A Study on the Safety and Efficacy of an Innovative Hydrophilic Dialysis Membrane
Abstract
1. Introduction
2. Materials and Methods
- Toraylight NV 2.1 in HD.
- Toraylight NV 2.1 in post-dilution HDF with Qi 50 mL/min.
- Toraylight NV 2.1 in post-dilution HDF with Qi 75 mL/min.
- Toraylight NV 2.1 in post-dilution HDF with Qi in auto-substitution.
- FX CorAL 800 in post-dilution HDF with Qi in auto-substitution.
3. Results and Discussion
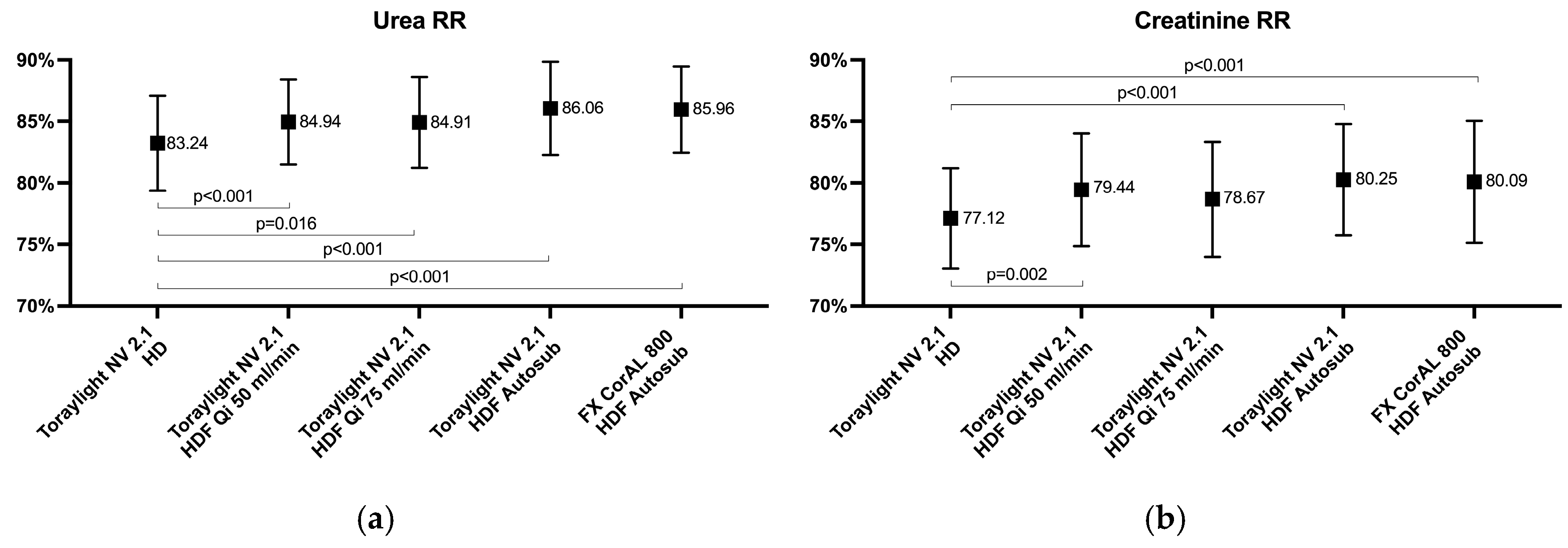
3.1. Small-Sized Molecules
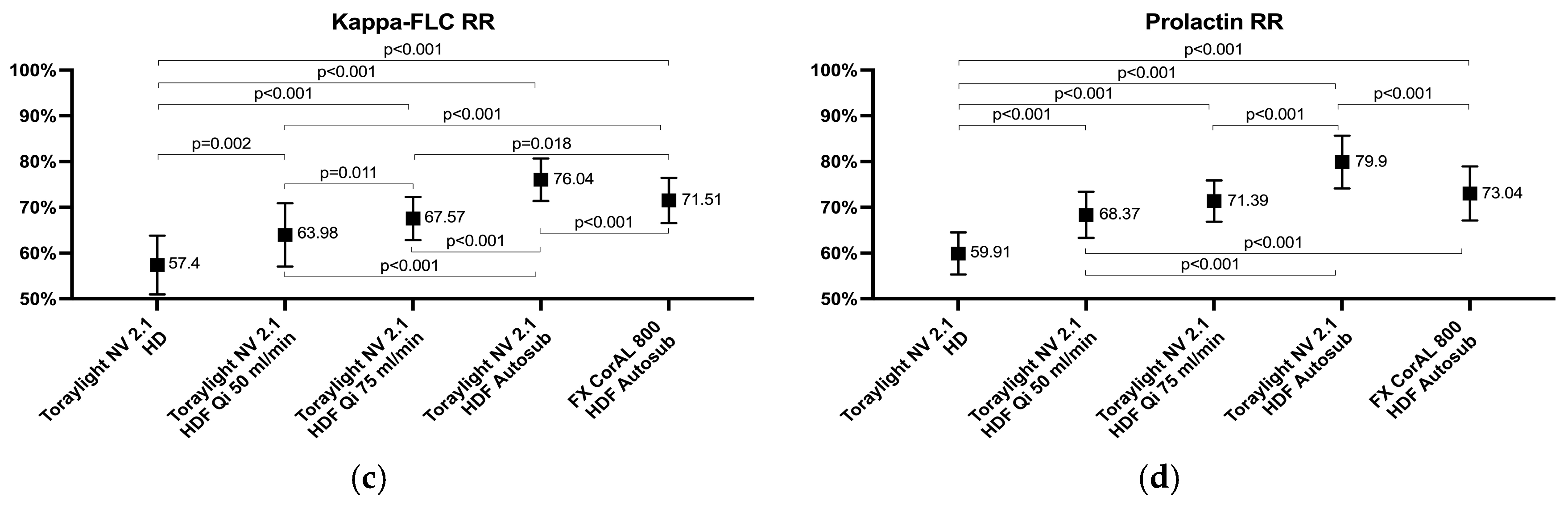
3.2. Medium-Sized Molecules
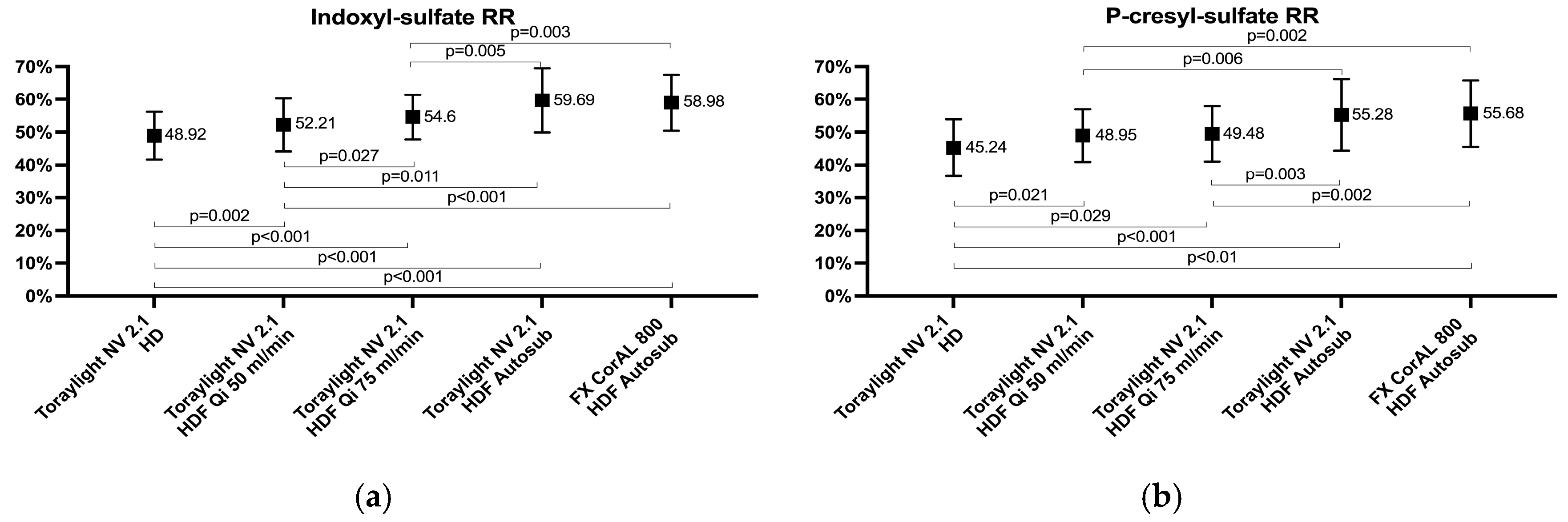
3.3. Protein-Bound Uremic Toxins
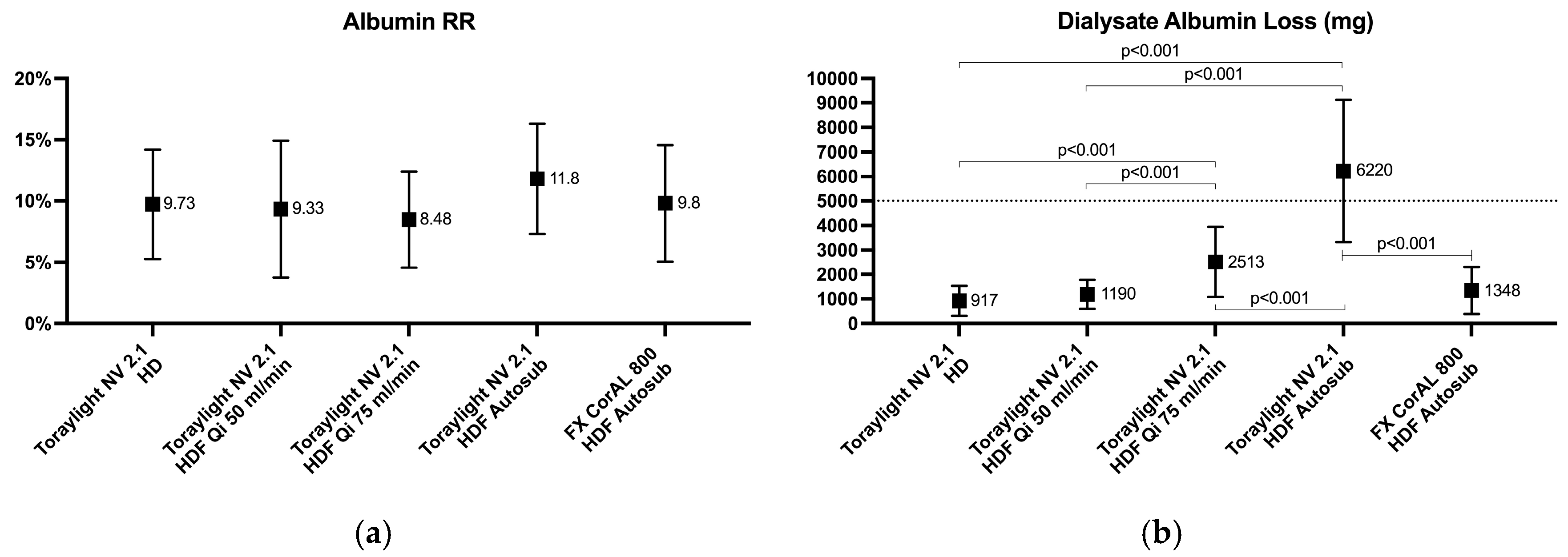
3.4. Albumin Loss in Blood and Dialysate
3.5. Global Removal Score
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of hydrophilicity and blood compatibility on polyethersulfone membrane by adding polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Wang, J.; Xue, L. Polysulfone hemodiafiltration membranes with enhanced antifouling and hemocompatibility modified by poly(vinyl pyrrolidone) via in situ cross-linked polymerization. Mater. Sci. Eng. C 2017, 74, 159–166. [Google Scholar] [CrossRef]
- Sato, Y.; Horiuchi, H.; Fukasawa, S.; Takesawa, S.; Hirayama, J. Influences of the priming procedure and saline circulation conditions on polyvinylpyrrolidone in vitro elution from polysulfone membrane dialyzers. Biochem. Biophys. Rep. 2021, 28, 101140. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, A.; Sánchez-Villanueva, R.; Domínguez-Ortega, J.; Álvarez, L.; Fiandor, A.; Nozal, P.; Sanz, P.; Pizarro-Sánchez, M.-S.; Andrés, E.; Cabezas, A.; et al. Characterization of hypersensitivity reactions to polysulfone hemodialysis membranes. Ann. Allergy Asthma Immunol. 2022, 128, 713–720. [Google Scholar] [CrossRef]
- Sánchez-Villanueva, R.J.; González, E.; Quirce, S.; Díaz, R.; Álvarez, L.; Menéndez, D.; Rodríguez-Gayo, L.; Bajo, M.A.; Selgas, R. Hypersensitivity reactions to synthetic haemodialysis membranes. Nefrologia 2014, 34, 520–525. [Google Scholar]
- Alvarez-de Lara, M.A.; Martín-Malo, A. Hypersensitivity reactions to synthetic haemodialysis membranes an emerging issue? Nefrologia 2014, 34, 698–702. [Google Scholar]
- Delgado-Córdova, M.; Blanco, N.; Azaña, N. Hypotension in hemodialysis secondary to a reaction to synthetic membranes. Nefrologia 2018, 38, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Yamaka, T.; Ichikawa, K.; Saito, M.; Watanabe, K.; Nakai, A.; Higuchi, N.; Igarashi, N.; Yoshimoto, H. Biocompatibility of the new anticoagulant dialyzer Toraylight NV. Sci. Postprint 2014, 1, e00020. [Google Scholar] [CrossRef]
- Hidaka, S.; Kobayashi, S.; Maesato, K.; Mochida, Y.; Ishioka, K.; Oka, M.; Moriya, H.; Ohtake, T.; Nomura, S. Hydrophilic polymer-coated polysulfone membrane improves endothelial function of hemodialysis patients: A pilot study. J. Clin. Nephrol. Res. 2015, 2, 1020. [Google Scholar]
- Kakuta, T.; Komaba, H.; Takagi, N.; Takahashi, Y.; Suzuki, H.; Hyodo, T.; Nagaoka, M.; Tanaka, R.; Iwao, S.; Ishida, M.; et al. A prospective multicenter randomized controlled study on interleukin-6 removal and induction by a new hemodialyzer with improved biocompatibility in hemodialysis patients: A pilot study. Ther. Apher. Dial. 2016, 20, 569–578. [Google Scholar] [CrossRef]
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement activation by dialysis membranes and its association with secondary membrane formation and surface charge. Artif. Organs 2021, 45, 770–778. [Google Scholar] [CrossRef]
- Hayama, M.; Yamamoto, K.-I.; Kohori, F.; Sakai, K. How polysulfone dialysis membranes containing polyvinylpyrrolidone achieve excellent biocompatibility? J. Memb. Sci. 2004, 234, 41–49. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Zhang, D.; Wang, G.; Zeng, Z.; Xue, Q. Negatively charged polysulfone membranes with hydrophilicity and antifouling properties based on in situ cross-linked polymerization. J. Colloid. Interface Sci. 2017, 498, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Rodas, L.M.; Gómez, M.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; Salgado, M.d.C.; Rico, N.; et al. Comparison of efficacy and safety of the new generation helixone dialyzers. Nefrologia 2024, 44, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Wehle, B. No change in corrected β2-microglobulin concentration after cuprophane hemodialysis. Lancet 1987, 1, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Rodas, L.; Broseta, J.J.; Gomez, M.; Xipell, M.; Guillen, E.; Montagud-Marrahi, E.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; et al. Medium Cut-Off Dialyzer versus Eight Hemodiafiltration Dialyzers: Comparison Using a Global Removal Score. Blood Purif. 2019, 48, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef]
- Mercadal, L.; Franck, J.E.; Metzger, M.; Ureña Torres, P.; de Cornelissen, F.; Edet, S.; Béchade, C.; Vigneau, C.; Drüeke, T.; Jacquelinet, C.; et al. Hemodiafiltration versus hemodialysis and survival in patients with ESRD: The French renal epidemiology and information network (REIN) registry. Am. J. Kidney Dis. 2015, 68, 247–255. [Google Scholar] [CrossRef] [PubMed]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient survival on haemodiafiltration and haemodialysis: A cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol. Dial. Transplant. 2019, 34, 326–338. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. CONVINCE Scientific Committee Investigators. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef]
- Oshihara, W.; Ueno, Y.; Fujieda, H. New Polysulfone Membrane Dialyzer, NV, with Low-Fouling Antithrombotic Properties. In Scientific Aspects of Dialysis Therapy: JSDT/ISBP Anniversary Edition; Kawanishi, H., Takemoto, Y., Eds.; S.Karger AG: Basel, Switzerland, 2017; Volume 189, pp. 222–229. [Google Scholar]
- Abe, M.; Masakane, I.; Wada, A.; Nakai, S.; Nitta, K.; Nakamoto, H. Dialyzer classification and mortality in hemodialysis patients: A 3-year nationwide cohort study. Front. Med. 2021, 8, 740461. [Google Scholar] [CrossRef] [PubMed]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Del Risco, J.; Rodas, L.M.; Arias-Guillén, M.; Vera, M.; Fontseré, N.; Salgado, M.d.C.; Rico, N. Comparison of four medium cut-off dialyzers. Clin. Kidney J. 2022, 15, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, A.; de la Flor, J.C.; Coll, E.; Iglesias, E.; Reque, J.; Valga, F. Expanded hemodialysis: What’s up, Doc? Clin. Kidney J. 2023, 16, 1071–1080. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Nalesso, F.; Zanella, M.; Cal, M.D.; Corradi, V.; Virzì, G.M.; Ferrari, F.; Garzotto, F.; Lorenzin, A.; et al. Prospective, randomized, multicenter, controlled trial (TRIATHRON 1) on a new antithrombogenic hydrophilic dialysis membrane. Int. J. Artif. Organs 2017, 40, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Ehlerding, G.; Erlenkötter, A.; Gauly, A.; Griesshaber, E.; Kennedy, J.; Rauber, L.; Ries, W.; Schmidt-Gürtler, H.; Stauss-Grabo, M.; Wagner, S.; et al. Performance and Hemocompatibility of a Novel Polysulfone Dialyzer: A Randomized Controlled Trial. Kidney360 2021, 2, 937–947. [Google Scholar] [CrossRef]
- Ehlerding, G.; Ries, W.; Kempkes-Koch, M.; Ziegler, E.; Erlenkötter Zawada, A.M.; Kennedy, J.P.; Ottillinger, B.; Stauss-Grabo, M.; Lang, T. Randomized comparison of three high-flux dialyzers during high-volume online hemodiafiltration-the comPERFORM study. Clin. Kidney J. 2022, 15, 672–680. [Google Scholar] [CrossRef]
- Kislikova, M.; Vega, A.; Verde, E.; Abad, S.; Vaca, M.; Acosta, A.; González, A.; Bascuñana, A.; Mijailova, A.; Nava, C.; et al. Depurative capacity toward medium molecules of the dialyzer Toray NV-U® HydrolinkTM: A new hydrophilic membrane to perform online hemodiafiltration. Int. J. Artif. Organs 2024, 47, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Potier, J.; Queffeulou, G.; Bouet, J. Are all dialyzers compatible with the convective volumes suggested for postdilution online hemodiafiltration? Int. J. Artif. Organs 2016, 39, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Boschetti-De-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO Membranes: Enhanced Selectivity in High-Flux Class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef] [PubMed]
- Masakane, I.; Sakurai, K. Current approaches to middle molecule removal: Room for innovation. Nephrol. Dial. Transplant. 2018, 33 (Suppl. S3), iii12–iii21. [Google Scholar] [CrossRef] [PubMed]




| Toraylight NV-21U | FX CorAL 800 | |
|---|---|---|
| Membrane | Polysulfone (Hydrolink membrane) | Helixone hydro (polysulfone) |
| KUF (mL/h/mmHg) | 52 | 73 |
| Wall thickness | 40 | 35 |
| Inner diameter | 200 | 210 |
| SC ß2-microglobulin | 0.93 | 1.00 |
| SC myoglobin | 0.55 | 0.60 |
| SC albumin | 0.005 | <0.001 |
| Surface (m2) | 2.1 | 2.0 |
| Sterilization | Gamma ray | Steam |
| Variable | Toraylight NV HD | Toraylight NV HDF 50 mL/min | Toraylight NV HDF 75 mL/min | Toraylight NV HDF Auto-Sub | FX Coral HDF Auto-Sub |
|---|---|---|---|---|---|
| Blood processed (L) | 123.3 ± 8.87 | 122.8 ± 8.87 | 123.5 ± 8.25 | 122.8 ± 9.17 | 123.2 ± 10.5 |
| Recirculation (%) | 15.8 ± 5.0 | 16.0 ± 4.8 | 16.8 ± 5.5 | 15.5 ± 4.9 | 15.5 ± 4.1 |
| Real dialysis time (min) | 285.2 ± 17.3 a | 284.2 ± 17.6 | 284.3 ± 17.6 a | 281.6 ± 17.6 | 282.9 ± 17.4 |
| Arterial pressure (mmHg) | −228 ± 39 | −221 ± 42 | −225 ± 31 | −216 ± 33 | −218 ± 30 |
| Venous pressure (mmHg) | 208 ± 29 | 204 ± 28 | 205 ± 33 | 199 ± 31 | 201 ± 27 |
| TMP (mmHg) | 25 ± 3 b | 51 ± 11 b | 71 ± 11 b | 159 ± 46 | 177 ± 35 |
| Initial hematocrit (%) | 28.75 ± 4.84 | 28.67 ± 4.63 | 29.84 ± 4.68 | 28.89 ± 4.96 | 28.92 ± 4.73 |
| Final hematocrit (%) | 32.46 ± 5.82 | 32.11 ± 4.73 | 34.76 ± 4.84 | 33.58 ± 6.15 | 32.76 ± 5.94 |
| Initial weight (kg) | 70.04 ± 12.16 | 69.82 ± 12.25 | 70.43 ± 12.47 | 70.08 ± 12.19 | 70.25 ± 12.00 |
| Final weight (kg) | 68.21 ± 11.98 | 68.14 ± 12.04 | 68.44 ± 12.12 | 68.13 ± 11.99 | 68.31 ± 11.72 |
| Weight gain (kg) | 1.83 ± 0.89 | 1.68 ± 0.91 | 1.98 ± 0.89 | 1.95 ± 0.84 | 1.94 ± 0.74 |
| Convective volume (L) | 2.33 ± 0.89 b | 16.53 ± 1.48 b | 23.55 ± 1.82 b | 36.08 ± 6.50 | 35.89 ± 5.77 |
| Filtration fraction (%) | NA | 13.5 ± 1.4 b | 19.1 ± 1.1 b | 29.8 ± 3.9 | 29.1 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maduell, F.; Escudero-Saiz, V.J.; Cuadrado-Payán, E.; Rodriguez-Garcia, M.; Gómez, M.; Rodas, L.M.; Fontseré, N.; Salgado, M.d.C.; Casals, G.; Rico, N.; et al. A Study on the Safety and Efficacy of an Innovative Hydrophilic Dialysis Membrane. Membranes 2025, 15, 30. https://doi.org/10.3390/membranes15010030
Maduell F, Escudero-Saiz VJ, Cuadrado-Payán E, Rodriguez-Garcia M, Gómez M, Rodas LM, Fontseré N, Salgado MdC, Casals G, Rico N, et al. A Study on the Safety and Efficacy of an Innovative Hydrophilic Dialysis Membrane. Membranes. 2025; 15(1):30. https://doi.org/10.3390/membranes15010030
Chicago/Turabian StyleMaduell, Francisco, Victor Joaquín Escudero-Saiz, Elena Cuadrado-Payán, Maria Rodriguez-Garcia, Miquel Gómez, Lida María Rodas, Néstor Fontseré, Maria del Carmen Salgado, Gregori Casals, Nayra Rico, and et al. 2025. "A Study on the Safety and Efficacy of an Innovative Hydrophilic Dialysis Membrane" Membranes 15, no. 1: 30. https://doi.org/10.3390/membranes15010030
APA StyleMaduell, F., Escudero-Saiz, V. J., Cuadrado-Payán, E., Rodriguez-Garcia, M., Gómez, M., Rodas, L. M., Fontseré, N., Salgado, M. d. C., Casals, G., Rico, N., & Broseta, J. J. (2025). A Study on the Safety and Efficacy of an Innovative Hydrophilic Dialysis Membrane. Membranes, 15(1), 30. https://doi.org/10.3390/membranes15010030







