolsky, S.N.; Politenkova, G.G.; et al. Structure and properties of helical fibers spun from cellulose solutions in [Bmim]Cl. Carbohydr. Polym. 2020, 235, 115866. [Google Scholar] [CrossRef]
- Goshadrou, A.; Lefsrud, M. Synergistic surfactant-assisted [EMIM]OAc pretreatment of lignocellulosic waste for enhanced cellulose accessibility to cellulase. Carbohydr. Polym. 2017, 166, 104–113. [Google Scholar] [CrossRef]
- Villanueva-Solís Luis, A.; Ruíz-Cuilty, K.; Camacho-Dávila, A.; Espinoza-Hicks, J.C.; González-Sánchez, G.; Ballinas-Casarrubias, L. Lignocellulosic waste pretreatment and esterification using green solvents. Sep. Purif. Technol. 2020, 250, 117102. [Google Scholar] [CrossRef]
- Nevstrueva, D.; Pihlajamaki, A.; Nikkola, J.; Manttari, M. Effect of precipitation temperature on the properties of cellulose ultrafiltration membranes prepared via immersion precipitation with ionic liquid as solvent. Membranes 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, L.V.; Bernardo, G.; Dalgliesh, R.; Mendes, A.; Parnell, S.R. Influence of the coagulation bath on the nanostructure of cellulose films regenerated from an ionic liquid solution. RSC Adv. 2024, 14, 12888–12896. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Baig, M.I.; de Vos, W.M.; Lindhoud, S. Preparation of sodium carboxymethyl cellulose–chitosan complex membranes through sustainable aqueous phase separation. ACS Appl. Polym. Mater. 2023, 5, 1810–1818. [Google Scholar] [CrossRef]
- Love, C.S.; Walker, A.; Damion, R.A.; Radhi, A.; Tanner, S.F.; Budtova, T.; Ries, M.E. Influence of cellulose on ion diffusivity in 1-ethyl-3-methyl-imidazolium acetate cellulose solutions. Biomacromolecules 2010, 11, 2927–2935. [Google Scholar]
- Lv, Y.; Wu, J.; Zhang, J.; Niu, Y.; Liu, C.-Y.; He, J.; Zhang, J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012, 53, 2524–2531. [Google Scholar] [CrossRef]


| Cellulose | [EMIM]OAc | Acetone | |
|---|---|---|---|
| 1:0 | 10 wt% | 90 wt% | 0 wt% |
| 4:1 | 10 wt% | 72 wt% | 18 wt% |
| 3:1 | 10 wt% | 67.5 wt% | 22.5 wt% |
| 2:1 | 10 wt% | 60 wt% | 30 wt% |
| Membrane | Initial Pure Water Permeability (L m−2 h−1 bar−1) | Fouling Rate (%) | Flux Recovery (%) |
|---|---|---|---|
| 1:0 | 14.30 | 91.12 | 87.03 |
| 4:1 | 28.09 | 89.75 | 91.44 |
| 3:1 | 40.04 | 91.68 | 95.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Kim, D.; de Vos, W.M. Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent. Membranes 2024, 14, 202. https://doi.org/10.3390/membranes14090202
Chen L, Kim D, de Vos WM. Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent. Membranes. 2024; 14(9):202. https://doi.org/10.3390/membranes14090202
Chicago/Turabian StyleChen, Luying, Dooli Kim, and Wiebe M. de Vos. 2024. "Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent" Membranes 14, no. 9: 202. https://doi.org/10.3390/membranes14090202
APA StyleChen, L., Kim, D., & de Vos, W. M. (2024). Enhancing the Separation Performance of Cellulose Membranes Fabricated from 1-Ethyl-3-methylimidazolium Acetate by Introducing Acetone as a Co-Solvent. Membranes, 14(9), 202. https://doi.org/10.3390/membranes14090202







