The Development and Analysis of a Preliminary Electrodialysis Process for the Purification of Complex Lithium Solutions for the Production of Li2CO3 and LiOH
Abstract
:1. Introduction
2. Materials and Methods
- First Stage: The objective was to concentrate the lithium-rich solution obtained from DLE to 1% Li by weight. This was achieved by an evaporation concentration process where the brines PC1 and LC1 were obtained, referred to as the initial solutions P1 and L1 after concentration.
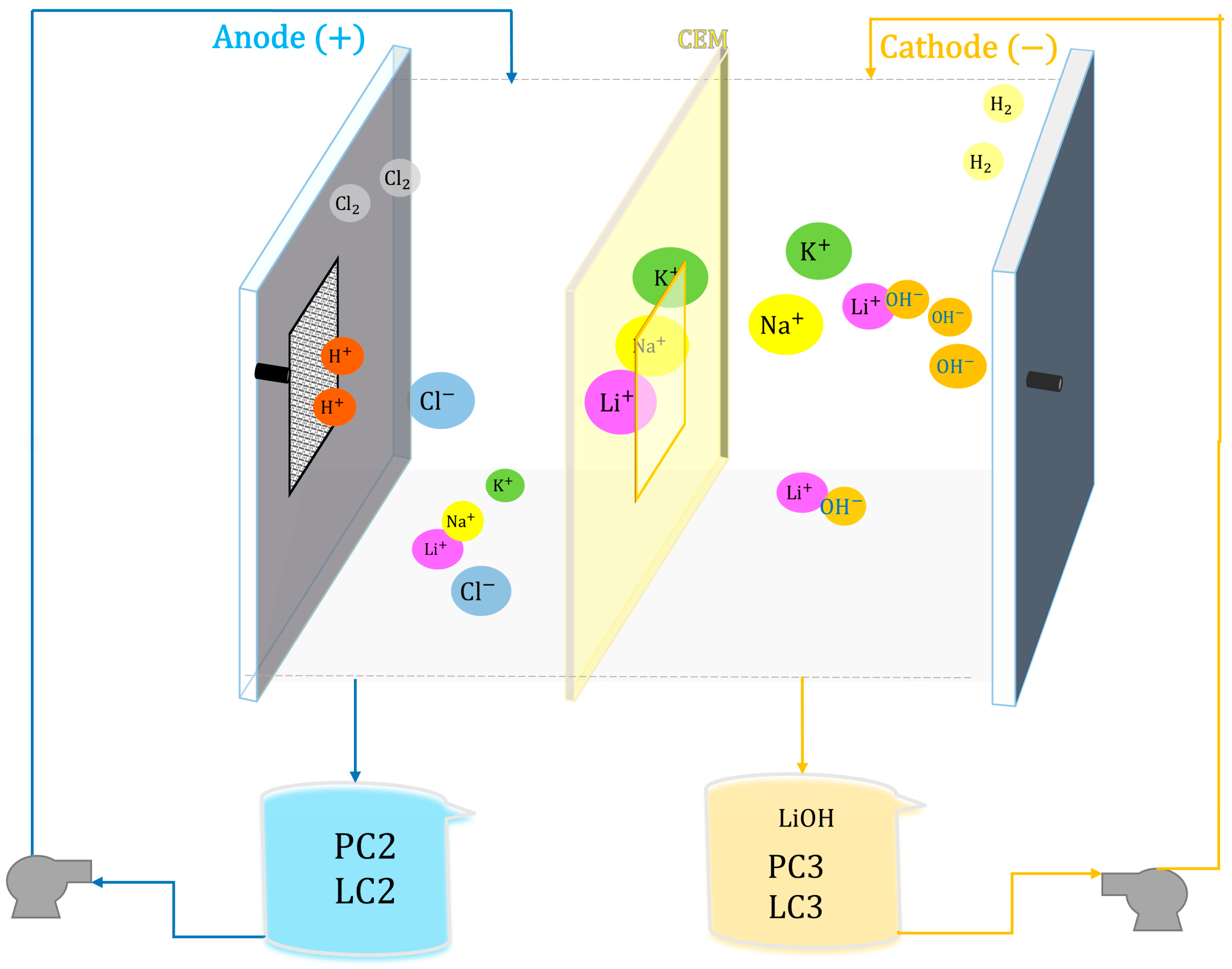
- Second Stage: A membrane electrodialysis process was used to alkalinize the acid solution, studying the removal of divalent cations (Mg2⁺ and Ca2⁺) by precipitation of Mg(OH)2 and Ca(OH)2. As a result, the brines PC2 and LC2 were obtained.
- Third Stage: The best result from the second stage was further worked with, evaluating the selectivity of the Na⁺ and K⁺ impurities still present alongside the Li⁺ ion. A commercial cationic membrane Nafion 117 (DuPont Co., Wilmington, DE, USA) and a monovalent selective cationic membrane Neosepta CMS (ASTOM Co., Tokyo, Japan) were used in a membrane electrolytic reactor prototype. The LiCl feed solution (PC2 and LC2) would serve as the anolyte, and the resulting LiOH solution would serve as the catholyte (PC3 and LC3), allowing for the evaluation of Li recovery and specific energy consumption.
2.1. Concentration by Evaporation
2.2. Electrochemical Alkalinization Process
2.3. Membrane Electrolysis Process
Statistical Analysis
3. Results
3.1. Concentration of Solutions
3.2. Electrodialysis Process for Mg and Ca Removal
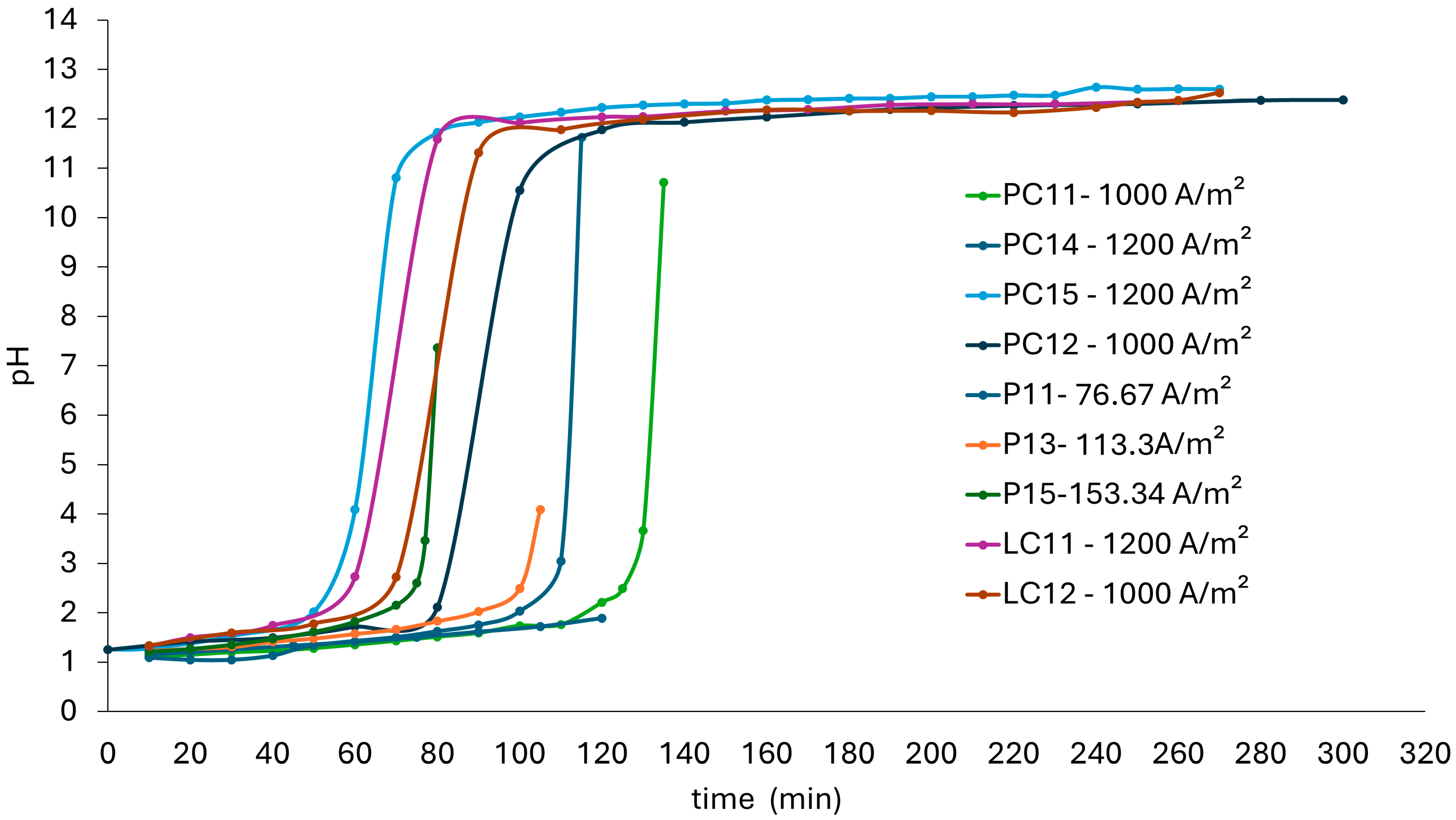
3.2.1. pH Evolution in Electrodialysis Process
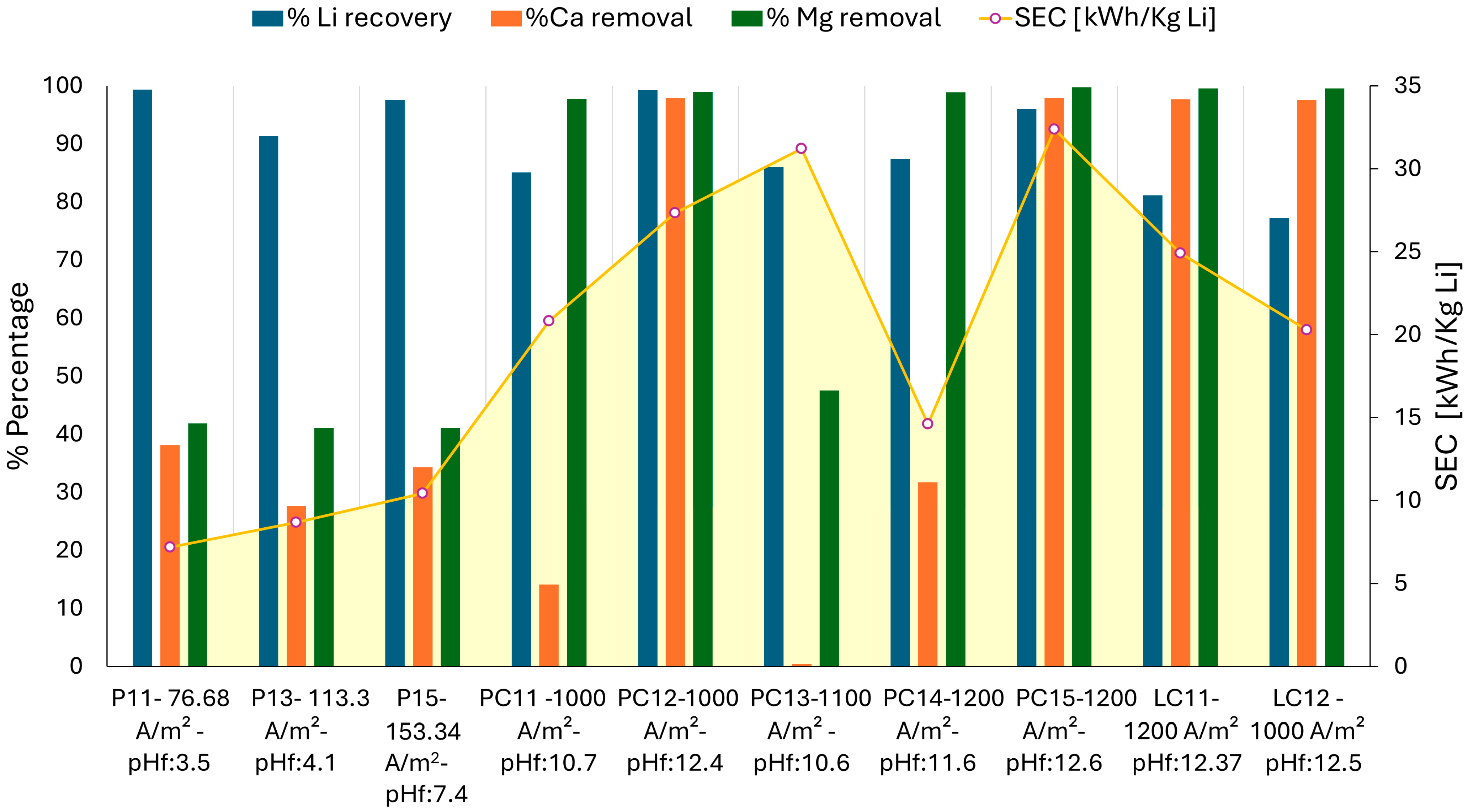
3.2.2. Li Recovery and Specific Energy Consumption
3.3. Membrane Electrolysis Process Results
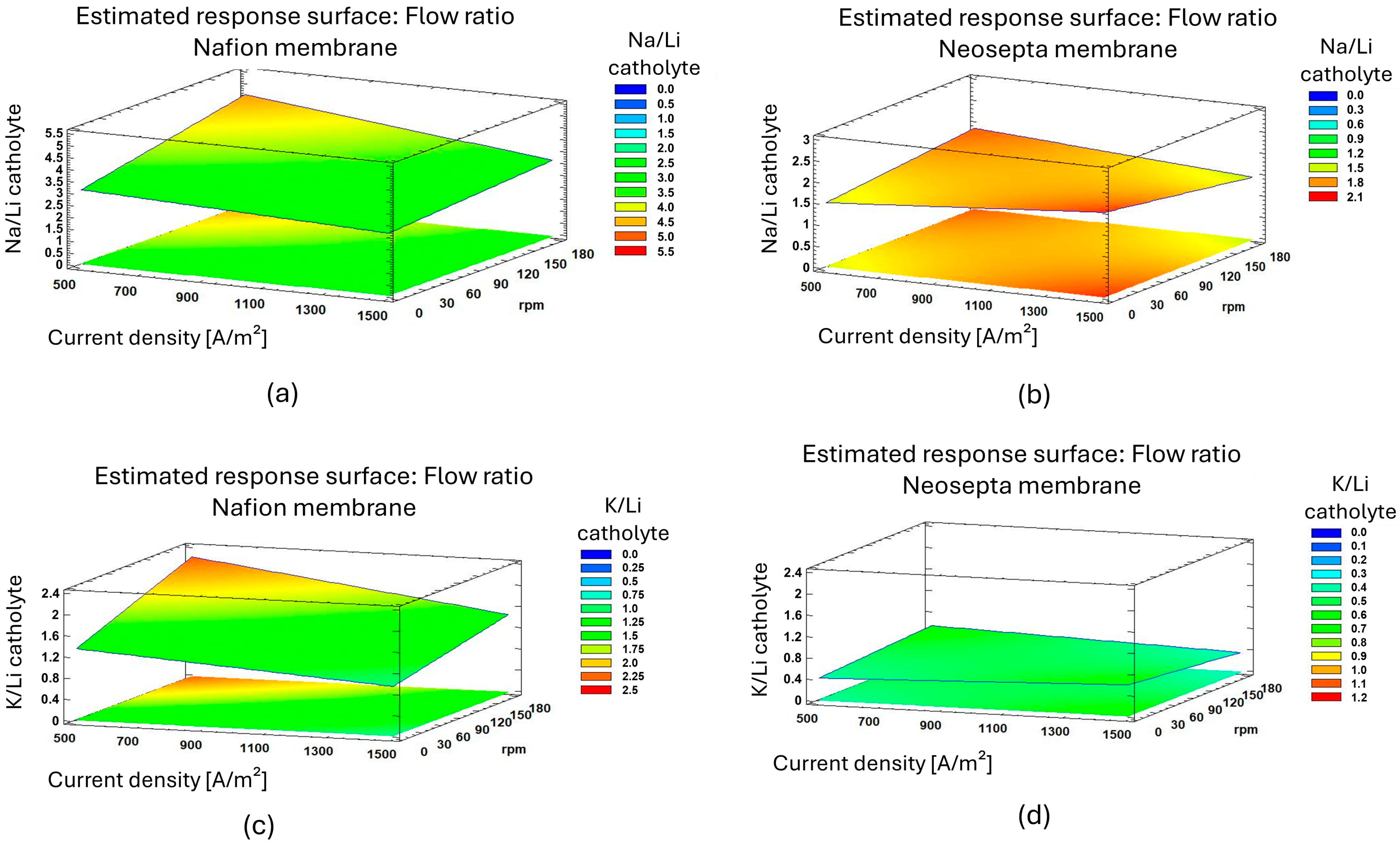
3.3.1. Selectivity and Migration Rate Selectivity of Na+, K+ and Li+
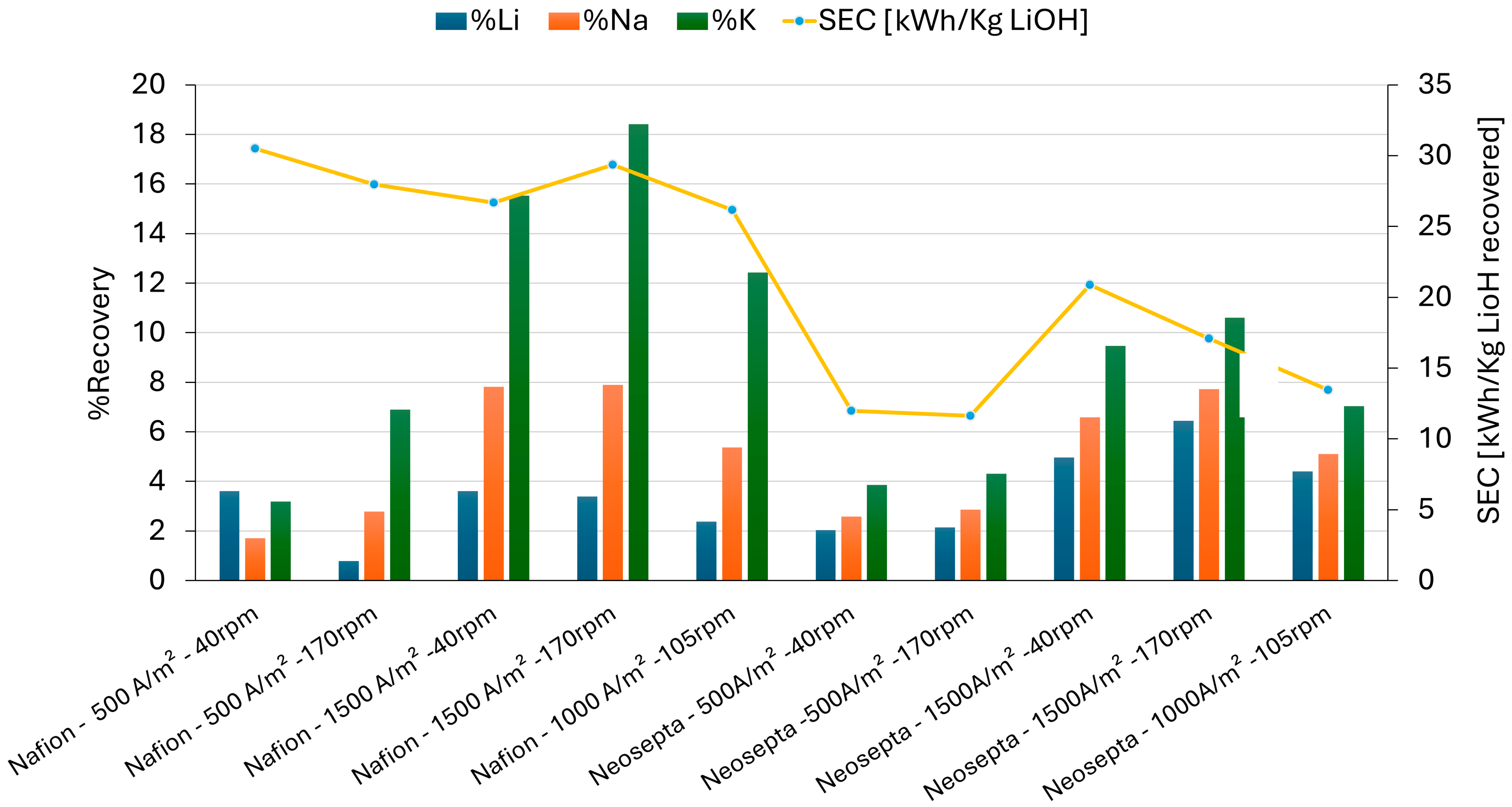
3.3.2. Percentage of Recovery and Electric Power Consumption
3.3.3. Statistical Analysis Results
3.4. Challenges and Future Prospects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, G.E. Alternativas de Extracción de Litio Para Salmueras de SQM Salar. Bachelor’s Thesis, Universidad de Concepción, Concepción, Chile, November 2018. [Google Scholar]
- SQM. Sustainability of Lithium Production in Chile. Available online: https://sqm.com/wp-content/uploads/2020/09/SQM_-_Sustainable_Lithium_-_English.pdf (accessed on 30 December 2024).
- Baudino, L.; Santos, C.; Pirri, C.F.; La Mantia, F.; Lamberti, A. Recent Advances in the Lithium Recovery from Water Resources: From Passive to Electrochemical Methods. Adv. Sci. 2022, 9, e2201380. [Google Scholar] [CrossRef] [PubMed]
- Dahlkamp, J.M.; Quintero, C.; Videla, A.; Rojas, R. Production processes for LiOH—A review. Hydrometallurgy 2023, 223, 106217. [Google Scholar] [CrossRef]
- Direct Lithium Extraction—Cleantech Lithium. 2024. Available online: https://ctlithium.com/about/direct-lithium-extraction (accessed on 26 December 2024).
- Villarroel, N.R.T.; Prat, U.A.; Albornoz, D.A.T.; de Chile, S.Q.Y.M. La Fuerza del Litio; SQM & Universidad Arturo Prat: Iquique, Chile, 2023; pp. 77–79. [Google Scholar] [CrossRef]
- Traviss, M. Chile Switches to Direct Lithium Extraction, Innovation News Network. 2023. Available online: https://www.innovationnewsnetwork.com/chile-switches-direct-lithium-extraction/33261/ (accessed on 26 December 2024).
- Farahbakhsh, J.; Arshadi, F.; Mofidi, Z.; Mohseni-Dargah, M.; Kök, C.; Assefi, M.; Soozanipour, A.; Zargar, M.; Asadnia, M.; Boroumand, Y.; et al. Direct lithium extraction: A new paradigm for lithium production and resource utilization. Desalination 2023, 575, 117249. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Nicolaci, H.; Young, P.; Bailey, E.; Chen, T.; Lin, Y.; Snowdon, N.; Zhang, J.; Rai, A.; Zheng, N.; Shi, R. Direct Lithium Extraction: A Potential Game Changing Technology. Goldman Sachs. Available online: https://www.goldmansachs.com/pdfs/insights/pages/gs-research/direct-lithium-extraction/report.pdf (accessed on 30 December 2024).
- Yuan, H.; Li, M.; Cui, L.; Wang, L.; Cheng, F. Electrochemical extraction technologies of lithium: Development and challenges. Desalination 2024, 598, 118419. [Google Scholar] [CrossRef]
- Harrison, S.; Blanchet, R. Proceso Para la Preparación de Carbonato de Litio de Alta Pureza. ES2475740T3, 26 March 2014. [Google Scholar]
- Grageda, M.; Gonzalez, A.; Quispe, A.; Ushak, S. Analysis of a process for producing battery grade lithium hydroxide by membrane electrodialysis. Membranes 2020, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Grágeda, M.; Quispe, A.; Ushak, S.; Sistat, P.; Cretin, M. Application and analysis of bipolar membrane electrodialysis for LIOH production at high electrolyte concentrations: Current scope and challenges. Membranes 2021, 11, 575. [Google Scholar] [CrossRef]
- Comportamiento FISICOQUÍMICO, Ventajas y Desventajas, de los Métodos: Tradicional Y Separativos de Innovación Tecnológica para la Extracción Directa Del Litio (EDL), a Partir de Salmueras Freáticas (no date) Revista Tecnológica. Available online: http://revistasbolivianas.umsa.bo/scielo.php?script=sci_arttext&pid=S1729-75322022000100005&lng=es&nrm=iso (accessed on 26 December 2024).
- Boroumand, Y.; Razmjou, A. Adsorption-type aluminium-based direct lithium extraction: The effect of heat, salinity and lithium content. Desalination 2024, 577, 117406. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ Concentration Aqueous Solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Z.; Li, H.; Ni, B. Advanced lithium ion-sieves for sustainable lithium recovery from brines. Sustain. Horiz. 2024, 9, 100093. [Google Scholar] [CrossRef]
- Kölbel, L.; Kölbel, T.; Herrmann, L.; Kaymakci, E.; Ghergut, I.; Poirel, A.; Schneider, J. Lithium extraction from geothermal brines in the Upper Rhine Graben: A case study of potential and current state of the art. Hydrometallurgy 2023, 221, 106131. [Google Scholar] [CrossRef]
- Al-Absi, R.S.; Abu-Dieyeh, M.H.; Ben-Hamadou, R.; Nasser, M.S.; Al-Ghouti, M.A. Novel composite materials of modified roasted date pits using ferrocyanides for the recovery of lithium ions from seawater reverse osmosis brine. Sci. Rep. 2021, 11, 18896. [Google Scholar] [CrossRef]
- Barksdale, A.C.; Yoon, J.; Kwon, H.J.; Han, J. Refinement of brine for lithium extraction using ion concentration polarization. Sep. Purif. Technol. 2021, 282, 120055. [Google Scholar] [CrossRef]
- Pell, R.; Tijsseling, L.; Goodenough, K.; Wall, F.; Dehaine, Q.; Grant, A.; Deak, D.; Yan, X.; Whattoff, P. Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Environ. 2021, 2, 665–679. [Google Scholar] [CrossRef]
- Zavahir, S.; Elmakki, T.; Gulied, M.; Ahmad, Z.; Al-Sulaiti, L.; Shon, H.K.; Chen, Y.; Park, H.; Batchelor, B.; Han, D.S. A review on lithium recovery using electrochemical capturing systems. Desalination 2020, 500, 114883. [Google Scholar] [CrossRef]
- Tran, K.T.; Van Luong, T.; An, J.; Kang, D.; Kim, M.; Tran, T. Recovery of magnesium from Uyuni salar brine as high purity magnesium oxalate. Hydrometallurgy 2013, 138, 93–99. [Google Scholar] [CrossRef]
- Firdiyono, F.; Lalasari, L.H.; Tarmizi, E.; Sulistiyono, E.; Andriyah, L.; Arini, T.; Natasha, N.C.; Yunita, F.E. The Degree of Lithium (Li) Stability Compared To Calcium (Ca) and Magnesium (Mg) From Low Lithium Grade Brine Water with Addition of Limestone and Oxalic Acid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012044. [Google Scholar] [CrossRef]
- Grágeda, M.; González, A.; Grágeda, M.; Ushak, S. Purification of brines by chemical precipitation and ion-exchange processes for obtaining battery-grade lithium compounds. Int. J. Energy Res. 2018, 42, 2386–2399. [Google Scholar] [CrossRef]
- Zhao, Z.; Si, X.; Liu, X.; He, L.; Liang, X. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials. Hydrometallurgy 2012, 133, 75–83. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zhao, Z.; Liang, X. Effect of Na+ on Li extraction from brine using LiFePO4/FePO4 electrodes. Hydrometallurgy 2014, 146, 24–28. [Google Scholar] [CrossRef]
- Xu, W.; Liu, D.; He, L.; Zhao, Z. A Comprehensive Membrane Process for Preparing Lithium Carbonate from High Mg/Li Brine. Membranes 2020, 10, 371. [Google Scholar] [CrossRef]
- Ji, Z.; Chen, Q.; Yuan, J.; Liu, J.; Zhao, Y.; Feng, W. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis. Sep. Purif. Technol. 2016, 172, 168–177. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, Z.; Liu, J.; Zhao, Y.; Wang, S.; Yuan, J. Development of recovering lithium from brines by selective-electrodialysis: Effect of coexisting cations on the migration of lithium. J. Membr. Sci. 2017, 548, 408–420. [Google Scholar] [CrossRef]
- Nieto CH, D.; Palacios, N.A.; Verbeeck, K.; Prévoteau, A.; Rabaey, K.; Flexer, V. Membrane electrolysis for the removal of Mg2+ and Ca2+ from lithium rich brines. Water Res. 2019, 154, 117–124. [Google Scholar] [CrossRef]
- Pan, X.; Dou, Z.; Meng, D.; Han, X.; Zhang, T. Electrochemical separation of magnesium from solutions of magnesium and lithium chloride. Hydrometallurgy 2019, 191, 105166. [Google Scholar] [CrossRef]
- Grágeda, M.; González, A.; Alavia, W.; Ushak, S. Development and optimization of a modified process for producing the battery grade LiOH: Optimization of energy and water consumption. Energy 2015, 89, 667–677. [Google Scholar] [CrossRef]
- Semerjian, L.; Ayoub, G. High-pH–magnesium coagulation–flocculation in wastewater treatment. Adv. Environ. Res. 2003, 7, 389–403. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation exchange membranes and process optimizations in electrodialysis for selective metal separation: A review. Membranes 2023, 13, 566. [Google Scholar] [CrossRef]
- Rottiers, T.; De Staelen, J.; Van Der Bruggen, B.; Pinoy, L. Permselectivity of cation-exchange membranes between different cations in aqueous alcohol mixtures. Electrochim. Acta 2016, 192, 489–496. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- García-Nieto, D.; Barragán, V.M. A comparative study of the electro-osmotic behavior of cation and anion exchange membranes in alcohol-water media. Electrochim. Acta 2014, 154, 166–176. [Google Scholar] [CrossRef]
- Stenina, I.; Sistat, P.; Rebrov, A.; Pourcelly, G.; Yaroslavtsev, A. Ion mobility in Nafion-117 membranes. Desalination 2004, 170, 49–57. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Golubenko, D.; Shevlyakova, N.; D’yakova, M.; Tverskoi, V.; Dammak, L.; Grande, D.; Yaroslavtsev, A. New cation-exchange membranes based on cross-linked sulfonated polystyrene and polyethylene for power generation systems. J. Membr. Sci. 2016, 515, 196–203. [Google Scholar] [CrossRef]
- Tuan, L.X.; Buess-Herman, C. Study of water content and microheterogeneity of CMS cation exchange membrane. Chem. Phys. Lett. 2006, 434, 49–55. [Google Scholar] [CrossRef]
- Firdaous, L.; Malériat, J.; Schlumpf, J.; Quéméneur, F. Transfer of monovalent and divalent cations in salt solutions by electrodialysis. Sep. Sci. Technol. 2007, 42, 931–948. [Google Scholar] [CrossRef]
- Ozkul, S.; Arbabzadeh, O.; Bisselink, R.; Kuipers, N.; Bruning, H.; Rijnaarts, H.; Dykstra, J. Selective adsorption in ion exchange membranes: The effect of solution ion composition on ion partitioning. Water Res. 2024, 254, 121382. [Google Scholar] [CrossRef]
- Saracco, G. Transport properties of monovalent-ion-permselective membranes. Chem. Eng. Sci. 1997, 52, 3019–3031. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Li, R.; Pan, W. A review for Ca(OH)2/CaO thermochemical energy storage systems. J. Energy Storage 2022, 50, 104612. [Google Scholar] [CrossRef]
- Gupta, A.; Armatis, P.D.; Sabharwall, P.; Fronk, B.M.; Utgikar, V. Thermodynamics of Ca(OH)2/CaO reversible reaction: Refinement of reaction equilibrium and implications for operation of chemical heat pump. Chem. Eng. Sci. 2020, 230, 116227. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Li, X. Research on the process, energy consumption and carbon emissions of different magnesium refining processes. Materials 2023, 16, 3340. [Google Scholar] [CrossRef]
- Ma, R.; Baradwaj, N.; Nomura, K.; Krishnamoorthy, A.; Kalia, R.K.; Nakano, A.; Vashishta, P. Alkali hydroxide (LiOH, NaOH, KOH) in water: Structural and vibrational properties, including neutron scattering results. J. Chem. Phys. 2024, 160, 134309. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Du, F.; Stolte, S.; Liu, X.; Wang, Y. Continuous Polarizability-Based separation of lithium iron phosphate and graphite using a dielectrophoretic particle separator. Langmuir 2025, 41, 1459–1468. [Google Scholar] [CrossRef]



| Solution | pH | %Li | %Na | %K | %Mg | %Ca | %LiCl | Na/Li | K/Li | Mg/Li | Ca/Li |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 1.2 | 0.076 | 0.318 | 0.095 | 0.007 | 0.006 | 0.47 | 4.184 | 1.25 | 0.095 | 0.075 |
| L1 | 1.3 | 0.055 | 0.002 | 0.004 | 0.002 | 0.006 | 0.34 | 0.04 | 0.074 | 0.038 | 0.109 |
| Experiment Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| P1 | 77 | 77 | 113 | 153 | 153 |
| PC1 | 1000 | 1000 | 1100 | 1200 | 1200 |
| LC1 | 1000 | 1000 | 1100 | 1200 | 1200 |
| N° Exp | Solution | Current Density (A/m2) | rpm | Membrane |
|---|---|---|---|---|
| 1 | PC2 | 500 | 40 | Nafion 117 |
| 2 | PC2 | 1500 | 40 | Nafion 117 |
| 3 | PC2 | 500 | 170 | Nafion 117 |
| 4 | PC2 | 1500 | 170 | Nafion 117 |
| 5 | PC2 | 1000 | 105 | Nafion 117 |
| 6 | PC2 | 500 | 40 | Neosepta CMS |
| 7 | PC2 | 1500 | 40 | Neosepta CMS |
| 8 | PC2 | 500 | 170 | Neosepta CMS |
| 9 | PC2 | 1500 | 170 | Neosepta CMS |
| 10 | PC2 | 1000 | 105 | Neosepta CMS |
| %Li | %Na | %K | %Mg | %Ca | |
|---|---|---|---|---|---|
| PC1 | 1.050 | 3.820 | 1.320 | 0.088 | 0.070 |
| LC1 | 1.144 | 0.047 | 0.085 | 0.044 | 0.126 |
| Solution | pH | %Li | %Na | %K | %LiCl | Na/Li | K/Li |
|---|---|---|---|---|---|---|---|
| PC2 | 12.5 | 1.05 | 3.82 | 1.32 | 6.41 | 3.64 | 1.26 |
| LC2 | 12 | 1.14 | 0.05 | 0.09 | 6.99 | 0.04 | 0.07 |
| Results for Ratio Na/Li | Results for Ratio K/Li | |||||
|---|---|---|---|---|---|---|
| Effect | Estimate | Stnd. Error | V.I.F. | Estimate | Stnd. Error | V.I.F. |
| Average ratio Na/Li | 7.65222 | 0.788233 | 4.99781 | 0.615292 | ||
| A: Current Density | 0.508602 | 1.76254 | 1.0 | −0.0650875 | 1.37584 | 1.0 |
| B: Flow | 3.5183 | 1.76254 | 1.0 | 3.26524 | 1.37584 | 1.0 |
| C: Type of Membrane | −3.99172 | 1.57647 | 1.0 | −4.60042 | 1.23058 | 1.0 |
| AB | −3.43075 | 1.76254 | 1.0 | −2.61201 | 1.37584 | 1.0 |
| AC | −0.668897 | 1.76254 | 1.0 | −0.196738 | 1.37584 | 1.0 |
| BC | −3.6652 | 1.76254 | 1.0 | −3.37461 | 1.37584 | 1.0 |
| Initial Solution | Unit | Evaporation | Alkalinization (Electrodialysis) | Membrane Electrolysis |
|---|---|---|---|---|
| L1 | kWh/kg Li | 52.45 (LC1) | 20.28 (LC12) | 22.21 (LC3) |
| P1 | kWh/kg Li | 52.38 (PC1) | 27.33 (PC12) | 33.46 (PC3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, A.; Choque, G.; Grágeda, M.; Ushak, S. The Development and Analysis of a Preliminary Electrodialysis Process for the Purification of Complex Lithium Solutions for the Production of Li2CO3 and LiOH. Membranes 2025, 15, 50. https://doi.org/10.3390/membranes15020050
González A, Choque G, Grágeda M, Ushak S. The Development and Analysis of a Preliminary Electrodialysis Process for the Purification of Complex Lithium Solutions for the Production of Li2CO3 and LiOH. Membranes. 2025; 15(2):50. https://doi.org/10.3390/membranes15020050
Chicago/Turabian StyleGonzález, Alonso, Geovanna Choque, Mario Grágeda, and Svetlana Ushak. 2025. "The Development and Analysis of a Preliminary Electrodialysis Process for the Purification of Complex Lithium Solutions for the Production of Li2CO3 and LiOH" Membranes 15, no. 2: 50. https://doi.org/10.3390/membranes15020050
APA StyleGonzález, A., Choque, G., Grágeda, M., & Ushak, S. (2025). The Development and Analysis of a Preliminary Electrodialysis Process for the Purification of Complex Lithium Solutions for the Production of Li2CO3 and LiOH. Membranes, 15(2), 50. https://doi.org/10.3390/membranes15020050






