Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica-Nanocoated GF Membranes
2.3. Membrane Characterizations
2.4. Emulsion Separation Experiments
2.5. Membrane Stability Tests
2.6. Membrane Antifouling Tests
3. Results and Discussion
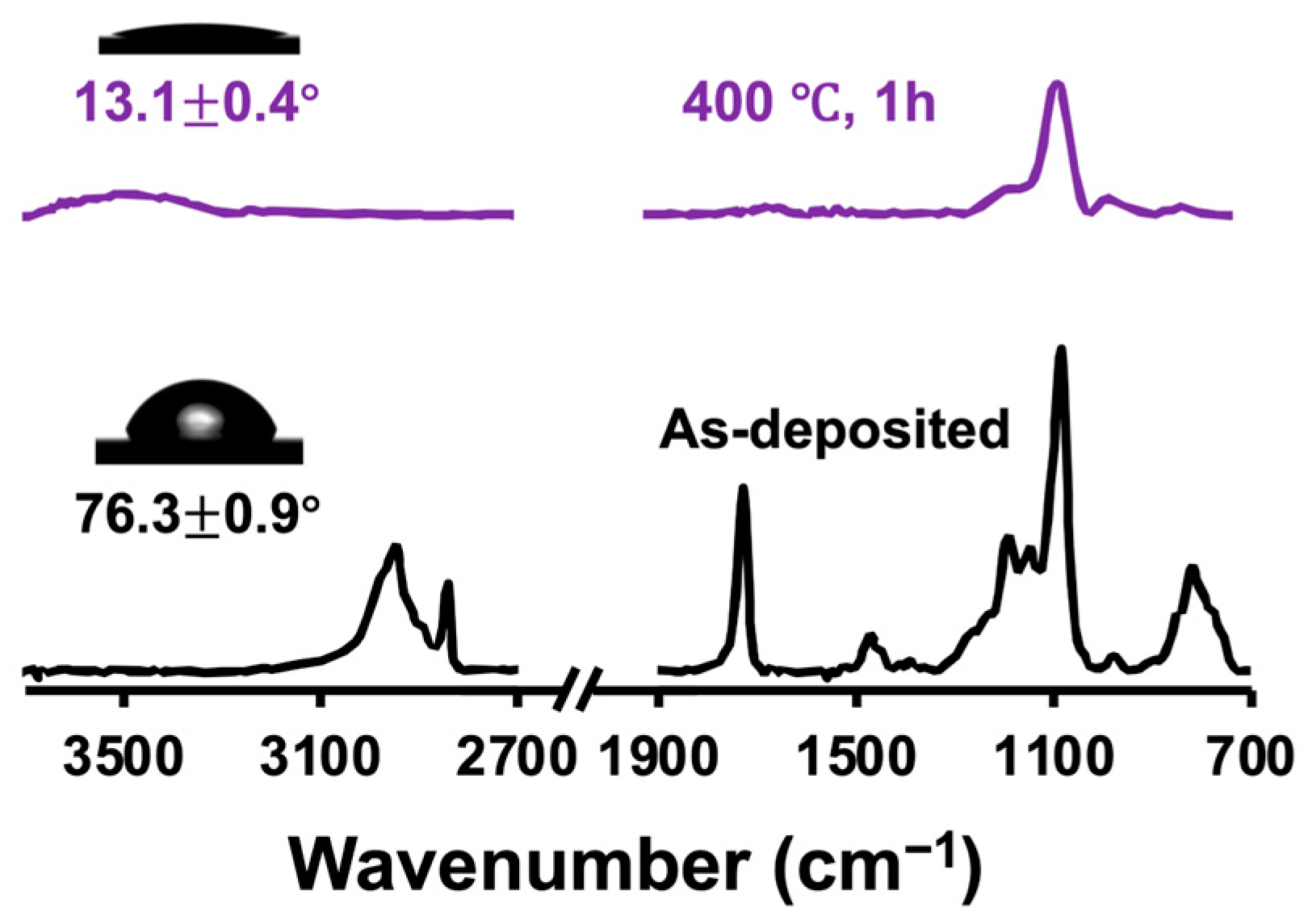
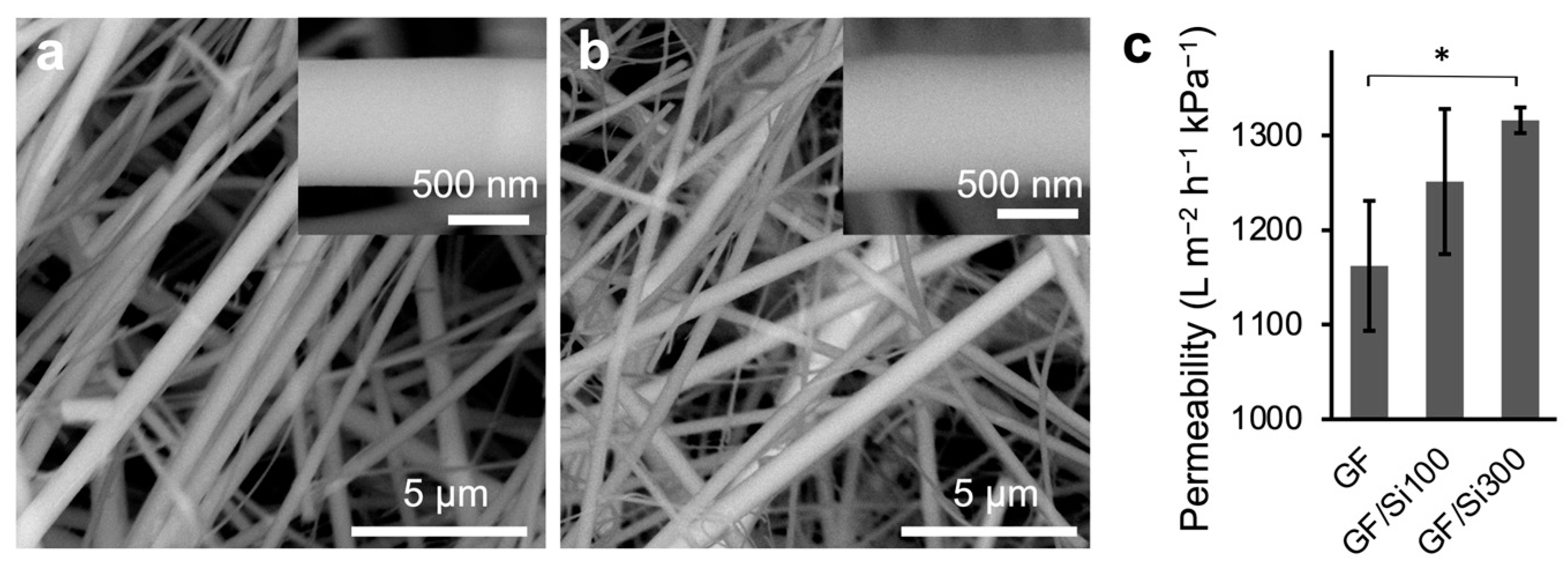
3.1. Membrane Preparation and Characterization
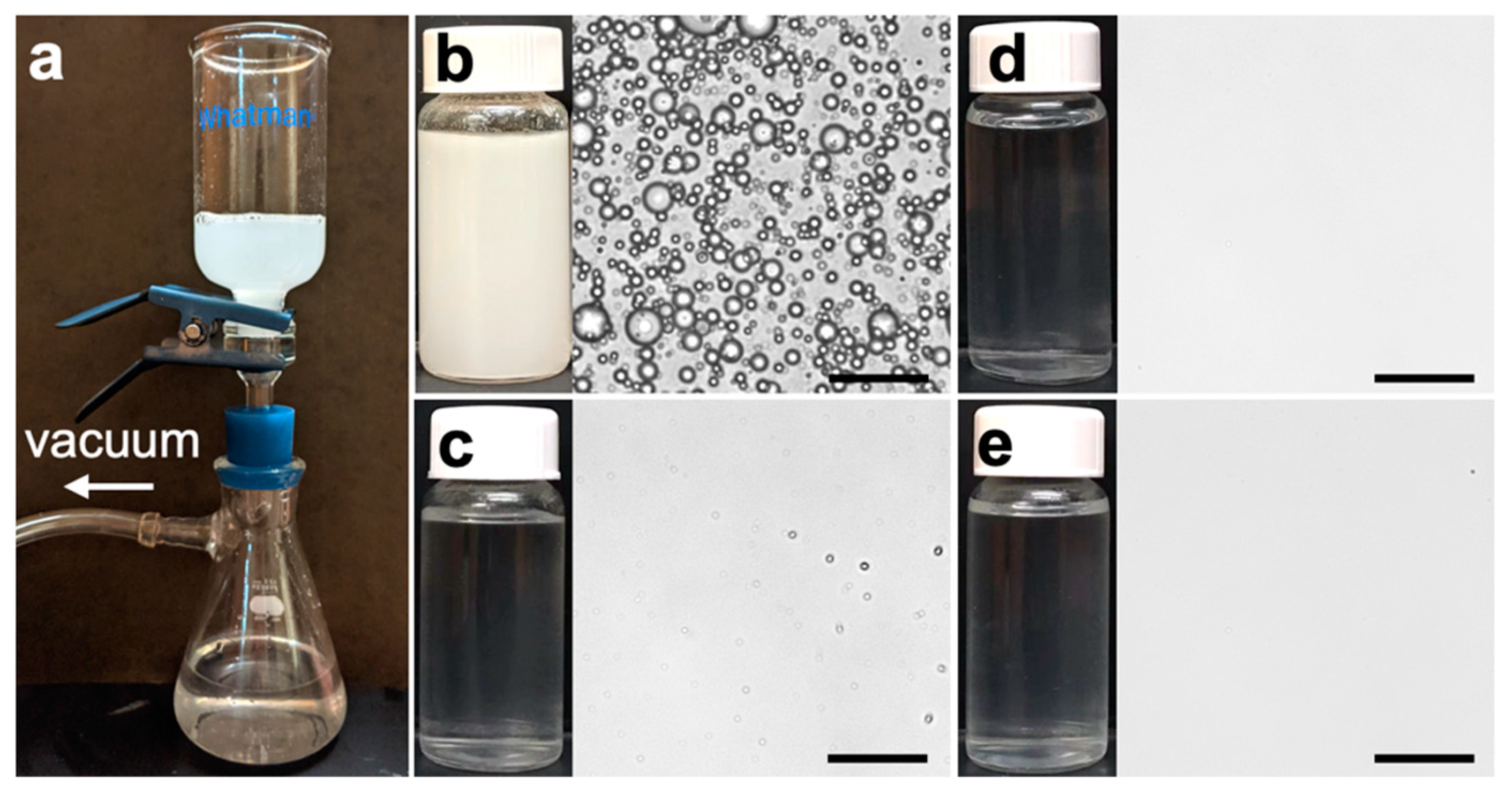
3.2. Emulsion Separation Performance
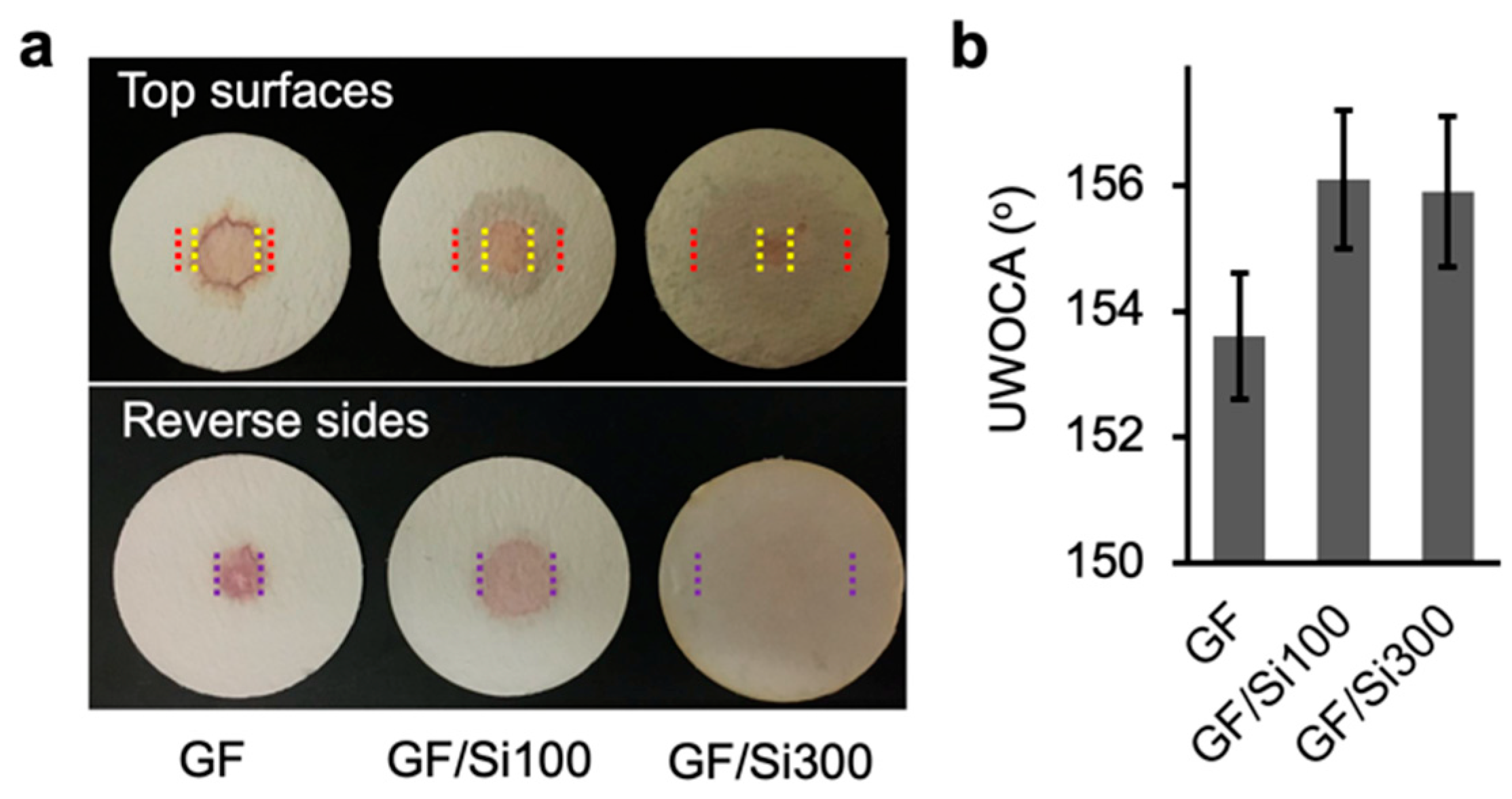
3.3. Membrane Stability
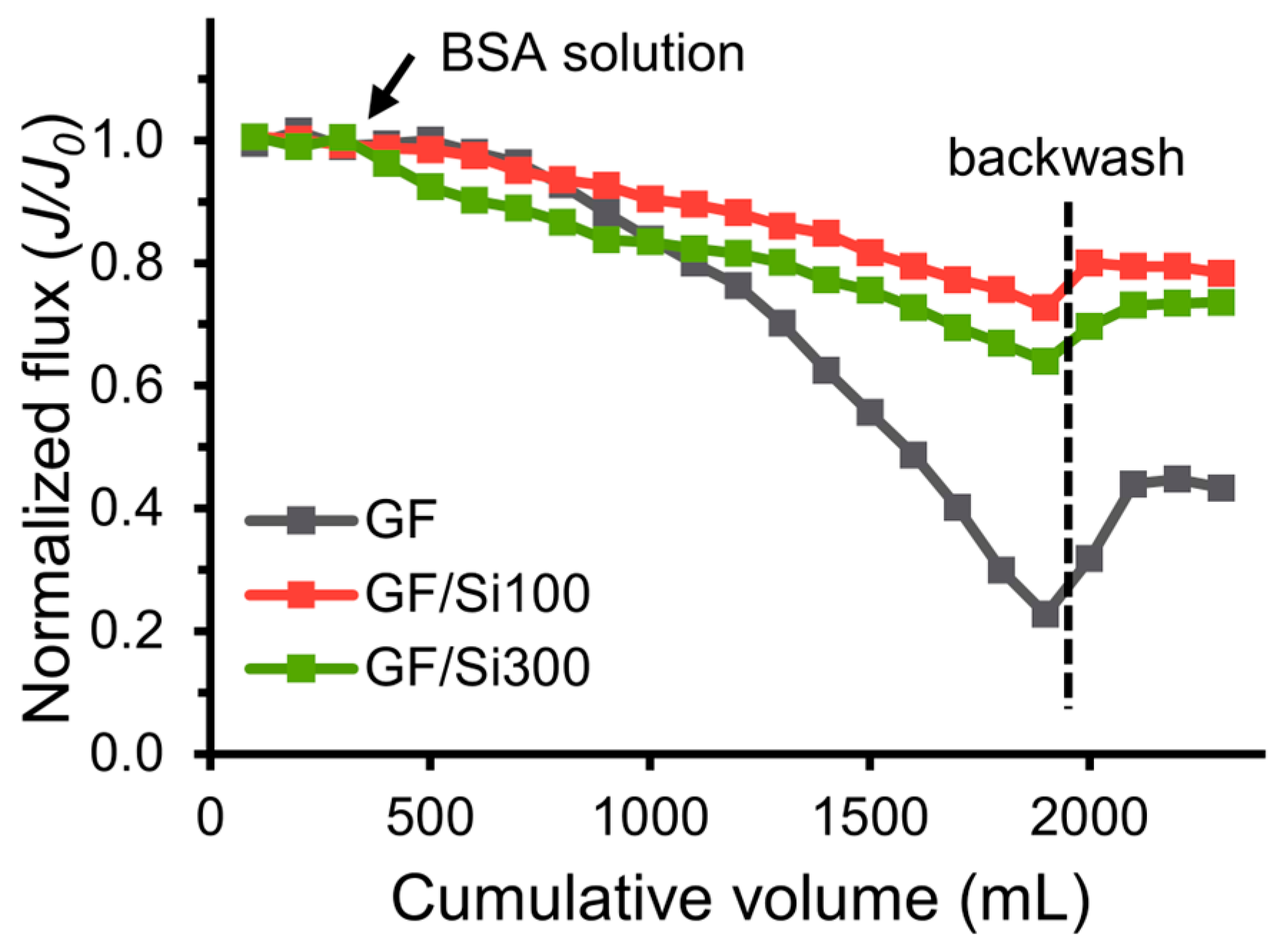
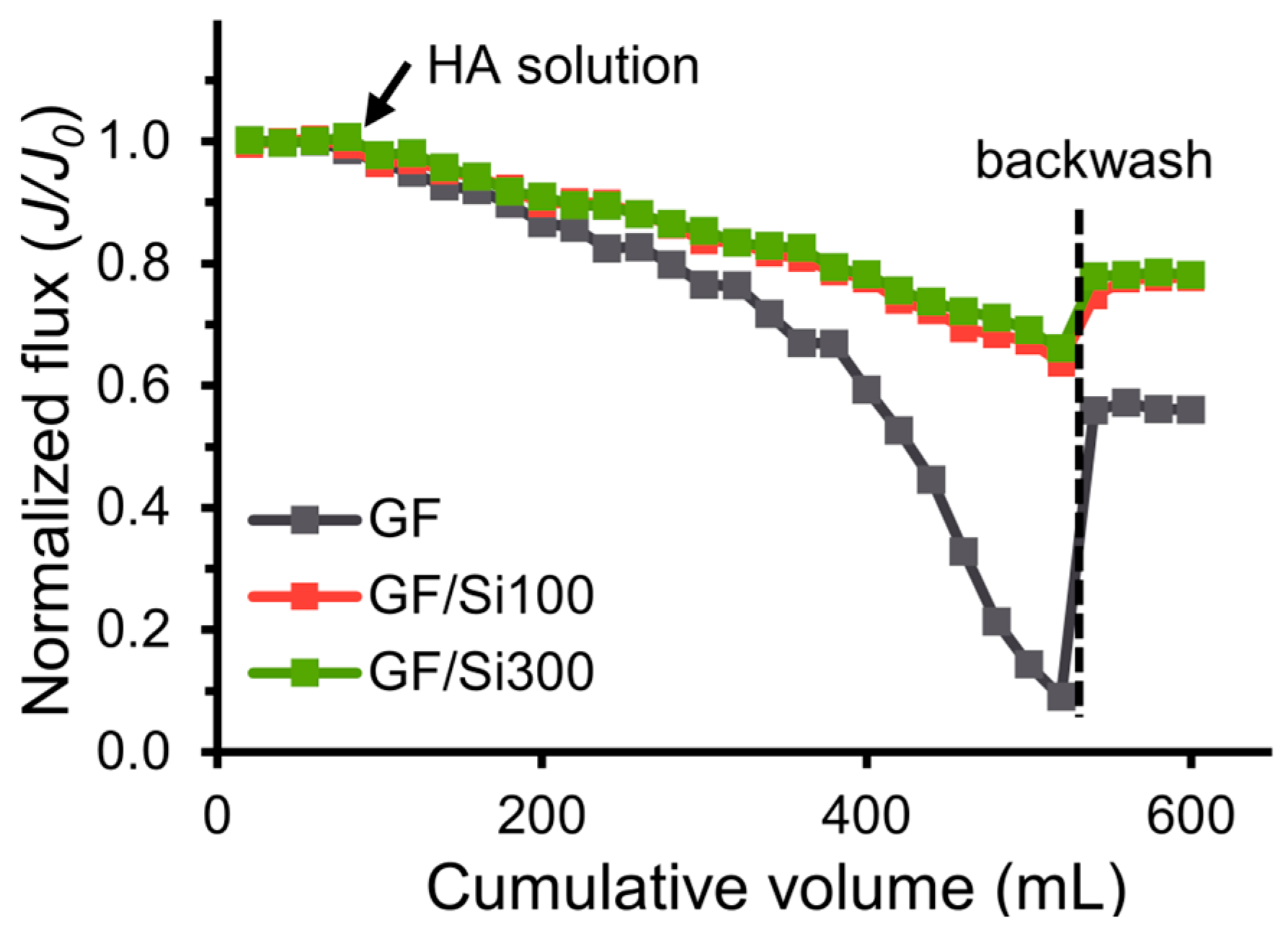
3.4. Fouling Resistance to Oil, BAS, and HA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrope, M. Oil Spill: Deep Wounds. Nature 2011, 472, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- McMahon, P.B.; Kulongoski, J.T.; Vengosh, A.; Cozzarelli, I.M.; Landon, M.K.; Kharaka, Y.K.; Gillespie, J.M.; Davis, T.A. Regional Patterns in the Geochemistry of Oil-Field Water, Southern San Joaquin Valley, California, USA. Appl. Geochem. 2018, 98, 127–140. [Google Scholar] [CrossRef]
- Kingston, P.F. Long-Term Environmental Impact of Oil Spills. Spill Sci. Technol. Bull. 2002, 7, 53–61. [Google Scholar] [CrossRef]
- Aguilera, F.; Méndez, J.; Pásaro, E.; Laffon, B. Review on the Effects of Exposure to Spilled Oils on Human Health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude Oil Emulsion: A Review on Formation, Classification and Stability of Water-in-Oil Emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-Based Separation for Oily Wastewater: A Practical Perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Liu, G. Rapid and Efficient Separation of Oil from Oil-in-Water Emulsions Using a Janus Cotton Fabric. Angew. Chem. Int. Ed. 2016, 55, 1291–1294. [Google Scholar] [CrossRef]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of Membrane Technology for Oil Field and Refinery Produced Water Treatment—A Review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane Technology Enhancement in Oil–Water Separation. A Review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, B.; Wu, X.; Yang, C.; Li, B. Different Types of Membrane Materials for Oil-Water Separation: Status and Challenges. Colloids Interface Sci. Commun. 2024, 59, 100772. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic Polymer-Based Membrane for Oily Wastewater Treatment: A Review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Li, J.-J.; Zhou, Y.-N.; Luo, Z.-H. Polymeric Materials with Switchable Superwettability for Controllable Oil/Water Separation: A Comprehensive Review. Prog. Polym. Sci. 2018, 87, 1–33. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A Review on Inorganic Membranes for Desalination and Wastewater Treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Kumbharkar, S.C.; Kim, J.F.; Bhole, Y.; Livingston, A.G. Beyond Polyimide: Crosslinked Polybenzimidazole Membranes for Organic Solvent Nanofiltration (OSN) in Harsh Environments. J. Membr. Sci. 2014, 457, 62–72. [Google Scholar] [CrossRef]
- Cao, Z.; Hao, T.; Wang, P.; Zhang, Y.; Cheng, B.; Yuan, T.; Meng, J. Surface Modified Glass Fiber Membranes with Superior Chemical and Thermal Resistance for O/W Separation. Chem. Eng. J. 2017, 309, 30–40. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Zhdanov, V.P.; Besenbacher, F. Enhancement of Protein Adsorption Induced by Surface Roughness. Langmuir 2006, 22, 10885–10888. [Google Scholar] [CrossRef]
- Woo, S.H.; Min, B.R.; Lee, J.S. Change of Surface Morphology, Permeate Flux, Surface Roughness and Water Contact Angle for Membranes with Similar Physicochemical Characteristics (except Surface Roughness) during Microfiltration. Sep. Purif. Technol. 2017, 187, 274–284. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Mohanty, S.; Nayak, S.K. Antifouling, Fouling Release and Antimicrobial Materials for Surface Modification of Reverse Osmosis and Nanofiltration Membranes. J. Mater. Chem. A 2018, 6, 313–333. [Google Scholar] [CrossRef]
- Zhou, X.; He, C. Tailoring the Surface Chemistry and Morphology of Glass Fiber Membranes for Robust Oil/Water Separation Using Poly(Dimethylsiloxanes) as Hydrophobic Molecular Binders. J. Mater. Chem. A 2018, 6, 607–615. [Google Scholar] [CrossRef]
- Phiri, I.; Eum, K.Y.; Kim, J.W.; Choi, W.S.; Kim, S.H.; Ko, J.M.; Jung, H. Simultaneous Complementary Oil-Water Separation and Water Desalination Using Functionalized Woven Glass Fiber Membranes. J. Ind. Eng. Chem. 2019, 73, 78–86. [Google Scholar] [CrossRef]
- Liu, Q.; Patel, A.A.; Liu, L. Superhydrophilic and Underwater Superoleophobic Poly(Sulfobetaine Methacrylate)-Grafted Glass Fiber Filters for Oil–Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 8996–9003. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Pi, J.-K.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Huang, X.-J.; Xu, Z.-K. Silica-Decorated Polypropylene Microfiltration Membranes with a Mussel-Inspired Intermediate Layer for Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2014, 6, 12566–12572. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In Situ Silica Growth for Superhydrophilic-Underwater Superoleophobic Silica/PVA Nanofibrous Membrane for Gravity-Driven Oil-in-Water Emulsion Separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Knowles, B.R.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Silica Nanoparticles Functionalized with Zwitterionic Sulfobetaine Siloxane for Application as a Versatile Antifouling Coating System. ACS Appl. Mater. Interfaces 2017, 9, 18584–18594. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High Permeation and Antifouling Polysulfone Ultrafiltration Membranes with in Situ Synthesized Silica Nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Small, C.; Ma, J.; Elimelech, M. Comparison of Organic Fouling Resistance of Thin-Film Composite Membranes Modified by Hydrophilic Silica Nanoparticles and Zwitterionic Polymer Brushes. J. Membr. Sci. 2017, 544, 135–142. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, M.; Chen, X.; Liu, T.; Xie, M.; Mao, Y. Flexible Membranes Fabricated by Initiated Chemical Vapor Deposition for Water Treatment, Battery, and Drug Delivery. Chem. Eng. J. 2023, 477, 146911. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, M.; Mao, Y. Solventless Polymer-Grafted Mesh for Rapid and Efficient Oil–Water Separation. ACS Appl. Polym. Mater. 2023, 5, 3801–3808. [Google Scholar] [CrossRef]
- Zhu, M.; Mao, Y. Large-Pore-Size Membranes Tuned by Chemically Vapor Deposited Nanocoatings for Rapid and Controlled Desalination. RSC Adv. 2020, 10, 40562–40568. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Cheng, X.; Lau, C.H.; Shao, L. Mussel-Inspired Hybrid Coatings That Transform Membrane Hydrophobicity into High Hydrophilicity and Underwater Superoleophobicity for Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 9534–9545. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly Hydrophilic Polyvinylidene Fluoride (PVDF) Ultrafiltration Membranes via Postfabrication Grafting of Surface-Tailored Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-N.; Au-Duong, A.-N.; Lee, C.-K. Facile Coating on Microporous Polypropylene Membrane for Antifouling Microfiltration Using Comb-Shaped Poly(N-Vinylpyrrolidone) with Multivalent Catechol. J. Membr. Sci. 2019, 574, 164–173. [Google Scholar] [CrossRef]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and Characterization of Low Fouling Novel Hybrid Ultrafiltration Membranes Based on the Blends of GO−TiO2 Nanocomposite and Polysulfone for Humic Acid Removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
- Mao, Y.; Gleason, K.K. Hot Filament Chemical Vapor Deposition of Poly(Glycidyl Methacrylate) Thin Films Using Tert-Butyl Peroxide as an Initiator. Langmuir 2004, 20, 2484–2488. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A. Short Natural-Fibre Reinforced Polyethylene and Natural Rubber Composites: Effect of Silane Coupling Agents and Fibres Loading. Compos. Sci. Technol. 2007, 67, 1627–1639. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Liso, M.J.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the Reaction of γ—Methacryloxypropyltrimethoxysilane (γ—MPS) with Slate Surfaces. J. Mater. Sci. 1999, 34, 3867–3873. [Google Scholar] [CrossRef]
- Gunji, T.; Makabe, Y.; Takamura, N.; Abe, Y. Preparation and Characterization of Organic–Inorganic Hybrids and Coating Films from 3-Methacryloxypropylpolysilsesquioxane. Appl. Organomet. Chem. 2001, 15, 683–692. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Tissot, I.; Lefebvre, F. Synthesis and Characterization of SiOH-Functionalized Polymer Latexes Using Methacryloxy Propyl Trimethoxysilane in Emulsion Polymerization. Macromolecules 2002, 35, 6185–6191. [Google Scholar] [CrossRef]
- Innocenzi, P.; Falcaro, P.; Grosso, D.; Babonneau, F. Order−Disorder Transitions and Evolution of Silica Structure in Self-Assembled Mesostructured Silica Films Studied through FTIR Spectroscopy. J. Phys. Chem. B 2003, 107, 4711–4717. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yaranova, M.A.; Batueva, I.S.; Bal’zhinimaev, B.S. A FTIR Study of Silanol Nests over Mesoporous Silicate Materials. Microporous Mesoporous Mater. 2019, 288, 109582. [Google Scholar] [CrossRef]
- Kanani, D.M.; Fissell, W.H.; Roy, S.; Dubnisheva, A.; Fleischman, A.; Zydney, A.L. Permeability–Selectivity Analysis for Ultrafiltration: Effect of Pore Geometry. J. Membr. Sci. 2010, 349, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, L.; Zhang, X.; Huang, H. Temporal Evolution of the Selectivity-Permeability Relationship during Porous Membrane Filtration of Protein Solutions. J. Membr. Sci. 2016, 514, 385–397. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. Outlining the Roles of Membrane-Foulant and Foulant-Foulant Interactions in Organic Fouling During Microfiltration and Ultrafiltration: A Mini-Review. Front. Chem. 2020, 8, 417. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Cheng, Z.; Lau, C.H.; Ma, J.; Shao, L. Surface Manipulation for Prevention of Migratory Viscous Crude Oil Fouling in Superhydrophilic Membranes. Nat. Commun. 2023, 14, 2679. [Google Scholar] [CrossRef]
- Yuan, S.; Li, J.; Zhu, J.; Volodine, A.; Li, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Hydrophilic Nanofiltration Membranes with Reduced Humic Acid Fouling Fabricated from Copolymers Designed by Introducing Carboxyl Groups in the Pendant Benzene Ring. J. Membr. Sci. 2018, 563, 655–663. [Google Scholar] [CrossRef]
- Shallan, A.; Guijt, R.; Breadmore, M. Capillary Electrophoresis: Basic Principles. In Encyclopedia of Forensic Sciences, 2nd ed.; Siegel, J.A., Saukko, P.J., Houck, M.M., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 549–559. ISBN 978-0-12-382166-9. [Google Scholar]
- Bao, Y.; Wang, B.; Du, C.; Shi, Q.; Xu, W.; Wang, Z. 2D Nano-Mica Sheets Assembled Membranes for High-Efficiency Oil/Water Separation. Nanomaterials 2022, 12, 2895. [Google Scholar] [CrossRef]





| Membrane | Flux (L m−2 h−1 bar−1) | Oil Removal Rate | Fouling | Stability | Ref |
|---|---|---|---|---|---|
| Nano-mica-coated PVDF membrane | ~720 | 99.5% | - | - | [49] |
| Silica-nanoparticle-modified polysulfone membrane | 400–1000 | - | flux recovery 55–75% after BSA | [27] | |
| Polyethyleneimine-modified glass fiber membrane | 900–1000 | 99.7% | - | sustained chemical solvent and boiling treatment | [17] |
| Silica & PDMS-coated glass fiber membrane | ~10,000 | - | - | - | [21] |
| Silica-nanocoated glass fiber membrane | 26,445–25,747 | 99.2–99.4% | flux recovery 79.3% after BSA | sustained chemical solvent and boiling treatment | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Huang, C.; Mao, Y. Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation. Membranes 2025, 15, 41. https://doi.org/10.3390/membranes15020041
Zhu M, Huang C, Mao Y. Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation. Membranes. 2025; 15(2):41. https://doi.org/10.3390/membranes15020041
Chicago/Turabian StyleZhu, Mengfan, Chengqian Huang, and Yu Mao. 2025. "Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation" Membranes 15, no. 2: 41. https://doi.org/10.3390/membranes15020041
APA StyleZhu, M., Huang, C., & Mao, Y. (2025). Silica-Nanocoated Membranes with Enhanced Stability and Antifouling Performance for Oil-Water Emulsion Separation. Membranes, 15(2), 41. https://doi.org/10.3390/membranes15020041






