The Nexus Between Sperm Membrane Integrity, Sperm Motility, and DNA Fragmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Experimental Design
2.2. Statistical Analysis
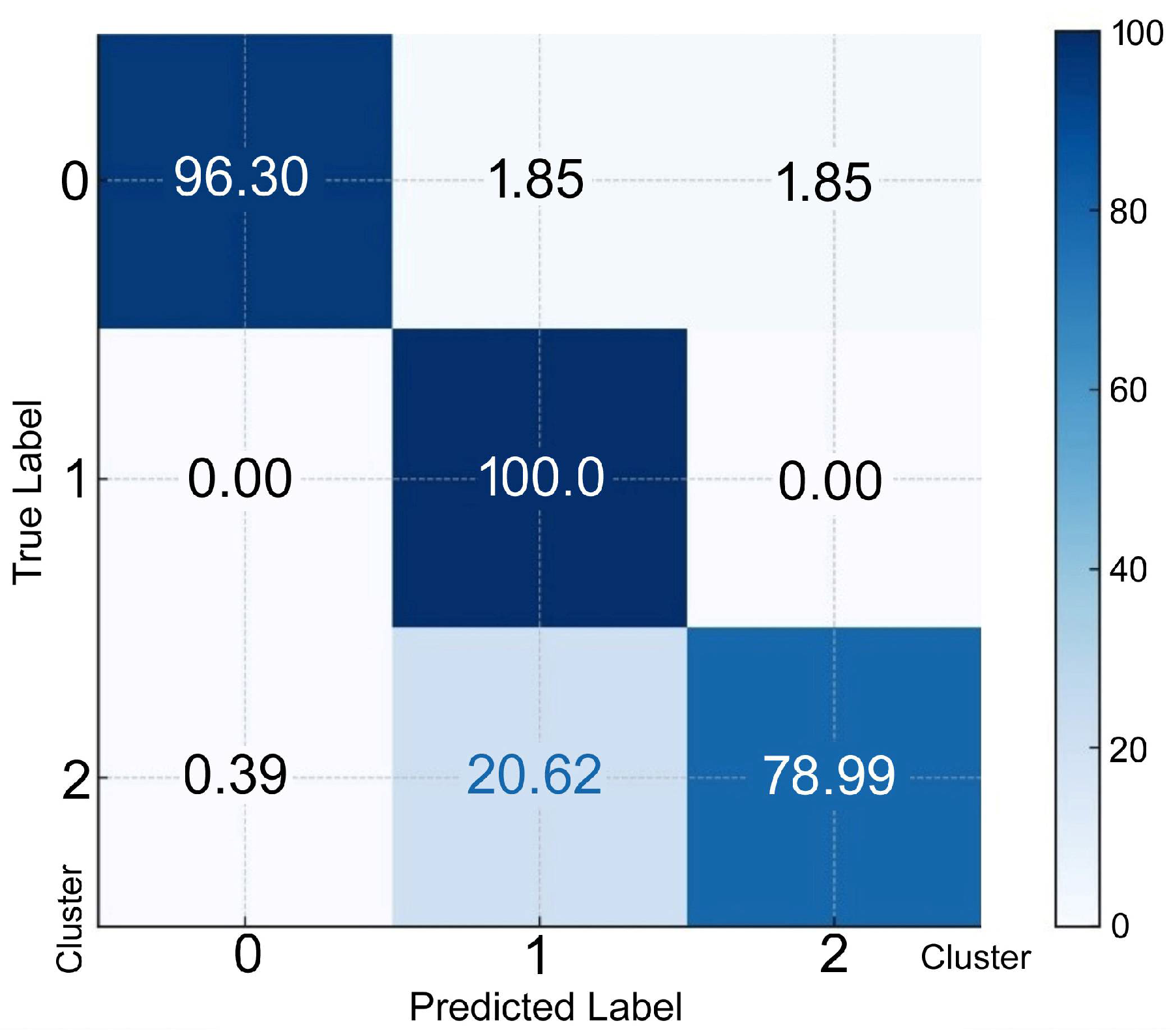
3. Results
4. Discussion
Towards a Holistic View of the Seminogram
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/343208 (accessed on 25 December 2024).
- Love, C.C.; Thompson, J.A.; Brinsko, S.P.; Rigby, S.L.; Blanchard, T.L.; Lowry, V.K.; Varner, D.D. Relationship between stallion sperm motility and viability as detected by two fluorescence staining techniques using flow cytometry. Theriogenology 2003, 60, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Sati, L.; Huszar, G. Sperm motility and viability: Overview of the cellular and physiological aspects that support these functions. EMJ Reprod. Health 2015, 1, 74–80. [Google Scholar] [CrossRef]
- Gosálvez, J.; Cortés-Gutiérrez, E.I.; Nuñez, R.; Fernández, J.L.; Caballero, P.; López-Fernández, C.; Holt, W.V. A dynamic assessment of sperm DNA fragmentation versus sperm viability in proven fertile human donors. Fertil. Steril. 2009, 92, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.M.; Odriozola, A.; Candenas, L.; Subirán, N. The role of sperm membrane potential and ion channels in regulating sperm function. Int. J. Mol. Sci. 2023, 24, 6995. [Google Scholar] [CrossRef]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm lipid markers of male fertility in mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- McNeil, P.L.; Steinhardt, R.A. Plasma membrane disruption: Repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 2003, 19, 697–731. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Nicotera, P. The calcium ion and cell death. J. Neural Transm. Suppl. 1994, 43, 1–11. [Google Scholar] [PubMed]
- Kaltsas, A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Santiso, R.; Tamayo, M.; Gosálvez, J.; Johnston, S.; Mariño, A.; Fernández, C.; Losada, C.; Fernández, J.L. DNA fragmentation dynamics allows the assessment of cryptic sperm damage in human: Evaluation of exposure to ionizing radiation, hyperthermia, acidic pH and nitric oxide. Mutat. Res. 2012, 734, 41–49. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Liang, Z.; Wu, J.; Li, L.; Chen, C.; Jin, F.; Tian, Y. Sperm DNA fragmentation and male fertility: A retrospective study of 5114 men attending a reproductive center. J. Assist. Reprod. Genet. 2021, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, X.; Pan, F.; Chen, Y.; An, R. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci. Rep. 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, J.; Fernández, C.L.; Johnston, S.D.; Bartolomé-Nebreda, J. Role of DNase activity in human sperm DNA fragmentation. Biomolecules 2024, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Axxari, C.; Forti, G.; Baldi, E. Investigation on the origin of sperm DNA fragmentation: Role of apoptosis, immaturity and oxidative stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Cluster Analysis in R: Multivariate Analysis I. STHDA: 2017. Available online: http://www.sthda.com (accessed on 25 December 2024).
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod. Sci. 2022, 29, 7–25. [Google Scholar] [CrossRef]
- du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Vignoli, A.; Tenori, L.; Dall’Acqua, S.; Sut, S.; La Vignera, S.; Condorelli, R.A.; Giacone, F.; et al. Lipidomic profile of human sperm membrane identifies a clustering of lipids associated with semen quality and function. Int. J. Mol. Sci. 2023, 25, 297. [Google Scholar] [CrossRef]
- Foster, M.L.; Varner, D.D.; Hinrichs, K.; Teague, S.; Lacaze, K.; Blanchard, T.L.; Love, C.C. Agreement between measures of total motility and membrane integrity in stallion sperm. Theriogenology 2011, 75, 1499–1505. [Google Scholar] [CrossRef]
- Fraser, L.; Gorszczaruk, K.; Strzezek, J. Relationship between motility and membrane integrity of boar spermatozoa in media varying in osmolality. Reprod. Domest. Anim. 2001, 36, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.; Clapham, D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Aurich, C. Fine feathers make fine birds: The mammalian sperm membrane lipid composition and effects on assisted reproduction. Anim. Reprod. Sci. 2022, 246, 106884. [Google Scholar] [CrossRef]
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm protein carbonylation. Andrologia 2019, 51, e13233. [Google Scholar] [CrossRef]
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle, environmental and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 2011, 78, 951–961. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Omolaoye, V.A.; Kandasamy, R.K.; Hachim, M.Y.; Du Plessis, S.S. Omics and male infertility: Highlighting the application of transcriptomic data. Life 2022, 12, 280. [Google Scholar] [CrossRef]
- Wagner, A.O.; Turk, A.; Kunej, T. Towards a multi-omics of male infertility. World J. Mens. Health 2023, 41, 272–288. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Stavros, S.; Sofikitis, N.; Georgiou, I.; Zachariou, A. Integrative assessment of seminal plasma biomarkers: A narrative review bridging the gap between infertility research and clinical practice. J. Clin. Med. 2024, 13, 3147. [Google Scholar] [CrossRef]
- González-Martínez, M.; Sánchez-Martín, P.; López-Fernández, C.; Johnston, S.D.; Gosálvez, J. The relationship between DNA fragmentation and the intensity of morphologically abnormal human spermatozoa. Asian Pac. J. Reprod. 2024, 13, 22–27. [Google Scholar] [CrossRef]



| Mean | SD | Lower Quartile | Median | Upper Quartile | |
|---|---|---|---|---|---|
| % Immotile | 42.9 | 16.3 | 31 | 40 | 51 |
| % SDPM | 26.1 | 14.7 | 16 | 23 | 33 |
| % SDF | 19.5 | 16.3 | 16 | 15 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góngora, A.; Johnston, S.; Contreras, P.; López-Fernández, C.; Gosálvez, J. The Nexus Between Sperm Membrane Integrity, Sperm Motility, and DNA Fragmentation. Membranes 2025, 15, 109. https://doi.org/10.3390/membranes15040109
Góngora A, Johnston S, Contreras P, López-Fernández C, Gosálvez J. The Nexus Between Sperm Membrane Integrity, Sperm Motility, and DNA Fragmentation. Membranes. 2025; 15(4):109. https://doi.org/10.3390/membranes15040109
Chicago/Turabian StyleGóngora, Alfredo, Stephen Johnston, Pablo Contreras, Carmen López-Fernández, and Jaime Gosálvez. 2025. "The Nexus Between Sperm Membrane Integrity, Sperm Motility, and DNA Fragmentation" Membranes 15, no. 4: 109. https://doi.org/10.3390/membranes15040109
APA StyleGóngora, A., Johnston, S., Contreras, P., López-Fernández, C., & Gosálvez, J. (2025). The Nexus Between Sperm Membrane Integrity, Sperm Motility, and DNA Fragmentation. Membranes, 15(4), 109. https://doi.org/10.3390/membranes15040109






