Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation
Abstract
:1. Introduction
2. Results and Discussion
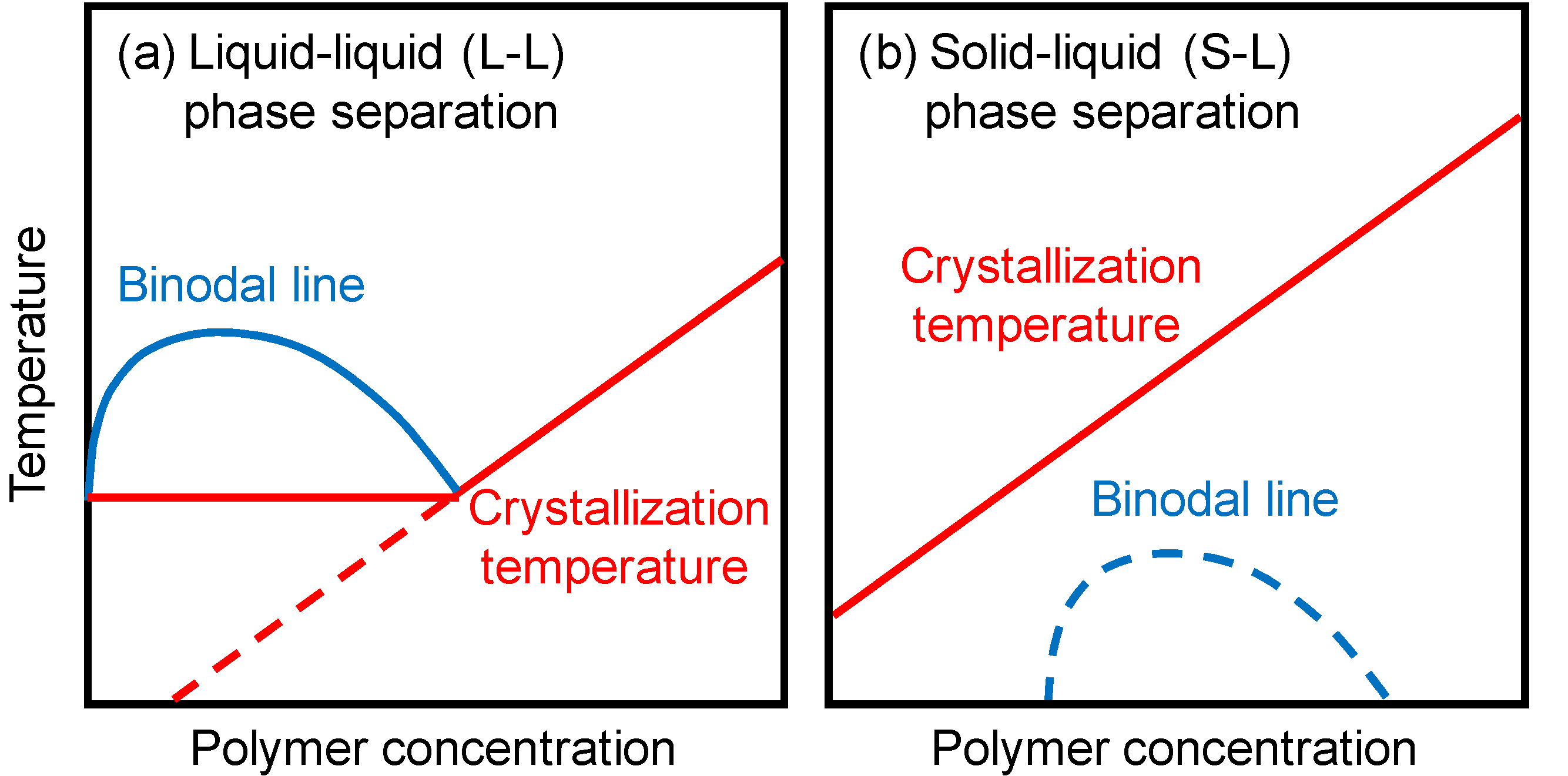
2.1. Phase Separation Behavior

2.2. Solidification Behavior of Polymer Solution during TIPS Process



3. Experimental Section
3.1. Materials
3.2. Crystallization Behavior

 is the melting enthalpy for 100% crystalline PVDF, 104.5 J/g [28], ΔHf(t) is the melting enthalpy of the solution at time t after the onset of crystallization, as measured by DSC, and φ is the weight fraction of PVDF in the solution.
is the melting enthalpy for 100% crystalline PVDF, 104.5 J/g [28], ΔHf(t) is the melting enthalpy of the solution at time t after the onset of crystallization, as measured by DSC, and φ is the weight fraction of PVDF in the solution.3.3. Observation of Phase Separation
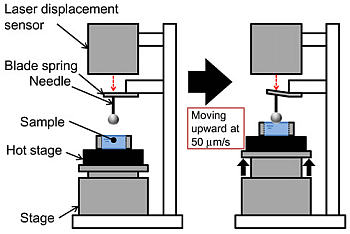
3.4. Membrane Stiffness Measurement


4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 75–140. [Google Scholar]
- Caneba, G.T.; Soong, D.S. Polymer membrane formation through the thermal-inversion process: experimental study of membrane structure formation. Macromolecules 1985, 18, 2538–2545. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kinzer, K.E.; Tseng, H.S. Microporous membrane formation via thermally induced phase separation. i. solid-liquid phase separation. J. Membr. Sci. 1990, 52, 239–261. [Google Scholar] [CrossRef]
- Tsai, F.J.; Torkelson, J.M. Microporous poly(methyl methacrylate) membranes. Effect of a low-viscosity solvent on the formation mechanism. Macromolecules 1990, 23, 4983–4989. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.; Kinzer, K.E. Microporous membrane formation via thermally induced phase separation. ii. liquid-liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Mehta, R.H.; Madsen, D.A.; Kalika, D.S. Microporous membranes based on poly(ether-ether ketone) via thermally-induced phase separation. J. Membr. Sci. 1995, 107, 93–106. [Google Scholar] [CrossRef]
- McGuire, K.S.; Laxminarayan, A.; Martula, D.S.; Lloyd, D.R. Kinetics of droplet growth in liquid-liquid phase separation of polymer-diluent systems. Model development. J. Colloid Interface Sci. 1996, 182, 46–58. [Google Scholar] [CrossRef]
- Matsuyama, H.; Berghmans, S.; Batarseh, M.T.; Lloyd, D.R. Effects of thermal history on anisotropic and asymmetric membranes formed by thermally induced phase separation. J. Membr. Sci. 1998, 142, 27–42. [Google Scholar] [CrossRef]
- Matsuyama, H.; Berghmans, S.; Lloyd, D.R. Formation of anisotropic membranes via thermally induced phase separation. Polymer 1999, 40, 2289–2301. [Google Scholar] [CrossRef]
- Matsuyama, H.; Kudari, S.; Kiyofuji, H.; Kitamura, Y. Kinetic studies of thermally induced phase separation in polymer-diluent system. J. Appl. Polym. Sci. 2000, 76, 1028–1036. [Google Scholar] [CrossRef]
- Ji, G.L.; Zhu, L.P.; Zhu, B.K.; Zhang, C.F.; Xu, Y.Y. Structure formation and characterization of pvdf hollow fiber membrane prepared via tips with diluent mixture. J. Membr. Sci. 2008, 319, 264–270. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Lee, S.Y.; Lee, J.M.; Woo, K.T.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Poly(vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Matsuyama, H.; Okafuji, H.; Maki, T.; Teramoto, M.; Kubota, N. Preparation of polyethylene hollow fiber membrane via thermally induced phase separation. J. Membr. Sci. 2003, 223, 119–126. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, C.K. Fabrication of polyethylene microporous membranes using triethylolpropane tris(2-ethylhexanoate) as a novel diluent by a thermally induced phase separation process. J. Membr. Sci. 2014, 449, 127–135. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, G.; Yang, J.; Wang, X.L. Formation of isotactic polypropylene membranes with bicontinuous structure and good strength via thermally induced phase separation method. Desalination 2009, 236, 8–15. [Google Scholar] [CrossRef]
- Vanegas, M.E.; Quijada, R.; Serafini, D. Microporous membranes prepared via thermally induced phase separation from metallocenic syndiotactic polypropylenes. Polymer 2009, 50, 2081–2086. [Google Scholar] [CrossRef]
- Tsai, F.J.; Torkelson, J.M. Roles of phase separation mechanism and coarsening in the formation of poly(methyl methacrylate) asymmetric membranes. Macromolecules 1990, 23, 775–784. [Google Scholar] [CrossRef]
- Funk, C.V.; Beavers, B.L.; Lloyd, D.R. Effect of particulate filler on cell size in membranes formed via liquid-liquid thermally induced phase separation. J. Membr. Sci. 2008, 325, 1–5. [Google Scholar] [CrossRef]
- Song, S.W.; Torkelson, J.M. Coarsening effects on the formation of microporous membranes produced via thermally induced phase separation of polystyrene-cyclohexanol solutions. J. Membr. Sci. 1995, 98, 209–222. [Google Scholar] [CrossRef]
- Qiangguo, B.L.; Yang, Y. The phase diagrams of mixtures of EVAL and PEG in relation to membrane formation. J. Membr. Sci. 2000, 180, 81–92. [Google Scholar] [CrossRef]
- Shang, M.; Matsuyama, H.; Maki, T.; Teramoto, M.; Lloyd, D.R. Effect of crystallization and liquid-liquid phase separation on phase-separation kinetics in poly(ethylene-co-vinyl alcohol)/glycerol solution. J. Polym. Sci. B Polym. Phys. 2003, 41, 194–201. [Google Scholar] [CrossRef]
- Bonyadi, S.; Chung, T.S.; Krantz, W.B. Investigation of corrugation phenomenon in the inner contour of hollow fibers during the non-solvent induced phase-separation process. J. Membr. Sci. 2007, 299, 200–210. [Google Scholar] [CrossRef]
- Yin, J.; Coutris, N.; Huang, Y. Experimental investigation of aligned groove formation on the inner surface of polyacrylonitrile hollow fiber membrane. J. Membr. Sci. 2012, 394, 57–68. [Google Scholar]
- Ishigami, T.; Nakatsuka, K.; Ohmukai, Y.; Kamio, E.; Maruyama, T.; Matsuyama, H. Solidification characteristics of polymer solution during polyvinylidene fluoride membrane preparation by nonsolvent-induced phase separation. J. Membr. Sci. 2013, 438, 77–82. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Maruyama, T.; Sotani, T.; Matsuyama, H. Preparation of PVDF hollow fiber membrane from a ternary polymer/solvent/nonsolvent system via thermally induced phase separation (TIPS) method. Sep. Purif. Technol. 2008, 63, 415–423. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly (vinylidene fluoride). J. Polym. Sci. B Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 126–130. [Google Scholar]
- Ma, W.; Zhang, J.; Wang, X.L.; Wang, S. Effect of PMMA on crystallization behavior and hydrophilicity of poly(vinylidene fluoride)/poly(methyl methacrylate) blend prepared in semi-dilute solutions. Appl. Surf. Sci. 2007, 253, 8377–8388. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishigami, T.; Nii, Y.; Ohmukai, Y.; Rajabzadeh, S.; Matsuyama, H. Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation. Membranes 2014, 4, 113-122. https://doi.org/10.3390/membranes4010113
Ishigami T, Nii Y, Ohmukai Y, Rajabzadeh S, Matsuyama H. Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation. Membranes. 2014; 4(1):113-122. https://doi.org/10.3390/membranes4010113
Chicago/Turabian StyleIshigami, Toru, Yoko Nii, Yoshikage Ohmukai, Saeid Rajabzadeh, and Hideto Matsuyama. 2014. "Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation" Membranes 4, no. 1: 113-122. https://doi.org/10.3390/membranes4010113
APA StyleIshigami, T., Nii, Y., Ohmukai, Y., Rajabzadeh, S., & Matsuyama, H. (2014). Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation. Membranes, 4(1), 113-122. https://doi.org/10.3390/membranes4010113