Carbon Nanotube- and Carbon Fiber-Reinforcement of Ethylene-Octene Copolymer Membranes for Gas and Vapor Separation
Abstract
:1. Introduction
- -
- energy savings;
- -
- environmental friendliness;
- -
- easy handling;
- -
- continuous process;
- -
- compact design and small footprint.
2. Experimental Section
2.1. Materials
2.2. Membrane Preparation
| EOC/MWCNT | EOC/CF | |||||
|---|---|---|---|---|---|---|
| wt % of MWCNT | vol % of MWCNT | DC Conductivity (S cm−1) | wt % of CF | vol % of CF | DC Conductivity (S cm−1) | |
| 2 | 1 | (3.16 ± 0.91) × 10−9 | 2 | 1 | (3.48 ± 0.85) × 10−9 | |
| 5 | 3 | (3.25 ± 0.83) × 10−9 | 5 | 2 | (3.54 ± 0.81) × 10−9 | |
| 10 | 5 | (4.08 ± 0.76) × 10−9 | 10 | 5 | (7.54 ± 0.44) × 10−6 | |
| 15 | 8 | (4.61 ± 0.30) × 10−3 | 15 | 7 | (1.46 ± 0.24) × 10−2 | |
| 20 | 11 | (6.32 ± 0.22) × 10−3 | 20 | 10 | (3.13 ± 0.19) × 10−2 | |
| 25 | 14 | (1.34 ± 0.24) × 10−2 | 25 | 13 | (1.60 ± 0.21) × 10−1 | |
| 30 | 18 | (2.53 ± 0.18) × 10−2 | 30 | 16 | (4.28 ± 0.14) × 10−1 | |
| 35 | 21 | (8.62 ± 0.15) × 10−2 | – | – | – | |
2.3. Membrane Characterization
2.3.1. Thickness and Morphology
2.3.2. Mechanical Tests
2.3.3. Gas and Vapor Permeability Measurements



3. Results and Discussion
3.1. Membrane Preparation
3.2. Membrane Morphology


3.3. Mechanical Tests
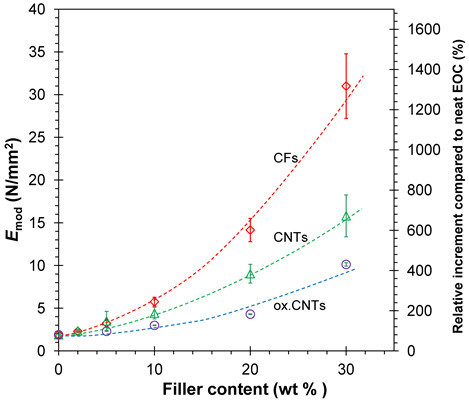
3.3.1. Stress-Strain Behavior


3.3.2. Frequency Dependence of the Young’s Modulus


| Filler content (wt %) | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| EOC + CNTs | EOC + ox-CNTs | EOC + CFs | ||||||
| K | n | K | n | K | n | |||
| 0 | 1.56 | 0.0837 | 1.56 | 0.0837 | 1.56 | 0.0837 | ||
| 5 | 2.87 | 0.0719 | 2.11 | 0.0710 | 3.04 | 0.0737 | ||
| 10 | 3.98 | 0.0650 | 2.55 | 0.0703 | 5.08 | 0.0730 | ||
| 20 | 7.51 | 0.0624 | 3.69 | 0.0732 | 11.9 | 0.0683 | ||
| 30 | 12.8 | 0.0644 | 8.44 | 0.0620 | 26.6 | 0.0437 | ||

3.4. Transport Properties
3.4.1. Gas Permeation Measurements



3.4.2. Vapor Permeation


4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Éigenson, A.S. Regularity in boiling point distribution of crude oil fractions. Chem. Technol. Fuels Oils 1973, 9, 3–8. [Google Scholar]
- Kimmerle, K.; Bell, C.M. Solvent recovery from air. J. Membr. Sci. 1988, 36, 477–488. [Google Scholar] [CrossRef]
- Ravanchia, M.T.; Kaghazchia, T.; Margarin, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Leemann, M.; Eigenberger, G.; Strathmann, H. Vapour permeation for the recovery of organic solvents from waste air streams: Separation capacities and process optimization. J. Membr. Sci. 1996, 113, 313–322. [Google Scholar] [CrossRef]
- Rebollar-Perez, G.; Carretier, E.; Lesage, N.; Moulin, P. Volatile organic compound (VOC) removal by vapor permeation at low VOC concentrations: Laboratory scale results and modeling for scale up. Membranes 2011, 1, 80–90. [Google Scholar] [CrossRef]
- Kim, H.J.; Nah, S.S.; Min, B.R. A new technique for preparation of PDMS pervaporation membrane for VOC removal. Adv. Environ. Res. 2002, 6, 255–264. [Google Scholar] [CrossRef]
- Majumdar, S.; Bhaumik, D.; Sirkar, K.K. Performance of commercial-size plasmapolymerized PDMS-coated hollow fiber modules in removing VOCs from N2/air. J. Membr. Sci. 2003, 214, 323–330. [Google Scholar] [CrossRef]
- Sohn, W.-I.; Ryu, D.-H.; Oh, S.-J.; Koo, J.-K. A study on the development of composite membranes for the separation of organic vapors. J. Membr. Sci. 2000, 175, 163–170. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Lawless, D. Separation of gasoline vapor from nitrogen by hollow fiber composite membranes for VOC emission control. J. Membr. Sci. 2006, 271, 114–124. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Pinnau, I.; Freeman, B. Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Šindelář, V.; Sysel, P.; Hynek, V.; Friess, K.; Šípek, M.; Castaneda, N. Transport of gases and organic vapors through membrane made of poly(amide-imide)s crosslinked with poly(ethyleneadipate). Collect. Czech. Chem. Commun. 2001, 66, 533–540. [Google Scholar] [CrossRef]
- Guizard, C.; Boutevin, B.; Guida, F.; Ratsimihety, A.; Amblard, P.; Lasere, J.C.; Naiglin, S. VOC vapor transport properties of new membranes based on cross-linked fluorinated elastomers. Sep. Purif. Technol. 2001, 22–23, 23–30. [Google Scholar]
- Togawa, J.; Kanno, T.; Horiuchi, J.-I.; Kobayashi, M. Gas permeability modification of polyolefin films induced by d-limonene swelling. J. Membr. Sci. 2001, 188, 39–48. [Google Scholar] [CrossRef]
- Tasselli, F.; Jansen, J.C.; Sidari, F.; Drioli, E. Morphology and transport property control of modified poly(ether ether ketone) (PEEKWC) hollow fiber membranes prepared from PEEKWC/PVP blends: Influence of the relative humidity in the air gap. J. Membr. Sci. 2005, 255, 13–22. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Jansen, A.E. CO2 separation with polyolefin membrane contactors and dedicated absorption liquids: Performances and prospects. Sep. Purif. Technol. 2002, 27, 231–242. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2005, 255, 13–22. [Google Scholar]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, reverse-selective nanocomposite membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Lengalova, A.; Saha, P. Compressive stress-electrical conductivity characteristics of multiwall carbon nanotube networks. J. Mater. Sci. 2011, 46, 3186–3190. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Benlikaya, R.; Svoboda, P.; Petras, D. A flexible multifunctional sensor based on carbon nanotube/polyurethane composite. IEEE Sens. J. 2013, 13, 4045–4048. [Google Scholar] [CrossRef]
- Petras, D.; Olejnik, R.; Slobodian, P.; Riha, P. Temperature dependence of electrical conductance of multi-walled carbon nanotube networks and their polystyrene composite. Key Eng. Mater. 2013, 543, 356–359. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Saha, P. A highly-deformable composite composed of an entangled network of electrically-conductive carbon-nanotubes embedded in elastic polyurethane. Carbon 2012, 50, 3446–3453. [Google Scholar] [CrossRef]
- Slobodian, P.; Kralova, D.; Lengalova, A.; Novotny, R.; Saha, P. Adaptation of polystyrene/multi-wall carbon nanotube composite properties in respect of its thermal stability. Polym. Compos. 2010, 31, 452–458. [Google Scholar]
- Slobodian, P.; Lengálová, A.; Šlouf, M.; Sáha, P. Poly(methyl methacrylate)/multi-wall carbon nanotubes composites prepared by solvent cast technique: composites electrical percolation threshold. J. Reinf. Plast. Compos. 2007, 26, 1705–1712. [Google Scholar] [CrossRef]
- Surya Murali, R.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas permeation behavior of pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 2012, 44, 1899–1901. [Google Scholar]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent developments in carbon nanotube membranes for water purification and gas separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Poongavalappil, S.; Svoboda, P.; Theravalappil, R.; Svobodova, D.; Danek, M.; Zatloukal, M. Study on the influence of electron beam irradiation on the thermal, mechanical, and rheological properties of ethylene-octene copolymer with high comonomer content. J. Appl. Polym. Sci. 2013, 128, 3026–3033. [Google Scholar] [CrossRef]
- Svoboda, P.; Theravalappil, R.; Poongavalappil, S.; Vilcakova, J.; Svobodova, D.; Mokrejs, P.; Blaha, A. A study on electrical and thermal conductivities of ethylene–octene copolymer/expandable graphite composites. Polym. Eng. Sci. 2012, 52, 1241–1249. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izak, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Clarizia, G.; Schauer, J.; Izák, P. High ionic liquid content polymeric gel membranes: preparation and performance. Macromolecules 2011, 44, 39–45. [Google Scholar] [CrossRef]
- Clarizia, G.; Algieri, C.; Drioli, E. Filler-polymer combination: A route to modify gas transport properties of a polymeric membrane. Polymer 2004, 45, 5671–5681. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Drioli, E. Organic vapour transport in glassy perfluoropolymer membranes: A simple semi-quantitative approach to analyze clustering phenomena by time lag measurements. J. Membr. Sci. 2011, 367, 141–151. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford Press: London, UK, 1990. [Google Scholar]
- Koda, S.; Mori, H.; Matsumoto, K.; Nomura, H. Ultrasonic degradation of water-soluble polymers. Polymers 1994, 35, 30–33. [Google Scholar]
- Taghizadeh, M.T.; Mehrdad, A. Calculation of the rate constant for the ultrasonic degradation of aqueous solutions of polyvinyl alcohol by viscometry. Ultrason. Sonochem. 2003, 10, 309–313. [Google Scholar] [CrossRef]
- Olejnik, R.; Liu, P.; Slobidian, P.; Zatloukal, M.; Saha, P. Characterization of carbon nanotube based polymer composites through rheology. AIP Conf. Proc. 2009, 1152, 204–209. [Google Scholar]
- Osazuwa, O.; Petrie, K.; Kontopoulou, M.; Xiang, P.; Ye, Z.; Docoslis, A. Characterization of non-covalently, non-specifically functionalized multi-wall carbon nanotubes and their melt compounded composites with an ethylene–octene copolymer. Compos. Sci. Technol. 2012, 73, 27–33. [Google Scholar] [CrossRef]
- Sliwa, F.; El Bounia, N.-E.; Charrier, F.; Marin, G.; Malet, F. Mechanical and interfacial properties of wood and bio-based thermoplastic composite. Compos. Sci. Technol. 2012, 72, 1733–1740. [Google Scholar] [CrossRef]
- Blume, I.; Schwering, P.J.F.; Mulder, M.H.V.; Smolders, C.A. Vapour sorption and permeation properties of poly(dimethylsiloxane) films. J. Membr. Sci. 1991, 61, 85–97. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sedláková, Z.; Clarizia, G.; Bernardo, P.; Jansen, J.C.; Slobodian, P.; Svoboda, P.; Kárászová, M.; Friess, K.; Izak, P. Carbon Nanotube- and Carbon Fiber-Reinforcement of Ethylene-Octene Copolymer Membranes for Gas and Vapor Separation. Membranes 2014, 4, 20-39. https://doi.org/10.3390/membranes4010020
Sedláková Z, Clarizia G, Bernardo P, Jansen JC, Slobodian P, Svoboda P, Kárászová M, Friess K, Izak P. Carbon Nanotube- and Carbon Fiber-Reinforcement of Ethylene-Octene Copolymer Membranes for Gas and Vapor Separation. Membranes. 2014; 4(1):20-39. https://doi.org/10.3390/membranes4010020
Chicago/Turabian StyleSedláková, Zuzana, Gabriele Clarizia, Paola Bernardo, Johannes Carolus Jansen, Petr Slobodian, Petr Svoboda, Magda Kárászová, Karel Friess, and Pavel Izak. 2014. "Carbon Nanotube- and Carbon Fiber-Reinforcement of Ethylene-Octene Copolymer Membranes for Gas and Vapor Separation" Membranes 4, no. 1: 20-39. https://doi.org/10.3390/membranes4010020
APA StyleSedláková, Z., Clarizia, G., Bernardo, P., Jansen, J. C., Slobodian, P., Svoboda, P., Kárászová, M., Friess, K., & Izak, P. (2014). Carbon Nanotube- and Carbon Fiber-Reinforcement of Ethylene-Octene Copolymer Membranes for Gas and Vapor Separation. Membranes, 4(1), 20-39. https://doi.org/10.3390/membranes4010020