ZIF-8-Embedded Cation-Exchange Membranes with Improved Monovalent Ion Selectivity for Capacitive Deionization
Abstract
:1. Introduction
2. Experimental
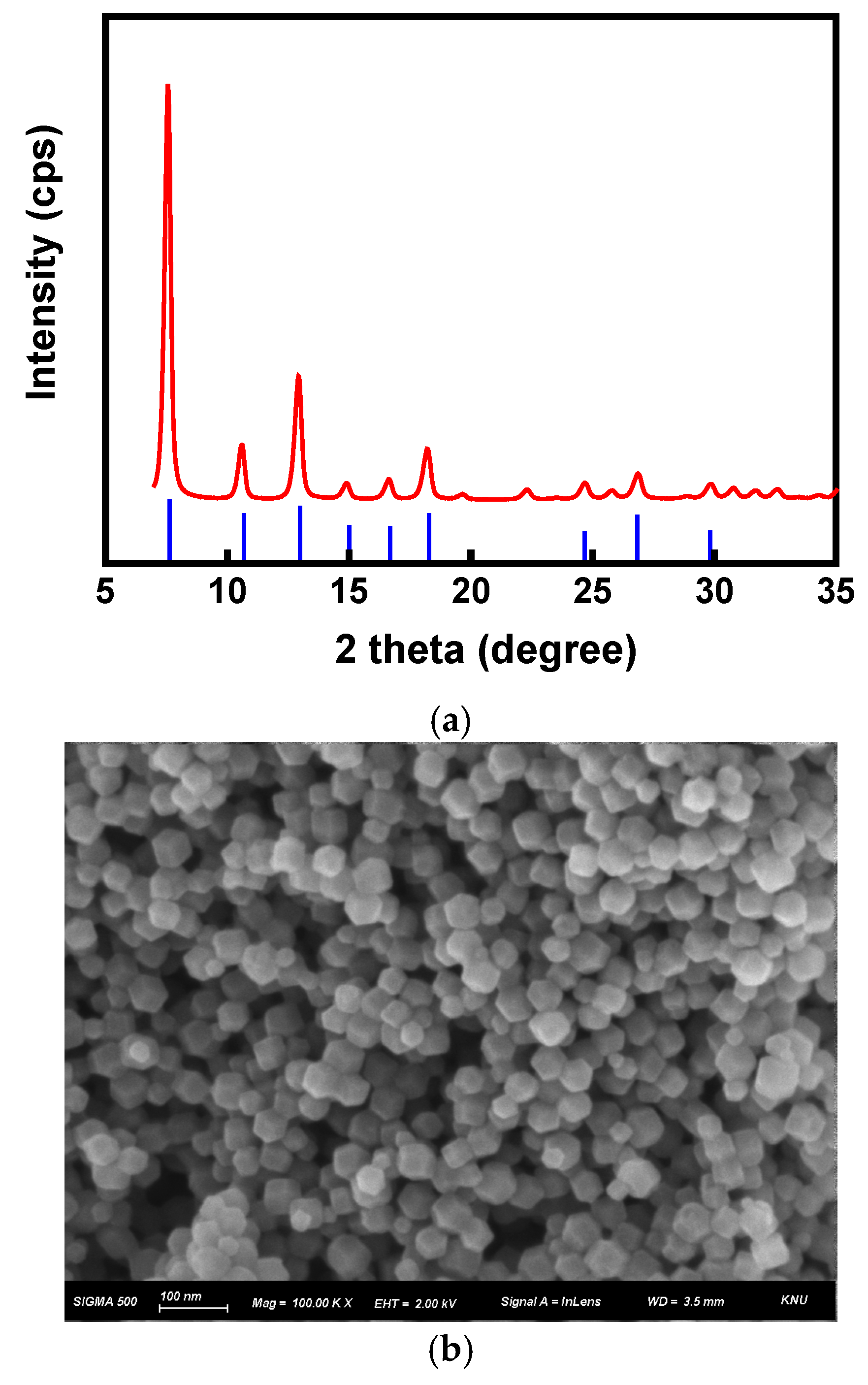
2.1. ZIF-8 Synthesis and Characterizations
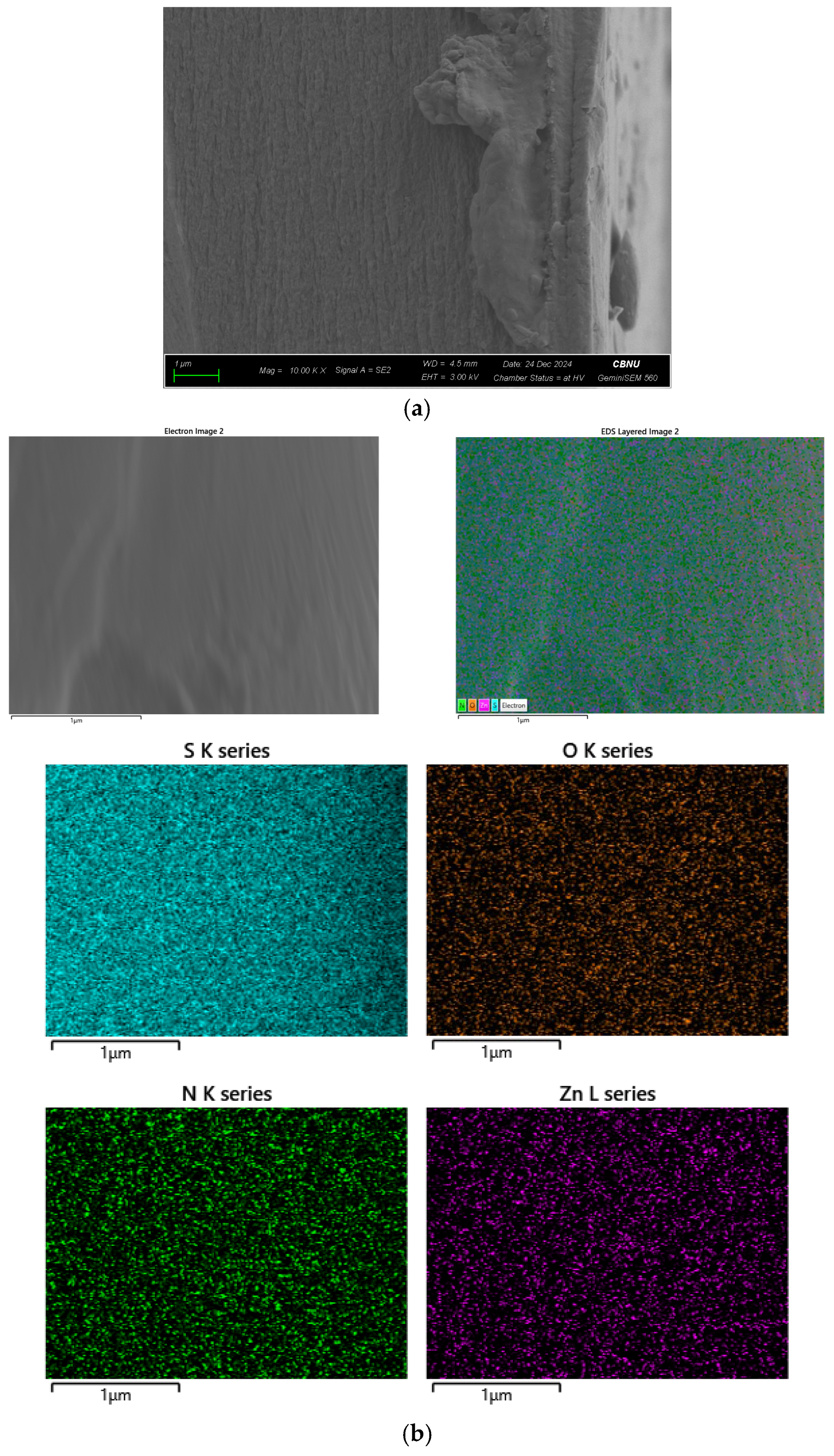
2.2. Membrane Fabrication and Characterizations
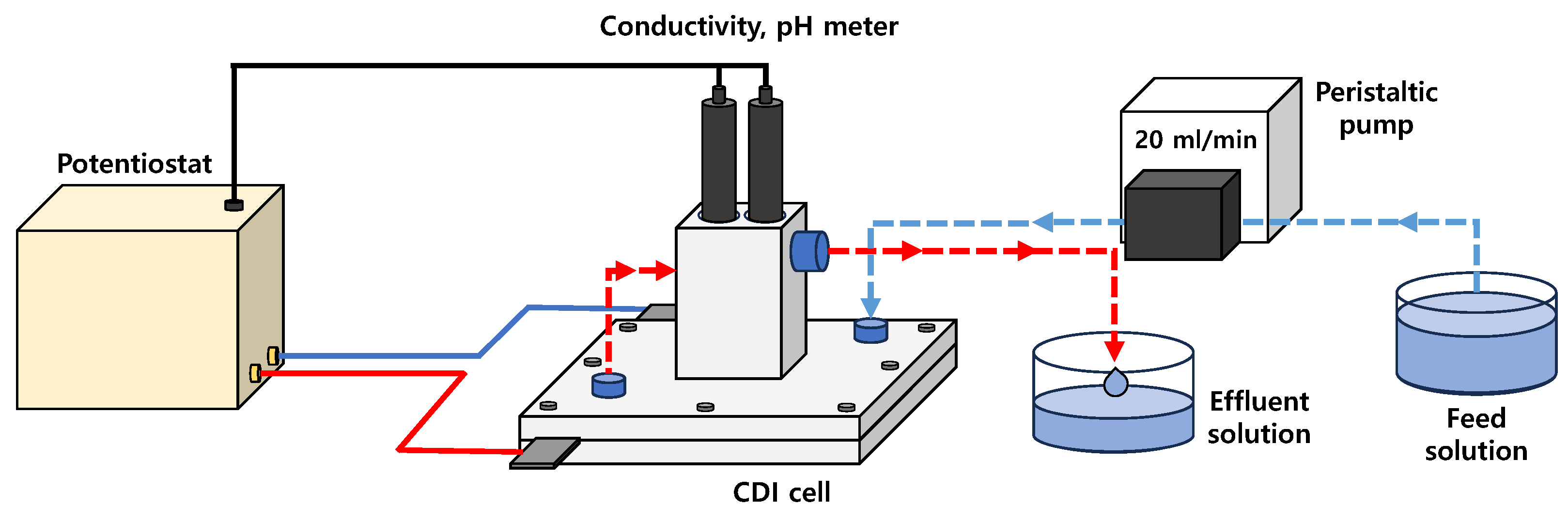
2.3. MCDI Cell Tests for Desalination
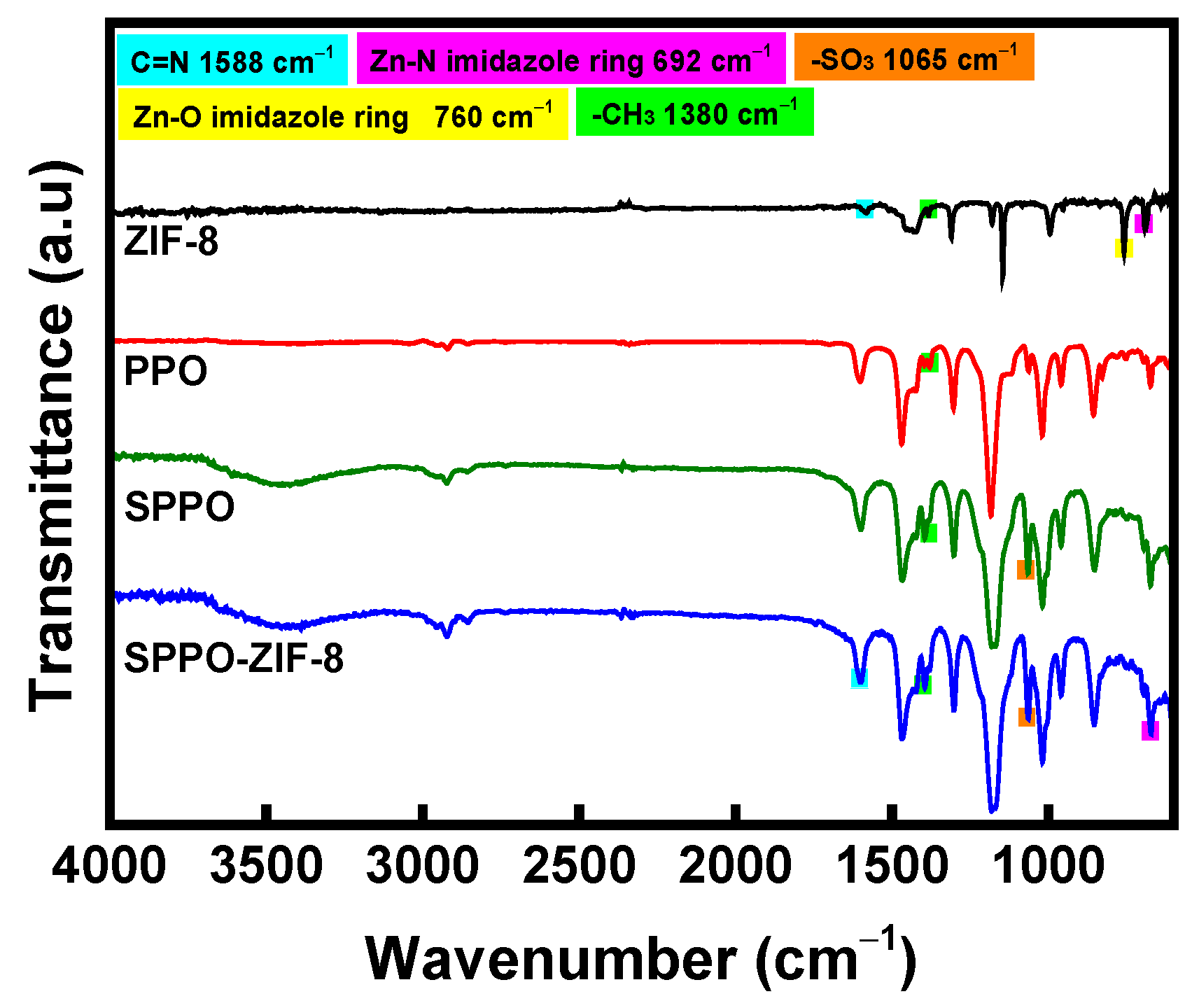
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar]
- Angeles, A.T.; Lee, J. Carbon-based capacitive deionization electrodes: Development techniques and its influence on electrode properties. Chem. Rec. 2021, 21, 820–840. [Google Scholar] [PubMed]
- Elisadiki, J.; Kibona, T.E.; Machunda, R.L.; Saleem, M.W.; Kim, W.; Jande, Y.A. Biomass-based carbon electrode materials for capacitive deionization: A review. Biomass Convers. Biorefin. 2020, 10, 1327–1356. [Google Scholar]
- Gaikwad, M.S.; Balomajumder, C. Capacitive deionization for desalination using nanostructured electrodes. Anal. Lett. 2016, 49, 1641–1655. [Google Scholar]
- Sivasubramanian, P.; Kumar, M.; Kirankumar, V.S.; Samuel, M.S.; Dong, C.; Chang, J. Capacitive deionization and electrosorption techniques with different electrodes for wastewater treatment applications. Desalination 2023, 559, 116652. [Google Scholar]
- Volfkovich, Y.M. Capacitive deionization of water (a review). Russ. J. Electrochem. 2020, 56, 18–51. [Google Scholar] [CrossRef]
- Pan, S.; Haddad, A.Z.; Kumar, A.; Wang, S. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar]
- Sufiani, O.; Elisadiki, J.; Machunda, R.L.; Jande, Y.A. Modification strategies to enhance electrosorption performance of activated carbon electrodes for capacitive deionization applications. J. Electroanal. Chem. 2019, 848, 113328. [Google Scholar]
- Zhang, X.; Zuo, K.; Zhang, X.; Zhang, C.; Liang, P. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review. Environ. Sci. Water Res. Technol. 2020, 6, 243–257. [Google Scholar]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar]
- Chen, R.; Sheehan, T.; Ng, J.L.; Brucks, M.; Su, X. Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci. Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- Bao, S.; Xin, C.; Zhang, Y.; Chen, B.; Ding, W.; Luo, Y. Application of capacitive deionization in water treatment and energy recovery: A review. Energies 2023, 16, 1136. [Google Scholar] [CrossRef]
- Gamaethiralalage, J.G.; Singh, K.; Sahin, S.; Yoon, J.; Elimelech, M.; Suss, M.E.; Liang, P.; Biesheuvel, P.M.; Zornitta, R.L.; Smet, L.C.P.M. Recent advances in ion selectivity with capacitive deionization. Energy Environ. Sci. 2021, 14, 1095–1120. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, R.; Wu, Y.; Xu, S.; Wang, J. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Hou, C.; Huang, C. A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 2013, 314, 124–129. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, Y.-E.; Park, J.-S.; Kang, M.-S. Capacitive deionization employing pore-filled cation-exchange membranes for energy-efficient removal of multivalent cations. Electrochim. Acta 2019, 295, 164–172. [Google Scholar] [CrossRef]
- Pang, X.; Tao, Y.; Xu, Y.; Pan, J.; Shen, J.; Gao, C. Enhanced monovalent selectivity of cation exchange membranes via adjustable charge density on functional layers. J. Membr. Sci. 2020, 595, 117544. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, B.; Tong, T.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Zhang, Q.; Wang, S.; Zhang, L.; Wu, Z.; Lu, G. Synthesis of ZIF-8 hollow spheres via MOF-to-MOF conversion. ChemistrySelect 2016, 1, 1763–1767. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Liu, Y.; Sarkar, A.K.; Bediako, J.K.; Kim, H.Y.; Yun, Y.-S. Super-stable, highly efficient, and recyclable fibrous metal–organic framework membranes for precious metal recovery from strong acidic solutions. Small 2019, 15, 1805242. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Zhang, X.; Du, S.; Zhang, J.; Su, B.; Gao, X.; Mandai, B. Amine-functionalized ZIF-8 nanoparticles as interlayer for the improvement of the separation performance of organic solvent nanofiltration (OSN) membrane. J. Membr. Sci. 2020, 614, 118433. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, Y.; Zhang, W.; Zhao, S.; Wang, Y. PVC-based hybrid membranes containing metal-organic frameworks for Li+/Mg2+ separation. J. Membr. Sci. 2020, 596, 117724. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, R.; Xiang, S.; Hu, J.; Zheng, X. Preparation and lithium-ion separation property of ZIF-8 membrane with excellent flexibility. Membranes 2023, 13, 500. [Google Scholar] [CrossRef]
- Wang, C.; Yang, R.; Wang, H. Synthesis of ZIF-8/fly ash composite for adsorption of Cu2+, Zn2+ and Ni2+ from aqueous solutions. Materials 2020, 13, 214. [Google Scholar] [CrossRef]
- Xiao, T.; Liu, D. The most advanced synthesis and a wide range of applications of MOF-74 and its derivatives. Microporous Mesoporous Mater. 2019, 283, 88–103. [Google Scholar] [CrossRef]
- Quijia, C.R.; Lima, C.; Silva, C.; Alves, R.C.; Frem, R.; Chorilli, M. Application of MIL-100 (Fe) in drug delivery and biomedicine. J. Drug Deliv. Sci. Technol. 2021, 61, 102217. [Google Scholar] [CrossRef]
- Qi, C.; Li, J.; Shi, Y.; Zhang, B.; Chen, T.; Wang, C.; Liu, Q.; Yang, X. ZIF-8 penetrating composite membrane for ion sieving. J. Solid State Chem. 2022, 313, 123281. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, S.; Xia, Q.; Wang, H.; Li, Z.; Li, J. Enhancement of CO2 adsorption and CO2/N2 selectivity on ZIF-8 via postsynthetic modification. AIChE J. 2013, 59, 2195–2206. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Park, W.-S.; Lim, H.; Ryi, S.-K.; Roh, S.-H.; Jung, H.-Y. Low permeable composite membrane based on sulfonated poly(phenylene oxide) (sPPO) and silica for vanadium redox flow battery. Int. J. Hydrogen Energy 2017, 42, 19035–19043. [Google Scholar] [CrossRef]
- Abdulla, S.A.; Sabouni, R.; Ghommem, M.; Alami, A.H. Synthesis and performance analysis of zeolitic imidazolate frameworks for CO2 sensing applications. Heliyon 2023, 9, 21349. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Soltani, R.D.C.; Bagheri, N.; Karimi, A.; Azar, A.E.F. Implementation of magnetic Fe3O4@ ZIF-8 nanocomposite to activate sodium percarbonate for highly effective degradation of organic compound in aqueous solution. J. Ind. Eng. Chem. 2018, 68, 406–415. [Google Scholar] [CrossRef]
- Ahmad, K.; Nde, D.T.; Khan, R.A.; Raza, W. Fabrication of Zeolitic imidazolate framework-8 (ZIF-8) modified screen-printed electrode and its catalytic role for the electrochemical determination of biological molecule (dopamine). Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134606. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Mohammadi, A.; Sadeghi, S.; Ghaderpoori, M.; Sahebi, S.; Alinejad, A. Arsenic adsorption over dodecahedra ZIF-8 from solution aqueous: Modelling, isotherms, kinetics and thermodynamics. Int. J. Environ. Anal. Chem. 2022, 102, 855–871. [Google Scholar] [CrossRef]
- Nagendra, B.; Salman, S.; Bloch, H.P.; Daniel, C.; Rizzo, P.; Fittipaldi, R.; Guerra, G. Poly(phenylene oxide) films with hydrophilic sulfonated amorphous phase and physically cross-linking hydrophobic crystalline phase. ACS Appl. Polym. Mater. 2023, 5, 3489–3498. [Google Scholar] [CrossRef]
- Si, Y.; Li, X.; Yang, G.; Mie, X.; Ge, L. Fabrication of a novel core–shell CQDs@ZIF-8 composite with enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 13049–13061. [Google Scholar]
- Liu, B.; Yang, Y.; Wu, H.; Wang, S.; Tian, J.; Dai, C.; Liu, T. Zeolitic imidazolate framework-8 triggers the inhibition of arginine biosynthesis to combat methicillin-resistant staphylococcus aureus. Small 2023, 19, 2205682. [Google Scholar]
- Petreanu, I.; Niculescu, V.-C.; Soare, A.; Iacob, C.; Teodorescu, M. Nanocomposite polyphenylene oxide with amino-functionalized silica: Structural characterization based on thermal analysis. J. Therm. Anal. Calorim. 2024, 149, 10671–10680. [Google Scholar] [CrossRef]
- Chirra, S.; Wang, L.-F.; Aggarwal, H.; Tsai, M.F.; Soorian, S.S.; Siliveri, S.; Goskula, S.; Gujjula, S.R.; Narayanan, V. Rapid synthesis of a novel nano-crystalline mesoporous faujasite type metal-organic framework, ZIF-8 catalyst, its detailed characterization, and NaBH4 assisted, enhanced catalytic Rhodamine B degradation. Mater. Today Commun. 2021, 26, 101993. [Google Scholar]
- Ji, Y.; Liu, X.; Li, H.; Jiao, X.; Yu, X.; Zhang, Y. Hydrophobic ZIF-8 covered active carbon for CO2 capture from humid gas. J. Ind. Eng. Chem. 2023, 121, 331–337. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Wang, X.; Duan, Z.; Lu, P.; Li, S.; Ji, D.; Wang, Z.; Li, G.; Yi, D.; et al. High-hydrophobic ZIF-8@ PLA composite aerogel and application for oil-water separation. Sep. Purif. Technol. 2021, 270, 118794. [Google Scholar]
- Zhang, H.; Snurr, R.Q. Computational study of water adsorption in the hydrophobic metal–organic framework ZIF-8: Adsorption mechanism and acceleration of the simulations. J. Phys. Chem. C 2017, 121, 24000–24010. [Google Scholar]
- Sann, E.E.; Pan, Y.; Gao, Z.; Zhan, S.; Xia, F. Highly hydrophobic ZIF-8 particles and application for oil-water separation. Sep. Purif. Technol. 2018, 206, 186–191. [Google Scholar]
- Sun, X.; Liu, Y.; Xu, R.; Chen, Y. MOF-derived nanoporous carbon incorporated in the cation exchange membrane for gradient power generation. Membranes 2022, 12, 322. [Google Scholar] [CrossRef]
- Han, B.; Sun, Z.; Jiang, H.; Sun, X.; Ma, J.; He, M.; Zhang, W. Thin and defect-free ZIF-8 layer assisted enhancement of the monovalent perm-selectivity for cation exchange membrane. Desalination 2022, 529, 115637. [Google Scholar]
- Yu, H.; Hossain, S.M.; Wang, C.; Choo, Y.; Naidu, G.; Han, D.S.; Shon, H.K. Selective lithium extraction from diluted binary solutions using metal-organic frameworks (MOF)-based membrane capacitive deionization (MCDI). Desalination 2023, 556, 116569. [Google Scholar]
- Dechnik, J.; Sumby, C.J.; Janiak, C. Enhancing mixed-matrix membrane performance with metal-organic framework additives. Cryst. Growth Des. 2017, 17, 4467–4488. [Google Scholar]
- Kim, J.S.; Rhim, J.W. Performance study of membrane capacitive deionization (MCDI) cell constructed with Nafion and aminated polyphenylene oxide (APPO). Membr. J. 2020, 30, 350–358. [Google Scholar]
- Tauk, M.; Bechelany, M.; Sistat, P.; Habchi, R.; Cretin, M.; Zaviska, F. Performance analysis of zeolitic imidazolate ion-selectivity advancements in capacitive deionization: A comprehensive review. Desalination 2024, 572, 117146. [Google Scholar]



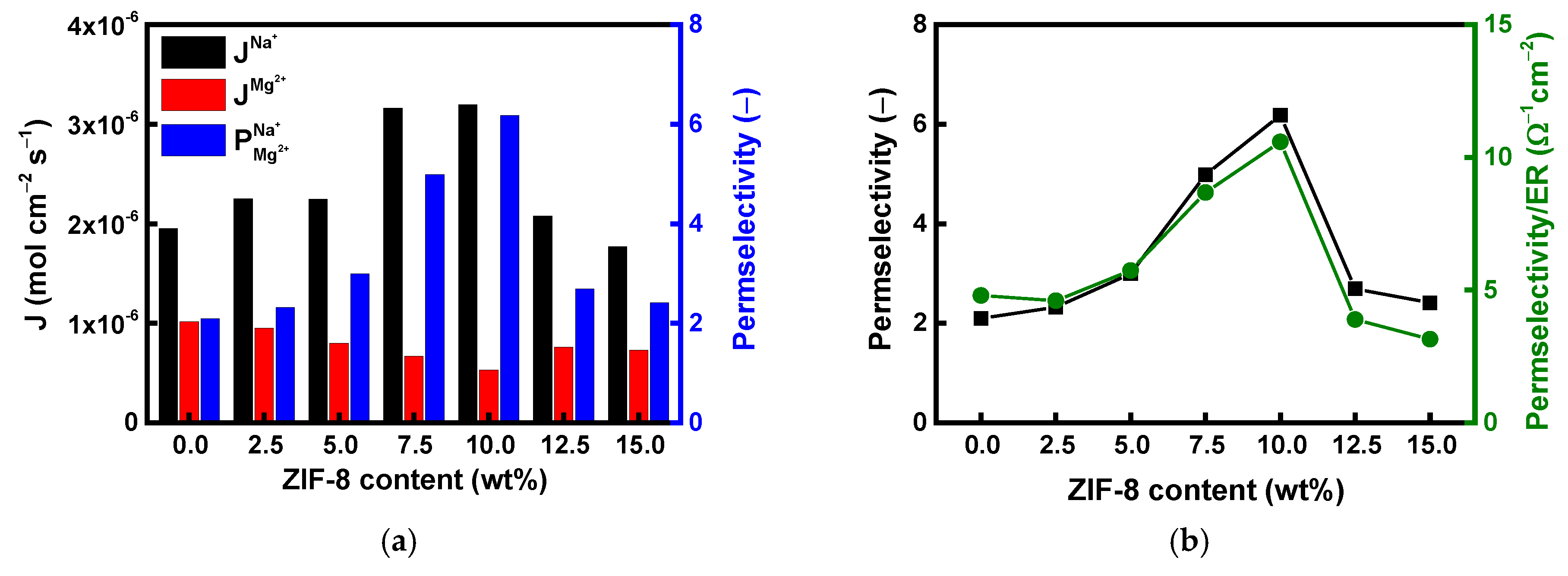
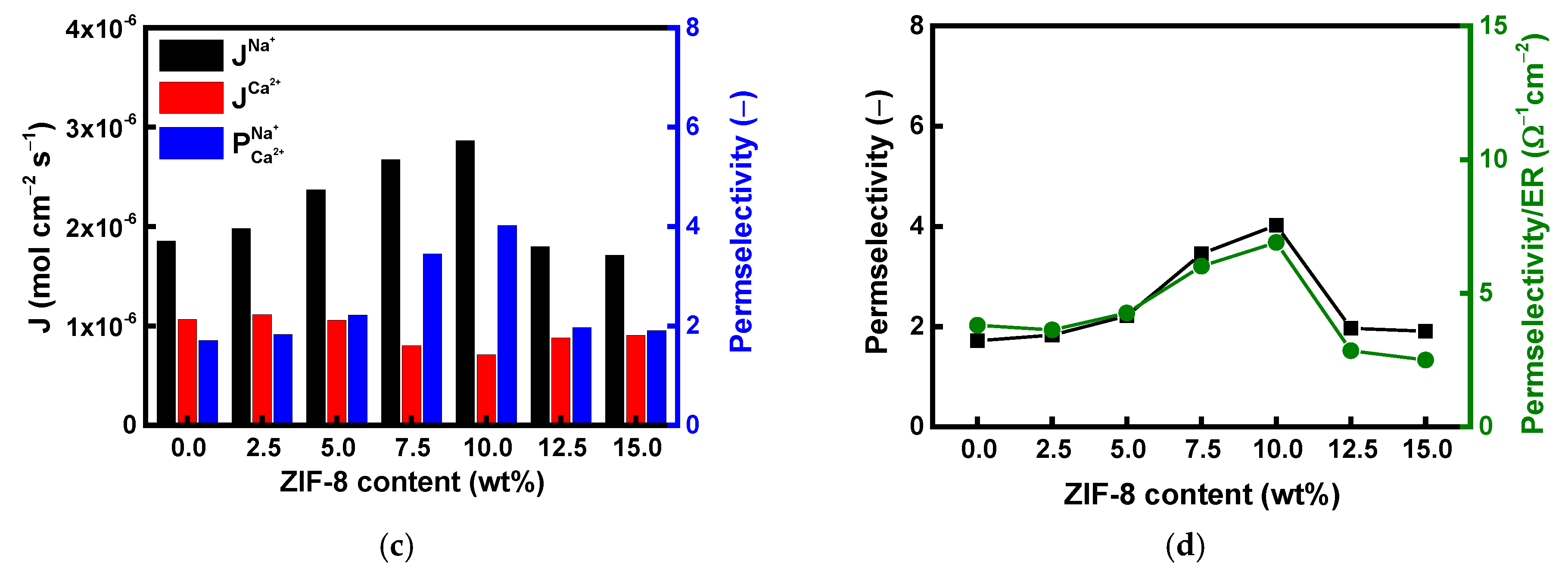
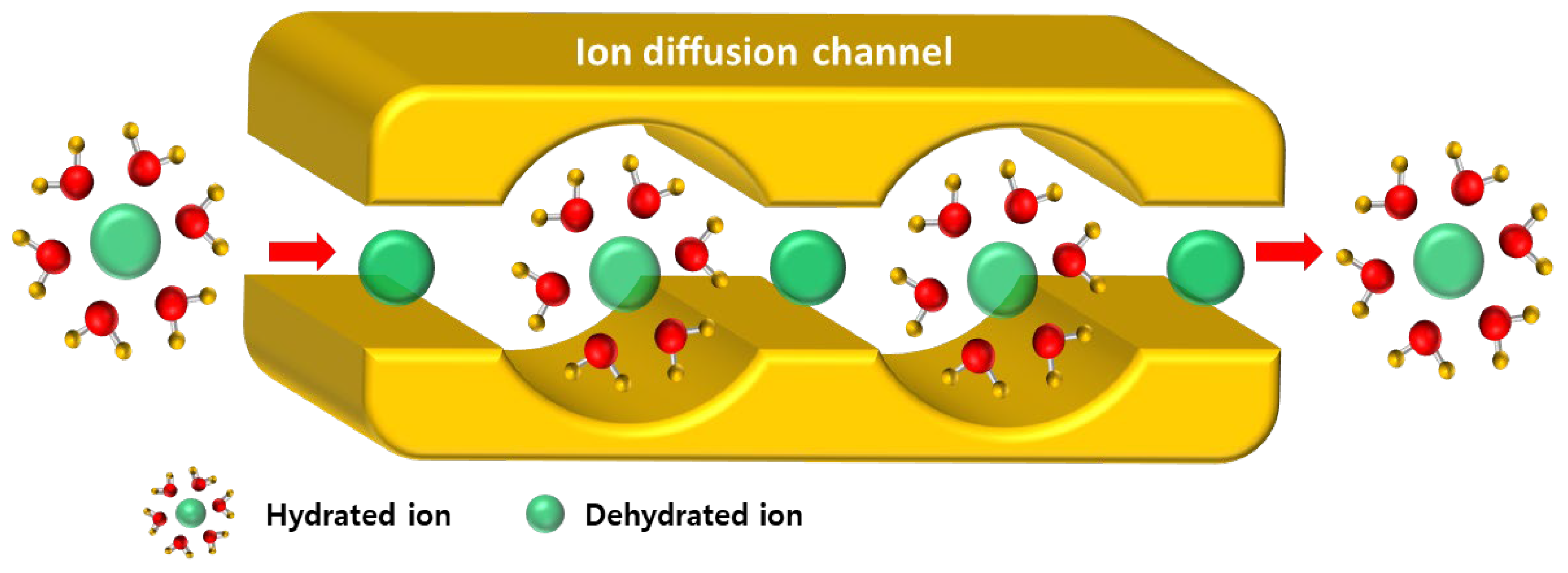
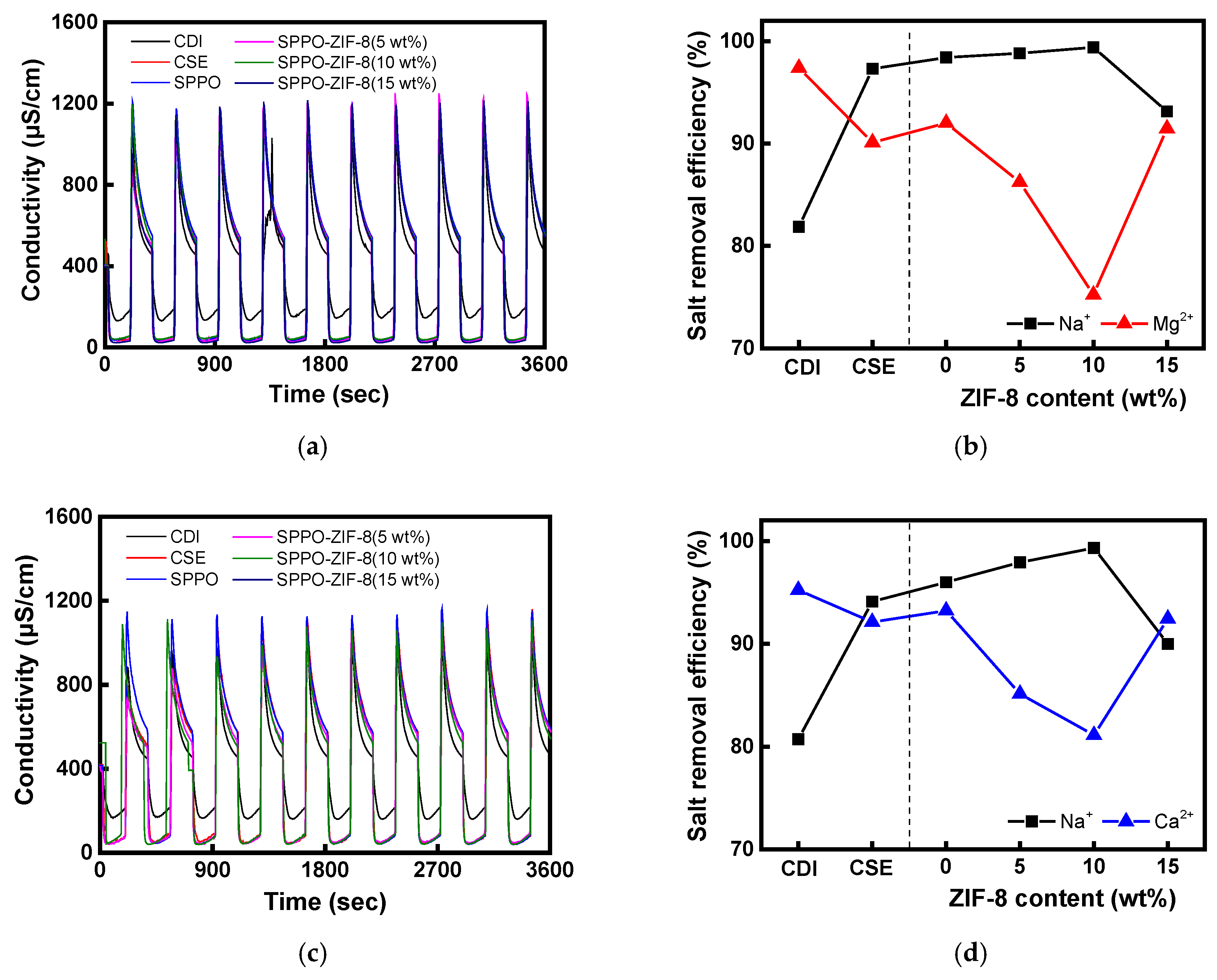
| Membranes | ER (Ω·cm2) | Conductivity (mS/cm) | TN (−) | IEC (meq./g) | WU (%) |
|---|---|---|---|---|---|
| CSE (Astom, Tokyo, Japan) | 1.87 | 7.65 | 0.983 | 2.20 | 29.80 |
| SPPO-ZIF-8 (0 wt%) | 0.431 | 8.50 | 0.966 | 1.99 | 28.20 |
| SPPO-ZIF-8 (1 wt%) | 0.502 | 7.24 | 0.970 | 1.98 | 28.04 |
| SPPO-ZIF-8 (2.5 wt%) | 0.504 | 7.06 | 0.968 | 1.96 | 27.82 |
| SPPO-ZIF-8 (5 wt%) | 0.521 | 6.87 | 0.971 | 1.89 | 26.94 |
| SPPO-ZIF-8 (7.5 wt%) | 0.574 | 6.31 | 0.962 | 1.91 | 27.60 |
| SPPO-ZIF-8 (10 wt%) | 0.584 | 6.03 | 0.973 | 1.88 | 26.88 |
| SPPO-ZIF-8 (12.5 wt%) | 0.691 | 5.41 | 0.969 | 1.64 | 24.10 |
| SPPO-ZIF-8 (15 wt%) | 0.764 | 4.84 | 0.967 | 1.52 | 23.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.-G.; Lee, J.-H.; Kang, M.-S. ZIF-8-Embedded Cation-Exchange Membranes with Improved Monovalent Ion Selectivity for Capacitive Deionization. Membranes 2025, 15, 19. https://doi.org/10.3390/membranes15010019
Han E-G, Lee J-H, Kang M-S. ZIF-8-Embedded Cation-Exchange Membranes with Improved Monovalent Ion Selectivity for Capacitive Deionization. Membranes. 2025; 15(1):19. https://doi.org/10.3390/membranes15010019
Chicago/Turabian StyleHan, Eui-Gyu, Ji-Hyeon Lee, and Moon-Sung Kang. 2025. "ZIF-8-Embedded Cation-Exchange Membranes with Improved Monovalent Ion Selectivity for Capacitive Deionization" Membranes 15, no. 1: 19. https://doi.org/10.3390/membranes15010019
APA StyleHan, E.-G., Lee, J.-H., & Kang, M.-S. (2025). ZIF-8-Embedded Cation-Exchange Membranes with Improved Monovalent Ion Selectivity for Capacitive Deionization. Membranes, 15(1), 19. https://doi.org/10.3390/membranes15010019







