RF Sputtering of Gold Nanoparticles in Liquid and Direct Transfer to Nafion Membrane for PEM Water Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanofluid
2.3. Fabrication of the Membrane
2.4. Preparation of the Pt/C Ink
2.5. Characterization Methods
2.5.1. Nanofluid
2.5.2. Membrane
2.6. Electrochemical Evaluation
3. Results and Discussion
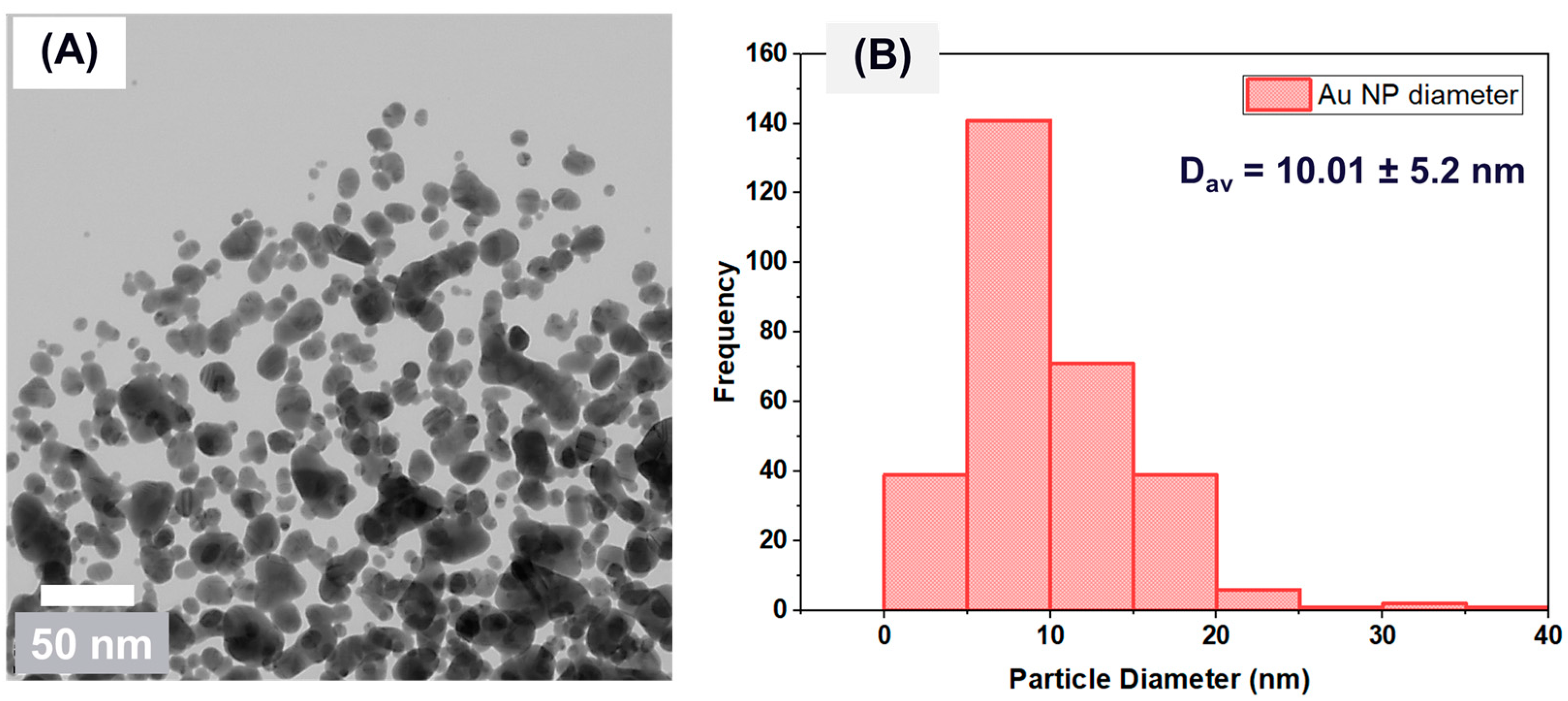
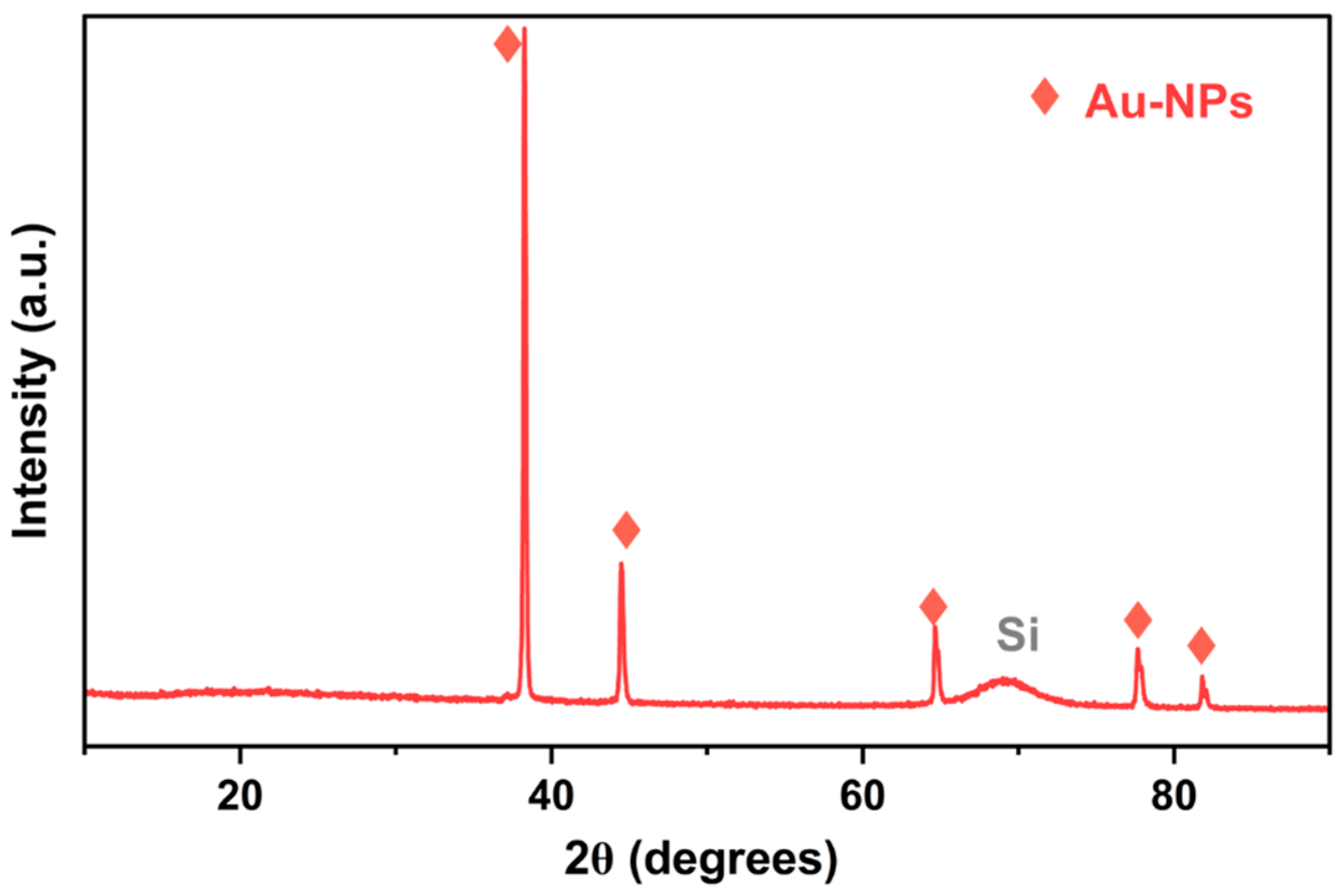
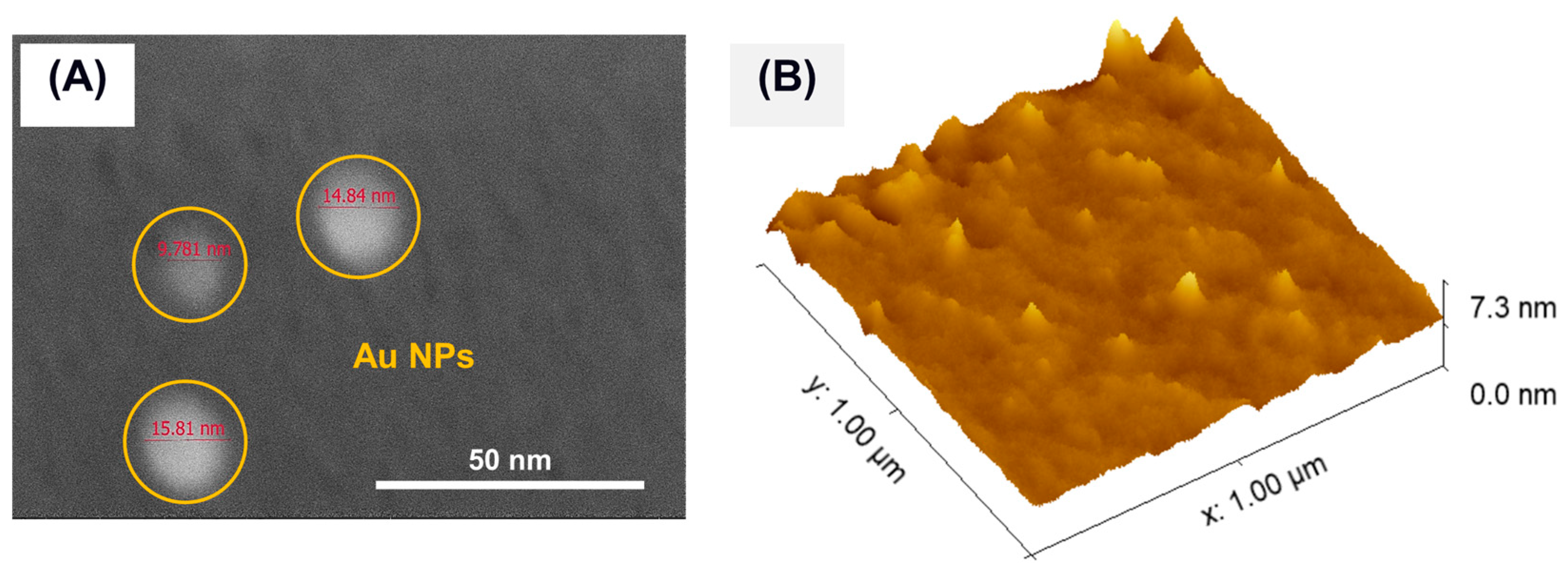
3.1. Nanofluid Analysis
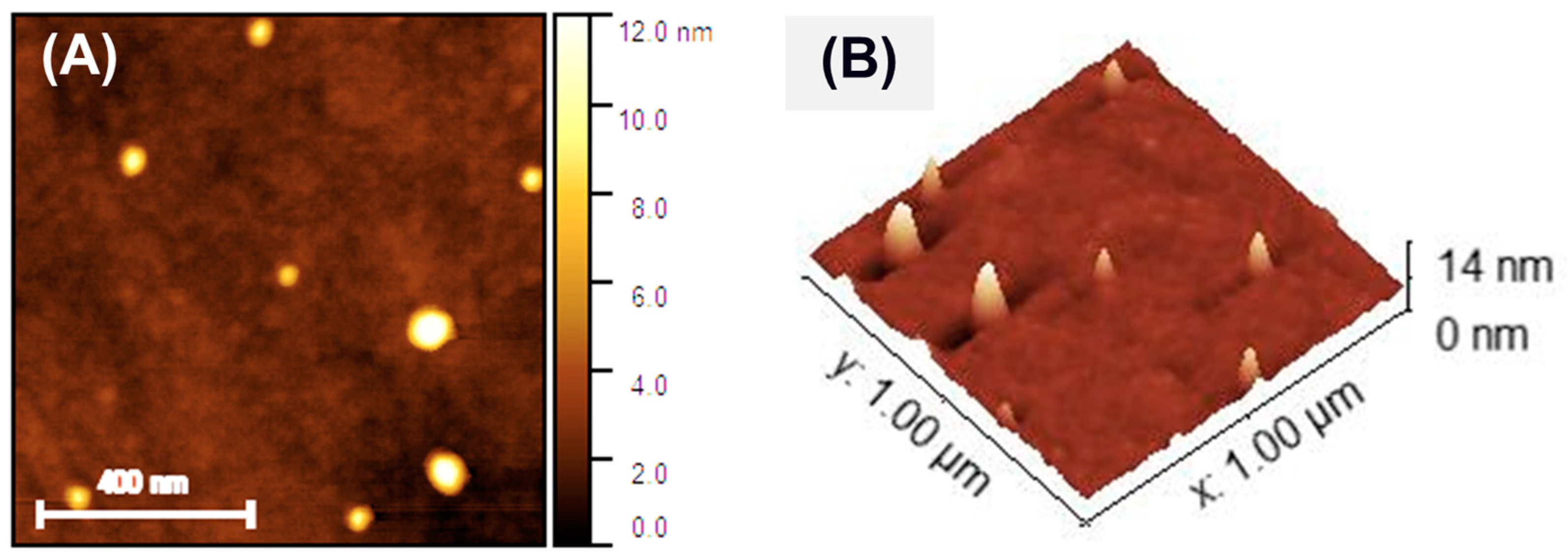
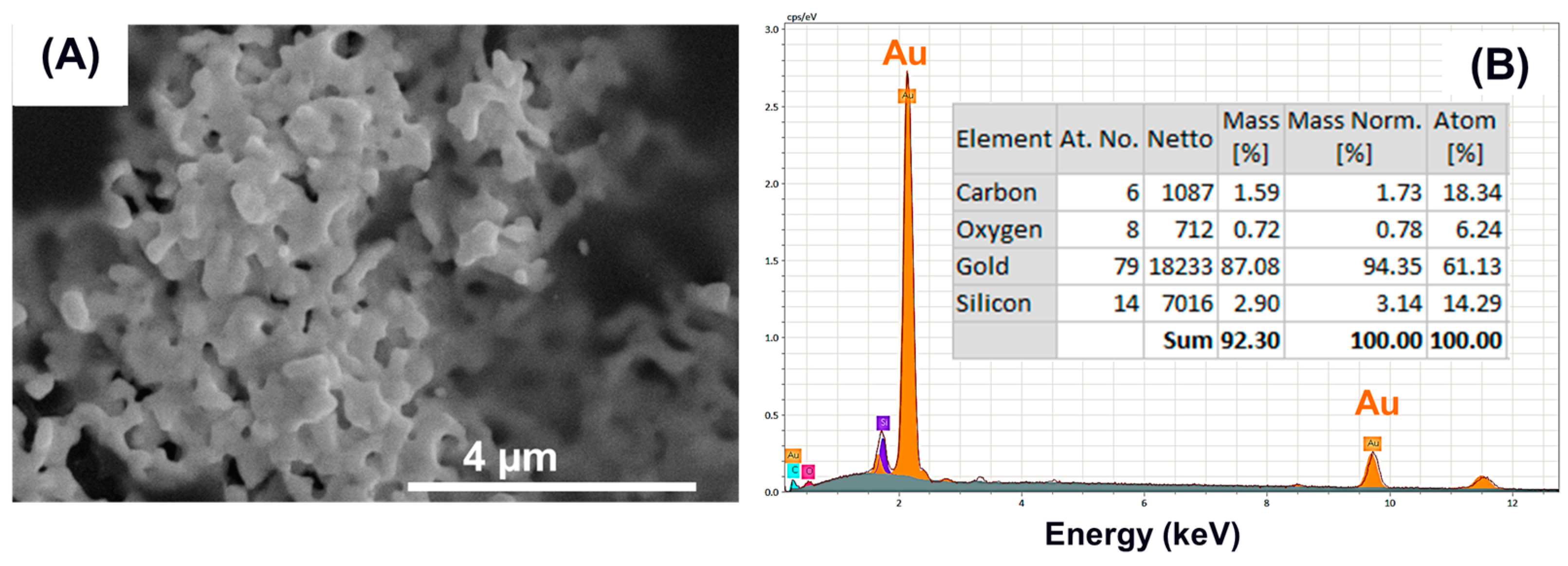
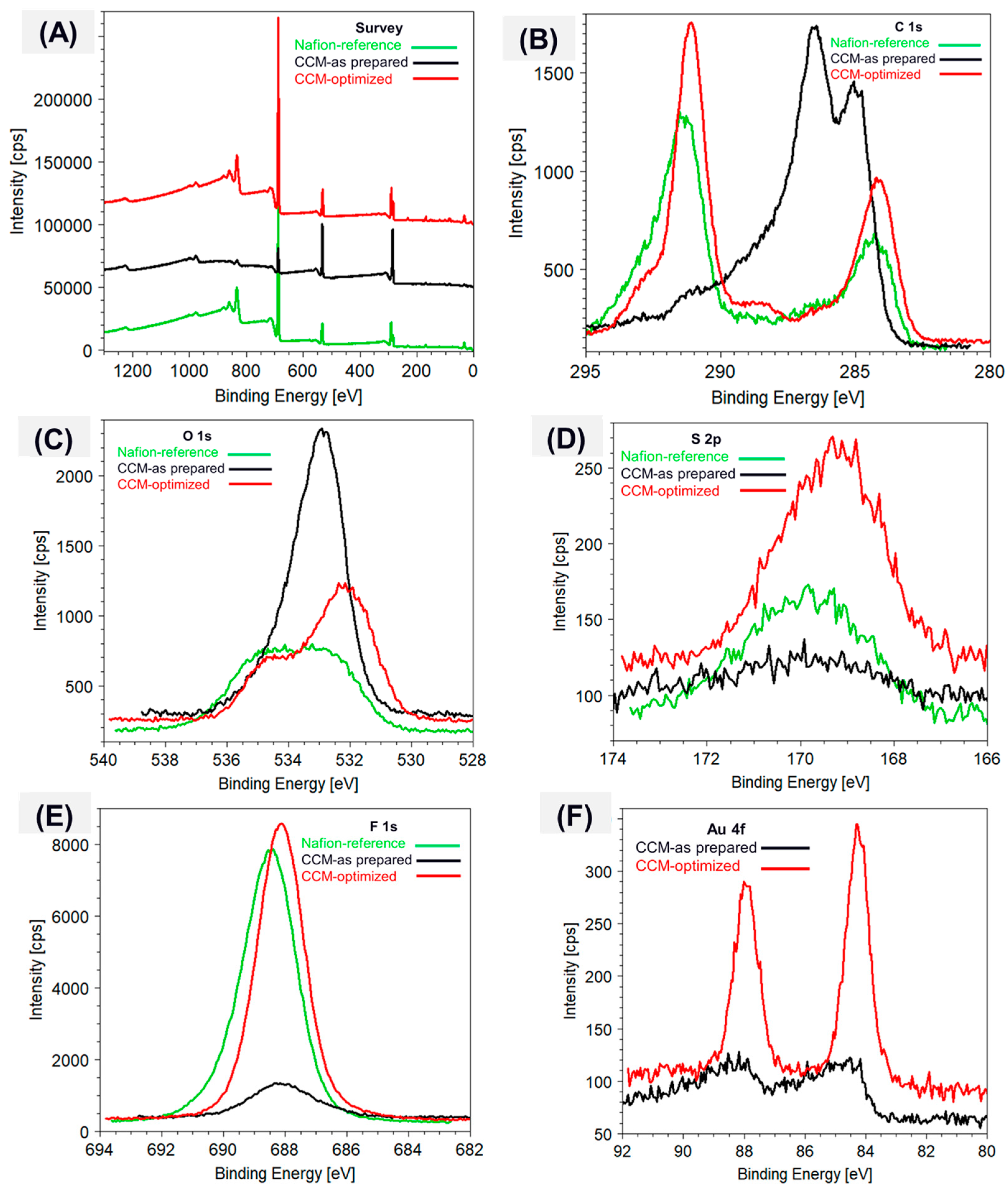
3.2. Nanomembrane Analysis
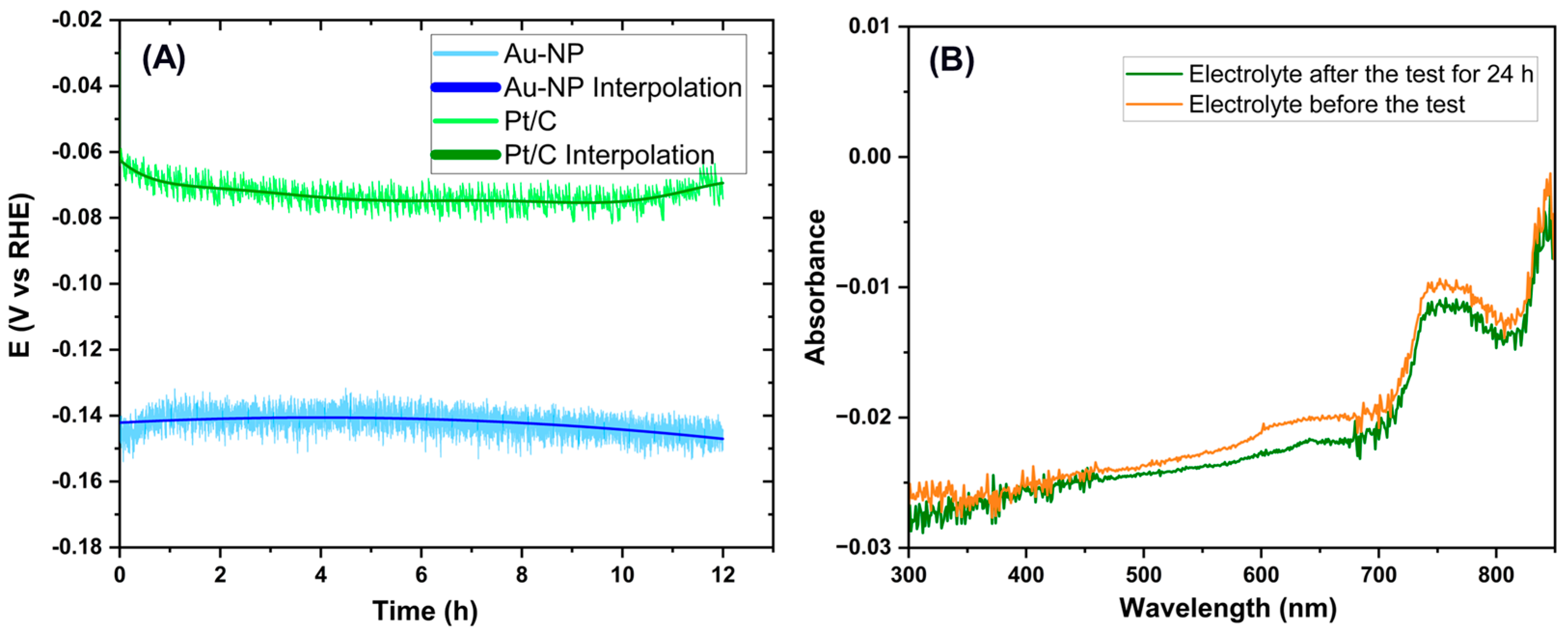
3.3. HER Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCM | Catalysts Coated Membrane |
| HER | Hydrogen Evolution Reaction |
| Au NPs | Gold Nanoparticles |
| PEG | Polyethylene glycol |
| B.E. | Binding Energy |
| PEMWE | Proton Exchange Membrane Water Electrolyzer |
References
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps Between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2023, 6, e12441. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen Production by Water Electrolysis Technologies: A Review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Arsad, S.R.; Arsad, A.Z.; Ker, P.J.; Hannan, M.A.; Tang, S.G.H.; Goh, S.M.; Mahlia, T.M.I. Recent Advancement in Water Electrolysis for Hydrogen Production: A Comprehensive Bibliometric Analysis and Technology Updates. Int. J. Hydrog. Energy 2024, 60, 780–801. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Kim, J.-D. High-Temperature Water Electrolysis Properties of Membrane Electrode Assemblies with Nafion and Crosslinked Sulfonated Polyphenylsulfone Membranes by Using a Decal Method. Membranes 2024, 14, 173. [Google Scholar] [CrossRef]
- Depuydt, S.; Van der Bruggen, B. Green Synthesis of Cation Exchange Membranes: A Review. Membranes 2024, 14, 23. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Lysova, A.A.; Voropaeva, D.Y.; Yaroslavtsev, A.B. Approaches to the Modification of Perfluorosulfonic Acid Membranes. Membranes 2023, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, T.; Meng, X.; He, D.; Li, L.; Song, K.; Jiang, J.; Sun, C. Advances in the Application of Sulfonated Poly(Ether Ether Ketone) (SPEEK) and Its Organic Composite Membranes for Proton Exchange Membrane Fuel Cells (PEMFCs). Polymers 2024, 16, 2840. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochimica Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, K.-Y.; Kim, H.-J.; Cho, E.; Lee, S.-Y.; Lim, T.-H.; Yoon, S.P.; Hwang, I.C.; Jang, J.H. The Effects of Nafion® Ionomer Content in PEMFC MEAs Prepared by a Catalyst-Coated Membrane (CCM) Spraying Method. Int. J. Hydrog. Energy 2010, 35, 2119–2126. [Google Scholar] [CrossRef]
- Vinodh, R.; Palanivel, T.; Sharanappa Kalanur, S.; Pollet, B.G. Recent Advancements in Catalyst Coated Membranes for Water Electrolysis: A Critical Review. Energy Adv. 2024, 3, 1144–1166. [Google Scholar] [CrossRef]
- Liu, C.; Luo, M.; Zeis, R.; Abel Chuang, P.-Y.; Zhang, R.; Du, S.; Sui, P.-C. Fabrication of Catalyst Layer for Proton Exchange Membrane Water Electrolyzer: I. Effects of Dispersion on Particle Size Distribution and Rheological Behavior. Int. J. Hydrog. Energy 2024, 52, 1143–1154. [Google Scholar] [CrossRef]
- Choo, M.-J.; Oh, K.-H.; Kim, H.-T.; Park, J.-K. Modulated Ionomer Distribution in the Catalyst Layer of Polymer Electrolyte Membrane Fuel Cells for High Temperature Operation. ChemSusChem 2014, 7, 2335–2341. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Voropaeva, D.Y.; Safronov, D.V.; Stretton, N.; Parshina, A.V.; Yaroslavtsev, A.B. Correlation between Nafion Morphology in Various Dispersion Liquids and Properties of the Cast Membranes. Membranes 2023, 13, 13. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. Catalyst Coated Membrane Electrodes for the Gas Phase CO2 Electroreduction to Formate. Catal. Today 2020, 346, 58–64. [Google Scholar] [CrossRef]
- Duman, H.; Akdaşçi, E.; Eker, F.; Bechelany, M.; Karav, S. Gold Nanoparticles: Multifunctional Properties, Synthesis, and Future Prospects. Nanomaterials 2024, 14, 1805. [Google Scholar] [CrossRef]
- Alba-Molina, D.; Santiago, A.R.P.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Martín-Romero, M.T.; Camacho, L.; Luque, R.; Cano, M. Tailoring the ORR and HER Electrocatalytic Performances of Gold Nanoparticles through Metal–Ligand Interfaces. J. Mater. Chem. A 2019, 7, 20425–20434. [Google Scholar] [CrossRef]
- Rodriguez, P.; Koper, M.T.M. Electrocatalysis on Gold. Phys. Chem. Chem. Phys. 2014, 16, 13583–13594. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, M.T.T.; Le, H.V.; Nguyen, D.N.; Truong, Q.D.; Tran, P.D. Gold Nanoparticles as an Outstanding Catalyst for the Hydrogen Evolution Reaction. Chem. Commun. 2018, 54, 3363–3366. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, Y.; Tang, Z. Atomically precise Au nanoclusters for electrochemical hydrogen evolution catalysis: Progress and perspectives. Polyoxometalates 2023, 2, 9140031. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, J.; Han, G.H.; Guo, W.; Hong, S.; Park, H.S.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Dendritic Gold-Supported Iridium/Iridium Oxide Ultra-Low Loading Electrodes for High-Performance Proton Exchange Membrane Water Electrolyzer. Appl. Catal. B Environ. 2021, 283, 119596. [Google Scholar] [CrossRef]
- Li, T.; Cui, L.; Kuhnert, M.; McLaren, T.I.; Pandey, R.; Liu, H.; Wang, W.; Xu, Z.; Xia, A.; Dalal, R.C.; et al. A Comprehensive Review of Soil Organic Carbon Estimates: Integrating Remote Sensing and Machine Learning Technologies. J. Soils Sediments 2024, 24, 3556–3571. [Google Scholar] [CrossRef]
- Kapil, N.; Cardinale, F.; Weissenberger, T.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Gold Nanoparticles with Tailored Size through Ligand Modification for Catalytic Applications. Chem. Commun. 2021, 57, 10775–10778. [Google Scholar] [CrossRef] [PubMed]
- Tepale, N.; Fernández-Escamilla, V.V.A.; Carreon-Alvarez, C.; González-Coronel, V.J.; Luna-Flores, A.; Carreon-Alvarez, A.; Aguilar, J. Nanoengineering of Gold Nanoparticles: Green Synthesis, Characterization, and Applications. Crystals 2019, 9, 612. [Google Scholar] [CrossRef]
- Alexandridis, P. Gold Nanoparticle Synthesis, Morphology Control, and Stabilization Facilitated by Functional Polymers. Chem. Eng. Technol. 2011, 34, 15–28. [Google Scholar] [CrossRef]
- Minati, L.; Benetti, F.; Chiappini, A.; Speranza, G. One-Step Synthesis of Star-Shaped Gold Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 623–628. [Google Scholar] [CrossRef]
- Alexeeva, O.K.; Fateev, V.N. Application of the Magnetron Sputtering for Nanostructured Electrocatalysts Synthesis. Int. J. Hydrog. Energy 2016, 41, 3373–3386. [Google Scholar] [CrossRef]
- Palmer, R.E.; Cai, R.; Vernieres, J. Synthesis without Solvents: The Cluster (Nanoparticle) Beam Route to Catalysts and Sensors. Acc. Chem. Res. 2018, 51, 2296–2304. [Google Scholar] [CrossRef]
- Sergievskaya, A.; Chauvin, A.; Konstantinidis, S. Sputtering onto Liquids: A Critical Review. Beilstein J. Nanotechnol. 2022, 13, 10–53. [Google Scholar] [CrossRef]
- Wender, H.; Gonçalves, R.V.; Feil, A.F.; Migowski, P.; Poletto, F.S.; Pohlmann, A.R.; Dupont, J.; Teixeira, S.R. Sputtering onto Liquids: From Thin Films to Nanoparticles. J. Phys. Chem. C 2011, 115, 16362–16367. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. A Review on Nanofluids—Part II: Experiments and Applications. Braz. J. Chem. Eng. 2008, 25, 631–648. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Yonezawa, T. Sputtering onto a Liquid: Interesting Physical Preparation Method for Multi-Metallic Nanoparticles. Sci. Technol. Adv. Mater. 2018, 19, 883–898. [Google Scholar] [CrossRef]
- Manjón, A.G.; Löffler, T.; Meischein, M.; Meyer, H.; Lim, J.; Strotkötter, V.; Schuhmann, W.; Ludwig, A.; Scheu, C. Sputter Deposition of Highly Active Complex Solid Solution Electrocatalysts into an Ionic Liquid Library: Effect of Structure and Composition on Oxygen Reduction Activity. Nanoscale 2020, 12, 23570–23577. [Google Scholar] [CrossRef] [PubMed]
- Lönn, B.; Strandberg, L.; Roth, V.; Luneau, M.; Wickman, B. Fuel Cell Catalyst Layers with Platinum Nanoparticles Synthesized by Sputtering onto Liquid Substrates. ACS Omega 2024, 9, 43725–43733. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene Glycol and Solutions of Polyethylene Glycol as Green Reaction Media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Turtayeva, Z.; Xu, F.; Dillet, J.; Mozet, K.; Peignier, R.; Celzard, A.; Maranzana, G. The Influence of Ink Formulation and Preparation on the Performance of Proton-Exchange Membrane Fuel Cell. Energies 2023, 16, 7519. [Google Scholar] [CrossRef]
- Sasikumar, G.; Ihm, J.W.; Ryu, H. Dependence of Optimum Nafion Content in Catalyst Layer on Platinum Loading. J. Power Sources 2004, 132, 11–17. [Google Scholar] [CrossRef]
- Speranza, G.; Canteri, R. RxpsG a New Open Project for Photoelectron and Electron Spectroscopy Data Processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, e1806296. [Google Scholar] [CrossRef]
- Creel, E.B.; Lyu, X.; McCool, G.; Ouimet, R.J.; Serov, A. Protocol for Screening Water Oxidation or Reduction Electrocatalyst Activity in a Three-Electrode Cell for Alkaline Exchange Membrane Electrolysis. Front. Energy Res. 2022, 10, 871604. [Google Scholar] [CrossRef]
- Friedman, A.K.; Shi, W.; Losovyj, Y.; Siedle, A.R.; Baker, L.A. Mapping Microscale Chemical Heterogeneity in Nafion Membranes with X-Ray Photoelectron Spectroscopy. J. Electrochem. Soc. 2018, 165, H733. [Google Scholar] [CrossRef]
- Hepperle, P.; Herman, A.; Khanbabaee, B.; Baek, W.Y.; Nettelbeck, H.; Rabus, H. XPS Examination of the Chemical Composition of PEGMUA-Coated Gold Nanoparticles. Part. Part. Syst. Charact. 2022, 39, 2200070. [Google Scholar] [CrossRef]
- Li, L.; Tian, X.; Meng, D.; Collins, M.; Wang, J.; Patterson, R.; Nguyen, L.; Yi, X. Processing, Characterization, and Impact of Nafion Thin Film on Photonic Nanowaveguides for Humidity Sensing. Adv. Photonics Res. 2022, 3, 2100181. [Google Scholar] [CrossRef]
- Luo, W.; Gan, J.; Huang, Z.; Chen, W.; Qian, G.; Zhou, X.; Duan, X. Boosting HER Performance of Pt-Based Catalysts Immobilized on Functionalized Vulcan Carbon by Atomic Layer Deposition. Front. Mater. 2019, 6, 251. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Liao, H.; Sun, S.; Li, S.; Ager, J.W.; Xu, Z.J. Activation Effect of Electrochemical Cycling on Gold Nanoparticles towards the Hydrogen Evolution Reaction in Sulfuric Acid. Electrochimica Acta 2016, 209, 440–447. [Google Scholar] [CrossRef]



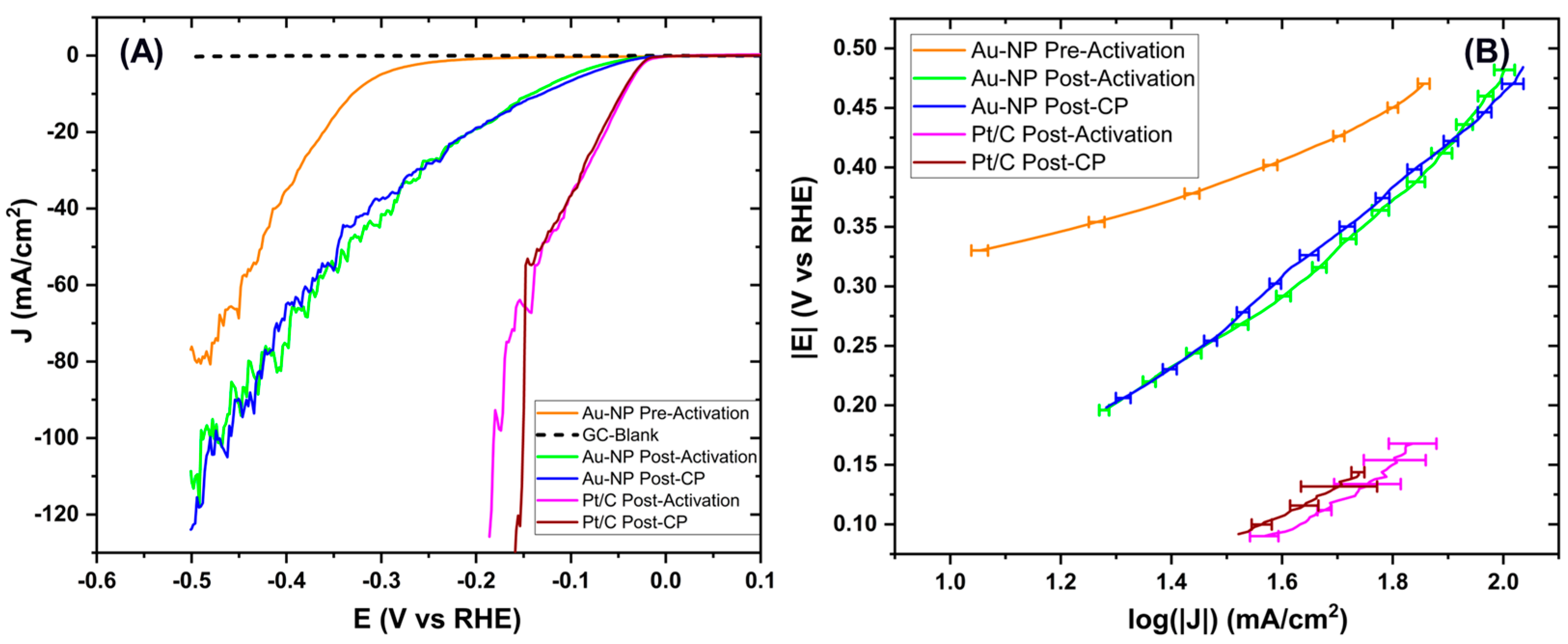
| Sample | LSV Test | Onset Potential (V vs. RHE) | Tafel Slope (mV/dec) |
|---|---|---|---|
| Au NPs | Pre-Activation | −0.29 | 17.7 ± 0.3 |
| Post-Activation | −0.05 | 38.6 ± 0.4 | |
| Post-CP | −0.05 | 38.1 ± 0.2 | |
| Pt/C | Post-Activation | −0.02 | 30.0 ± 0.5 |
| Post-CP | −0.02 | 23.6 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandraiahgari, C.R.; Gottardi, G.; Speranza, G.; Muzzi, B.; Dalessandro, D.; Pedrielli, A.; Micheli, V.; Bartali, R.; Laidani, N.B.; Testi, M. RF Sputtering of Gold Nanoparticles in Liquid and Direct Transfer to Nafion Membrane for PEM Water Electrolysis. Membranes 2025, 15, 115. https://doi.org/10.3390/membranes15040115
Chandraiahgari CR, Gottardi G, Speranza G, Muzzi B, Dalessandro D, Pedrielli A, Micheli V, Bartali R, Laidani NB, Testi M. RF Sputtering of Gold Nanoparticles in Liquid and Direct Transfer to Nafion Membrane for PEM Water Electrolysis. Membranes. 2025; 15(4):115. https://doi.org/10.3390/membranes15040115
Chicago/Turabian StyleChandraiahgari, Chandrakanth Reddy, Gloria Gottardi, Giorgio Speranza, Beatrice Muzzi, Domenico Dalessandro, Andrea Pedrielli, Victor Micheli, Ruben Bartali, Nadhira Bensaada Laidani, and Matteo Testi. 2025. "RF Sputtering of Gold Nanoparticles in Liquid and Direct Transfer to Nafion Membrane for PEM Water Electrolysis" Membranes 15, no. 4: 115. https://doi.org/10.3390/membranes15040115
APA StyleChandraiahgari, C. R., Gottardi, G., Speranza, G., Muzzi, B., Dalessandro, D., Pedrielli, A., Micheli, V., Bartali, R., Laidani, N. B., & Testi, M. (2025). RF Sputtering of Gold Nanoparticles in Liquid and Direct Transfer to Nafion Membrane for PEM Water Electrolysis. Membranes, 15(4), 115. https://doi.org/10.3390/membranes15040115








