Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6
Abstract
:1. Introduction
2. The Membrane Receptor that Mediates Cellular Uptake of Vitamin A from the Blood


3. STRA6’s Vitamin A Uptake Mechanism and Its Coupling to Intracellular Proteins

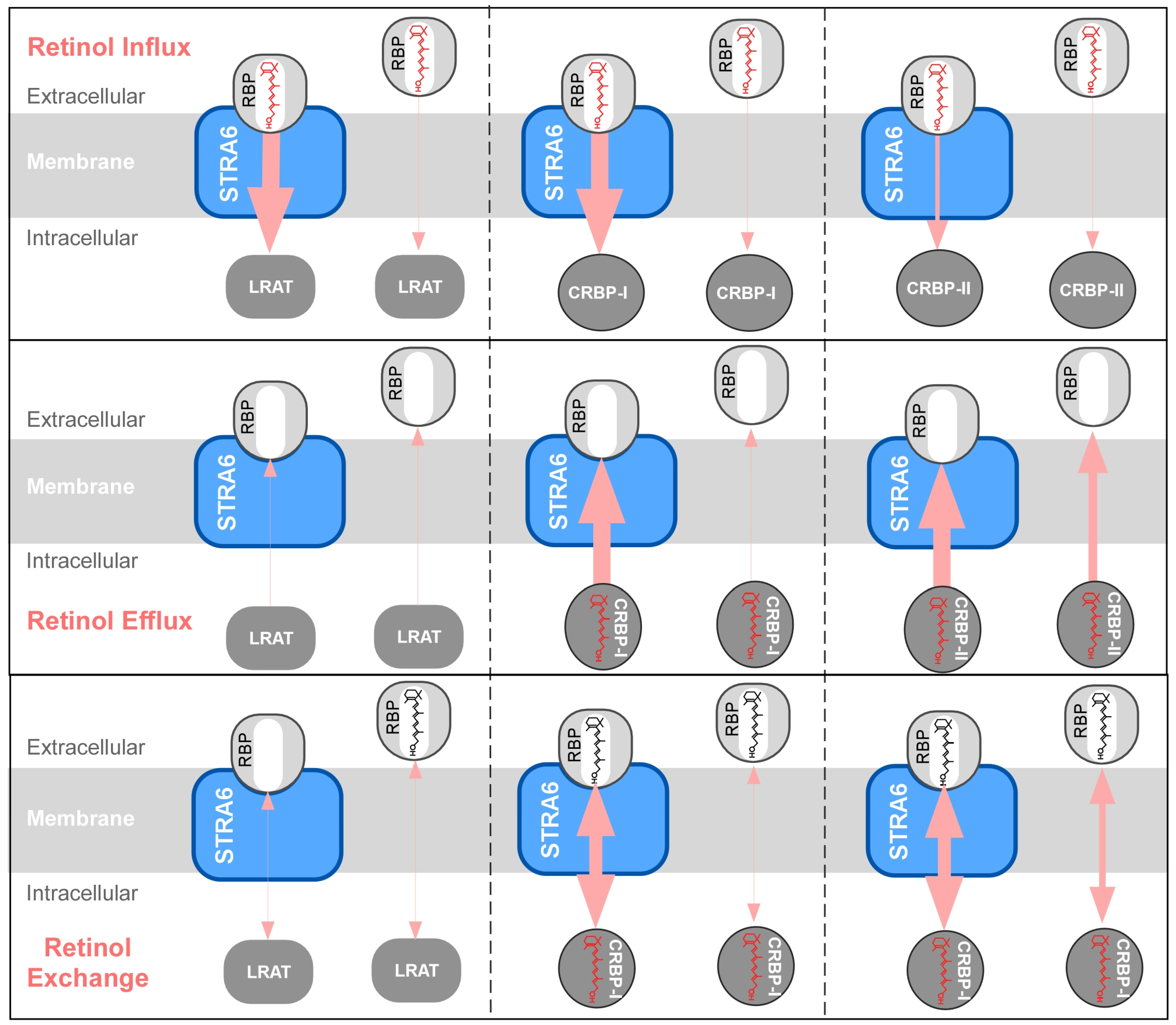
4. STRA6-Catalyzed Vitamin A Influx, Efflux and Exchange

5. Isomer-Specific Effects of Retinoids on STRA6 Activity

6. STRA6 as a Therapeutic Target for Small Molecule-Based Drugs
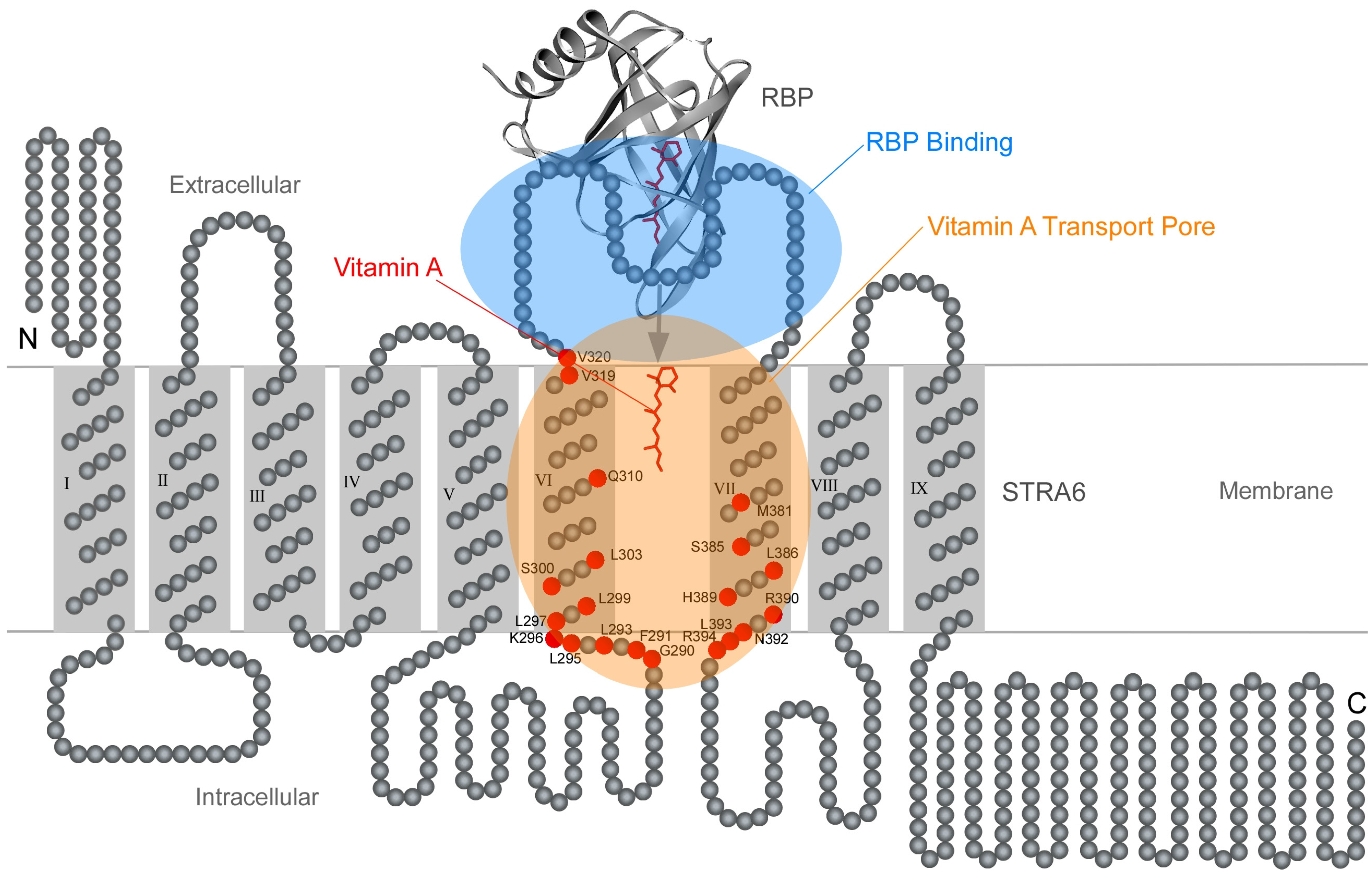
7. Transmembrane Pathway for Vitamin A Transport by STRA6
8. Comparing RBP/STRA6 Interaction with RBP/TTR Interaction

9. RBP-Dependent Pathway and RBP-Independent Pathway
10. Phenotypic Variability Caused by Mutations in RBP or STRA6
11. Points of Potential Confusion
12. Conclusion
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Wald, G. Molecular basis of visual excitation. Science 1968, 162, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.E. Night blindness. Sci. Am. 1966, 215, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Crouch, R.K.; Chader, G.J.; Wiggert, B.; Pepperberg, D.R. Retinoids and the visual process. Photochem. Photobiol. 1996, 64, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Kiser, P.D.; Golczak, M.; Palczewski, K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 2010, 35, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Ziouzenkova, O.; Orasanu, G.; Sharlach, M.; Akiyama, T.E.; Berger, J.P.; Viereck, J.; Hamilton, J.A.; Tang, G.; Dolnikowski, G.G.; Vogel, S.; et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 2007, 13, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin. Immunol. Immunopathol. 1996, 80, S52–S62. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect. 1994, 90, 105–117. [Google Scholar] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [PubMed]
- Maden, M. Role of retinoids in embryonic development. In Vitamin A in Health and Disease; Blomhoff, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 289–322. [Google Scholar]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Drager, U.C. Retinoic acid signaling in the functioning brain. Sci. STKE 2006, 2006, pe10. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C. Vitamin A status: Relationship to immunity and the antibody response. Proc. Soc. Exp. Biol. Med. 1992, 200, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wolgemuth, D.J. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome Res. 2004, 105, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Wolbach, S.R.; Howe, P.R. Tissue change following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K. The significance of vitamin A for the development and function of the lung. Forum Nutr. 2003, 56, 37–40. [Google Scholar] [PubMed]
- Ross, A.C. On the sources of retinoic acid in the lung: Understanding the local conversion of retinol to retinoic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L247–L248. [Google Scholar] [CrossRef] [PubMed]
- Vahlquist, A. Role of retinoids in normal and diseased skin. In Vitamin A in Health and Disease; Blomhoff, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 365–424. [Google Scholar]
- Zhou, G.B.; Zhang, J.; Wang, Z.Y.; Chen, S.J.; Chen, Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: A paradigm of synergistic molecular targeting therapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [PubMed]
- Goodman, D.S. Plasma retinol-binding protein. In The Retinoids; Sporn, M.B., Boberts, A.B., Goodman, D.S., Eds.; Academic Press, Inc.: New York, NY, USA, 1984; Volume 2, pp. 41–88. [Google Scholar]
- Rask, L.; Anundi, H.; Bohme, J.; Eriksson, U.; Fredriksson, A.; Nilsson, S.F.; Ronne, H.; Vahlquist, A.; Peterson, P.A. The retinol-binding protein. Scand. J. Clin. Lab. Invest. Suppl. 1980, 154, 45–61. [Google Scholar] [PubMed]
- Blomhoff, R.; Green, M.H.; Berg, T.; Norum, K.R. Transport and storage of vitamin A. Science 1990, 250, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Ong, D.E. Plasma retinol binding protein: Structure and function of the prototypic lipocalin. Biochim. Biophys. Acta 2000, 1482, 57–64. [Google Scholar] [CrossRef]
- Quadro, L.; Hamberger, L.; Colantuoni, V.; Gottesman, M.E.; Blaner, W.S. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol. Asp. Med. 2003, 24, 421–430. [Google Scholar] [CrossRef]
- Quadro, L.; Hamberger, L.; Gottesman, M.E.; Colantuoni, V.; Ramakrishnan, R.; Blaner, W.S. Transplacental delivery of retinoid: The role of retinol-binding protein and lipoprotein retinyl ester. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E844–E851. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, G.; Berni, R. Plasma retinol-binding protein: Structure and interactions with retinol, retinoids, and transthyretin. Vitam. Horm. 2004, 69, 271–295. [Google Scholar] [PubMed]
- Heller, J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J. Biol. Chem. 1975, 250, 3613–3619. [Google Scholar] [PubMed]
- Bok, D.; Heller, J. Transport of retinol from the blood to the retina: An autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp. Eye Res. 1976, 22, 395–402. [Google Scholar] [CrossRef]
- Rask, L.; Peterson, P.A. In vitro uptake of vitamin a from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J. Biol. Chem. 1976, 251, 6360–6366. [Google Scholar] [PubMed]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. Identification of a membrane receptor for retinol-binding protein functioning in the cellular uptake of retinol. Nutr. Rev. 2007, 65, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Yu, J.; Wiita, P.; Ter-Stepanian, M.; Sun, H. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry 2008, 47, 5387–5395. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Yu, J.; Wiita, P.; Honda, J.; Sun, H. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J. Biol. Chem. 2008, 283, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Maeda, A.; Bereta, G.; Maeda, T.; Kiser, P.D.; Hunzelmann, S.; von Lintig, J.; Blaner, W.S.; Palczewski, K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 2008, 283, 9543–9554. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Yu, J.; Ter-Stepanian, M.; Zhong, M.; Cheng, G.; Yuan, Q.; Jin, M.; Travis, G.H.; Ong, D.; Sun, H. Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem. Biol. 2011, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Isken, A.; Golczak, M.; Oberhauser, V.; Hunzelmann, S.; Driever, W.; Imanishi, Y.; Palczewski, K.; von Lintig, J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for matthew-wood syndrome. Cell Metab. 2008, 7, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Golczak, M.; Palczewski, K.; von Lintig, J. Lecithin:Retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 2012, 287, 24216–24227. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Gerke, V.; Palczewski, K. Retinosomes: New insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell. Biol. 2004, 166, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Blaner, W.S.; Hamberger, L.; Van Gelder, R.N.; Vogel, S.; Piantedosi, R.; Gouras, P.; Colantuoni, V.; Gottesman, M.E. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J. Biol. Chem. 2002, 277, 30191–30197. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Blaner, W.S.; Hamberger, L.; Novikoff, P.M.; Vogel, S.; Piantedosi, R.; Gottesman, M.E.; Colantuoni, V. The role of extrahepatic retinol binding protein in the mobilization of retinoid stores. J. Lipid Res. 2004, 45, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Qtaishat, N.M.; Redmond, T.M.; Pepperberg, D.R. Acute radiolabeling of retinoids in eye tissues of normal and RPE65-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.S.; Raz, A. Extraction and recombination studies of the interaction of retinol with human plasma retinol-binding protein. J. Lipid Res. 1972, 13, 338–347. [Google Scholar] [PubMed]
- Horwitz, J.; Heller, J. Interactions of all-trans, 9-, 11-, and 13-cis-retinal, all-trans-retinyl acetate, and retinoic acid with human retinol-binding protein and prealbumin. J. Biol. Chem. 1973, 248, 6317–6324. [Google Scholar] [PubMed]
- Horwitz, J.; Heller, J. Properties of the chromophore binding site of retinol-binding protein from human plasma. J. Biol. Chem. 1974, 249, 4712–4719. [Google Scholar] [PubMed]
- Berni, R.; Clerici, M.; Malpeli, G.; Cleris, L.; Formelli, F. Retinoids: In vitro interaction with retinol-binding protein and influence on plasma retinol. FASEB J. 1993, 7, 1179–1184. [Google Scholar] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. Differential and isomer-specific modulation of vitamin A transport and the catalytic activities of the RBP receptor by retinoids. J. Membr. Biol. 2013, 246, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Horwitz, J. Conformational changes following interaction between retinol isomers and human retinol-binding protein and between the retinol-binding protein and prealbumin. J. Biol. Chem. 1973, 248, 6308–6316. [Google Scholar] [PubMed]
- Amengual, J.; Zhang, N.; Kemerer, M.; Maeda, T.; Palczewski, K.; Von Lintig, J. STRA6 is critical for cellular vitamin a uptake and homeostasis. Hum. Mol. Genet. 2014, 23, 5402–5417. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Malpani, S.; Dyson, M.; Ono, M.; Coon, J.S.; Kim, J.J.; Schink, J.C.; Bulun, S.E.; Pavone, M.E. Fenretinide: A novel treatment for endometrial cancer. PLoS One 2014, 9, e110410. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ross, A.C. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J. Lipid Res. 2010, 51, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Nau, H.; Chahoud, I.; Dencker, L.; Lammer, E.J.; Scott, W.J. Teratogenicity of vitamin A and retinoids. In Vitamin A in Health and Disease; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 615–664. [Google Scholar]
- Collins, M.D.; Mao, G.E. Teratology of retinoids. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Sieving, P.A.; Chaudhry, P.; Kondo, M.; Provenzano, M.; Wu, D.; Carlson, T.J.; Bush, R.A.; Thompson, D.A. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.; Sakai, Y.; Zhang, J.; Koul, O.; Mineur, Y.; Crusio, W.E.; McCaffery, P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Szeto, W.; Jiang, W.; Tice, D.A.; Rubinfeld, B.; Hollingshead, P.G.; Fong, S.E.; Dugger, D.L.; Pham, T.; Yansura, D.G.; Wong, T.A.; et al. Overexpression of the retinoic acid-responsive gene STRA6 in human cancers and its synergistic induction by wnt-1 and retinoic acid. Cancer Res. 2001, 61, 4197–4205. [Google Scholar] [PubMed]
- Liu, J.R.; Sun, X.R.; Dong, H.W.; Sun, C.H.; Sun, W.G.; Chen, B.Q.; Song, Y.Q.; Yang, B.F. Beta-ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the sprague-dawley rat. Int. J. Cancer 2008, 122, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Cooma, I.; Mohammed, A.; Steele, V.E.; Rao, C.V. Beta-ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid x receptor-alpha in human colon cancer cells. Mol. Cancer Ther. 2008, 7, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Wood, E.J. Fenretinide and its relation to cancer. Cancer Treat. Rev. 1999, 25, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Ambalavanan, N. Retinoic acid combined with vitamin a synergizes to increase retinyl ester storage in the lungs of newborn and dexamethasone-treated neonatal rats. Neonatology 2007, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Li, N.Q. Retinol combined with retinoic acid increases retinol uptake and esterification in the lungs of young adult rats when delivered by the intramuscular as well as oral routes. J. Nutr. 2007, 137, 2371–2376. [Google Scholar] [PubMed]
- James, M.L.; Ross, A.C.; Bulger, A.; Philips, J.B., 3rd; Ambalavanan, N. Vitamin A and retinoic acid act synergistically to increase lung retinyl esters during normoxia and reduce hyperoxic lung injury in newborn mice. Pediatr. Res. 2010, 67, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wray, A.E.; Ross, A.C. Oral vitamin A and retinoic acid supplementation stimulates antibody production and splenic STRA6 expression in tetanus toxoid-immunized mice. J. Nutr. 2012, 142, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kawaguchi, R.; Ter-Stepanian, M.; Kassai, M.; Sun, H. Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS One 2013, 8, e73838. [Google Scholar] [CrossRef] [PubMed]
- Hille, B.; Schwarz, W. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 1978, 72, 409–442. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R.; Miller, C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science 1989, 245, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, M.; Jan, Y.N.; Jan, L.Y. Stabilization of ion selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proc. Natl. Acad. Sci. USA 1997, 94, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Lin, M.C.; Bannister, J.P.; Silverman, W.R.; Mock, A.F.; Roux, B.; Papazian, D.M. Atomic proximity between S4 segment and pore domain in shaker potassium channels. Neuron 2003, 39, 467–481. [Google Scholar] [CrossRef]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Pan, A.C.; Gagnon, D.G.; Dalmas, O.; Cordero-Morales, J.F.; Chakrapani, S.; Roux, B.; Perozo, E. Structural basis for the coupling between activation and inactivation gates in K(+) channels. Nature 2010, 466, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Labro, A.J.; Lacroix, J.J.; Villalba-Galea, C.A.; Snyders, D.J.; Bezanilla, F. Molecular mechanism for depolarization-induced modulation of kv channel closure. J. Gen. Physiol. 2012, 140, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; Labarca, C.G.; Charnet, P.; Davidson, N.; Lester, H.A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science 1988, 242, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Monaco, H.L.; Rizzi, M.; Coda, A. Structure of a complex of two plasma proteins: Transthyretin and retinol-binding protein. Science 1995, 268, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Naylor, H.M.; Newcomer, M.E. The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP. Biochemistry 1999, 38, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.M.; Nelson, C.; Tarle, S.A.; Pribila, J.T.; Bardakjian, T.; Woods, S.; Schneider, A.; Glaser, T. Biochemical basis for dominant inheritance, variable penetrance, and maternal effects in RBP4 congenital eye disease. Cell 2015, 161, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kawaguchi, R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int. Rev. Cell Mol. Biol. 2011, 288, 1–41. [Google Scholar] [PubMed]
- Berry, D.C.; Croniger, C.M.; Ghyselinck, N.B.; Noy, N. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor stra6. Mol. Cell. Biol. 2012, 32, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Blaner, W.S.; Salchow, D.J.; Vogel, S.; Piantedosi, R.; Gouras, P.; Freeman, S.; Cosma, M.P.; Colantuoni, V.; Gottesman, M.E. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999, 18, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Hamberger, L.; Gottesman, M.E.; Wang, F.; Colantuoni, V.; Blaner, W.S.; Mendelsohn, C.L. Pathways of vitamin a delivery to the embryo: Insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005, 146, 4479–4490. [Google Scholar] [CrossRef] [PubMed]
- D'Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A metabolism: An update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef] [PubMed]
- Bavik, C.; Ward, S.J.; Chambon, P. Developmental abnormalities in cultured mouse embryos deprived of retinoic by inhibition of yolk-sac retinol binding protein synthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 3110–3114. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.R.; Goodman, D.S. Vitamin A transport in human vitamin A toxicity. N. Engl. J. Med. 1976, 294, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Green, M.H.; Green, J.B. Dynamics and control of plasma retinol. In Vitamin A in Health and Disease; Blomhoff, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Ishibashi, S.; Perrey, S.; Chen, Z.; Osuga, J.; Shimada, M.; Ohashi, K.; Harada, K.; Yazaki, Y.; Yamada, N. Role of the low density lipoprotein (LDL) receptor pathway in the metabolism of chylomicron remnants. A quantitative study in knockout mice lacking the LDL receptor, apolipoprotein e, or both. J. Biol. Chem. 1996, 271, 22422–22427. [Google Scholar] [PubMed]
- Beisiegel, U.; Weber, W.; Bengtsson-Olivecrona, G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA 1991, 88, 8342–8346. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, A.; Gafvels, M.; Heeren, J.; Meyer, N.; Angelin, B.; Beisiegel, U. VLDL receptor mediates the uptake of human chylomicron remnants in vitro. J. Lipid Res. 1996, 37, 1733–1742. [Google Scholar] [PubMed]
- Hagen, E.; Myhre, A.M.; Smeland, S.; Halvorsen, B.; Norum, K.R.; Blomhoff, R. Uptake of vitamin a in macrophages from physiologic transport proteins: Role of retinol-binding protein and chylomicron remnants. J. Nutr. Biochem. 1999, 10, 345–352. [Google Scholar] [CrossRef]
- Blaner, W.S.; Obunike, J.C.; Kurlandsky, S.B.; al-Haideri, M.; Piantedosi, R.; Deckelbaum, R.J.; Goldberg, I.J. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J. Biol. Chem. 1994, 269, 16559–16565. [Google Scholar] [PubMed]
- Wassef, L.; Quadro, L. Uptake of dietary retinoids at the maternal-fetal barrier: In vivo evidence for the role of lipoprotein lipase and alternative pathways. J. Biol. Chem. 2011, 286, 32198–32207. [Google Scholar] [CrossRef] [PubMed]
- Mallia, A.K.; Smith, J.E.; Goodman, D.W. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis a in the rat. J. Lipid Res. 1975, 16, 180–188. [Google Scholar] [PubMed]
- Pasutto, F.; Sticht, H.; Hammersen, G.; Gillessen-Kaesbach, G.; Fitzpatrick, D.R.; Nurnberg, G.; Brasch, F.; Schirmer-Zimmermann, H.; Tolmie, J.L.; Chitayat, D.; et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 2007, 80, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Golzio, C.; Martinovic-Bouriel, J.; Thomas, S.; Mougou-Zrelli, S.; Grattagliano-Bessieres, B.; Bonniere, M.; Delahaye, S.; Munnich, A.; Encha-Razavi, F.; Lyonnet, S.; et al. Matthew-wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am. J. Hum. Genet. 2007, 80, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Lu, T.; Metlapally, R.; Katowitz, J.; Kherani, F.; Wang, T.Y.; Tran-Viet, K.N.; Young, T.L. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol. Vis. 2008, 14, 2458–2465. [Google Scholar] [PubMed]
- Chassaing, N.; Golzio, C.; Odent, S.; Lequeux, L.; Vigouroux, A.; Martinovic-Bouriel, J.; Tiziano, F.D.; Masini, L.; Piro, F.; Maragliano, G.; et al. Phenotypic spectrum of STRA6 mutations: From matthew-wood syndrome to non-lethal anophthalmia. Hum. Mutat. 2009, 30, E673–E681. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, R.; Babovic, N.; Lteif, A.; Eidem, B.; Kirmani, S.; Olson, T.; Babovic-Vuksanovic, D. Vitamin A deficiency in an infant with pagod syndrome. Am. J. Med. Genet. A 2009, 149A, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Segel, R.; Levy-Lahad, E.; Pasutto, F.; Picard, E.; Rauch, A.; Alterescu, G.; Schimmel, M.S. Pulmonary hypoplasia-diaphragmatic hernia-anophthalmia-cardiac defect (pdac) syndrome due to STRA6 mutations--what are the minimal criteria? J. Med. Genet. A 2009, 149A, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Bove, K.E.; Slavotinek, A.M. Two novel STRA6 mutations in a patient with anophthalmia and diaphragmatic eventration. Am. J. Med. Genet. A 2009, 149A, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.; Kawaguchi, R.; McGettigan, P.; Sun, H.; Morrissey, M.; Nielsen, J.; Conroy, J.; Regan, R.; Tormey, P.; Ni Chroinin, M.; et al. First implication of STRA6 mutations in isolated anophthalmia, microphthalmia and coloboma: Adding a new dimension to the STRA6 phenotype. Hum. Mutat. 2011, 32, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, M.W.; Biesalski, H.K.; Wissinger, B.; Gollnick, H.; Gielen, S.; Frank, J.; Beck, S.; Zrenner, E. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest. Ophthalmol. Vis. Sci. 1999, 40, 3–11. [Google Scholar] [PubMed]
- Folli, C.; Viglione, S.; Busconi, M.; Berni, R. Biochemical basis for retinol deficiency induced by the I41N and G75D mutations in human plasma retinol-binding protein. Biochem. Biophys. Res. Commun. 2005, 336, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Bok, D. The retinal pigment epithelium: a versatile partner in vision. J. Cell Sci. Suppl. 1993, 17, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sun, H. A genetic clog in the vitamin A transport machinery. Cell 2015, 161, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Banhegyi, G.; Braun, L.; Csala, M.; Puskas, F.; Mandl, J. Ascorbate metabolism and its regulation in animals. Free Radic. Biol. Med. 1997, 23, 793–803. [Google Scholar] [CrossRef]
- Yahyavi, M.; Abouzeid, H.; Gawdat, G.; de Preux, A.S.; Xiao, T.; Bardakjian, T.; Schneider, A.; Choi, A.; Jorgenson, E.; Baier, H.; et al. ALDH1a3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Hum. Mol. Genet. 2013, 22, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, H.; Favez, T.; Schmid, A.; Agosti, C.; Youssef, M.; Marzouk, I.; El Shakankiry, N.; Bayoumi, N.; Munier, F.L.; Schorderet, D.F. Mutations in ALDH1a3 represent a frequent cause of microphthalmia/anophthalmia in consanguineous families. Hum. Mutat. 2014, 35, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Matt, N.; Dupe, V.; Garnier, J.M.; Dennefeld, C.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005, 132, 4789–4800. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Chitayat, D.; Caron, V.; Chassaing, N.; Bitoun, P.; Patry, L.; Cordier, M.P.; Capo-Chichi, J.M.; Francannet, C.; Calvas, P.; et al. Recessive and dominant mutations in retinoic acid receptor beta in cases with microphthalmia and diaphragmatic hernia. Am. J. Hum. Genet. 2013, 93, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.B.; Dupe, V.; Dierich, A.; Messaddeq, N.; Garnier, J.M.; Rochette-Egly, C.; Chambon, P.; Mark, M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997, 41, 425–447. [Google Scholar] [PubMed]
- Sun, H.; Molday, R.S.; Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 1999, 274, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Tsybovsky, Y.; Molday, R.S.; Palczewski, K. The ATP-binding cassette transporter ABCA4: Structural and functional properties and role in retinal disease. Adv. Exp. Med. Biol. 2010, 703, 105–125. [Google Scholar] [PubMed]
- Quazi, F.; Lenevich, S.; Molday, R.S. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 2012, 3, 925. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mir, A.; Paloma, E.; Allikmets, R.; Ayuso, C.; del Rio, T.; Dean, M.; Vilageliu, L.; Gonzalez-Duarte, R.; Balcells, S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat. Genet. 1998, 18, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Cremers, F.P.; van de Pol, D.J.; van Driel, M.; den Hollander, A.I.; van Haren, F.J.; Knoers, N.V.; Tijmes, N.; Bergen, A.A.; Rohrschneider, K.; Blankenagel, A.; et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene abcr. Hum. Mol. Genet. 1998, 7, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Chou, S.; Desai, A.; Hoppel, C.L.; Matsuyama, S.; Palczewski, K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009, 284, 15173–15183. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Mark, M.; Jacobs, H.; Klopfenstein, M.; Hu, J.; Lloyd, M.; Habib, S.; Tosha, C.; Radu, R.A.; Ghyselinck, N.B.; et al. Retinoid content, visual responses and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Nau, H. Teratogenicity of isotretinoin revisited: Species variation and the role of all-trans-retinoic acid. J. Am. Acad. Dermatol. 2001, 45, S183–S187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.P.; Furr, H.C.; Tanumihardjo, S.A. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp. Biol. Med. (Maywood) 2008, 233, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. Apo-RBPp, holo-RBP, and insulin resistance. Mol. Cell Biol. 2014, 34, 2105–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. How free retinol behaves differently from rbp-bound retinol in rbp receptor-mediated vitamin A uptake. Mol. Cell Biol. 2014, 34, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jin, H.; Majumdar, A.; Noy, N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA 2011, 108, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; O'Byrne, S.M.; Vreeland, A.C.; Blaner, W.S.; Noy, N. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol. Cell Biol. 2012, 32, 3164–3175. [Google Scholar] [CrossRef] [PubMed]
- Erikstrup, C.; Mortensen, O.H.; Nielsen, A.R.; Fischer, C.P.; Plomgaard, P.; Petersen, A.M.; Krogh-Madsen, R.; Lindegaard, B.; Erhardt, J.G.; Ullum, H.; et al. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Motani, A.; Wang, Z.; Conn, M.; Siegler, K.; Zhang, Y.; Liu, Q.; Johnstone, S.; Xu, H.; Thibault, S.; Wang, Y.; et al. Identification and characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo. J. Biol. Chem. 2009, 284, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Preitner, F.; Mody, N.; Graham, T.E.; Peroni, O.D.; Kahn, B.B. Long-term fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1420–E1429. [Google Scholar] [CrossRef] [PubMed]
- Norseen, J.; Hosooka, T.; Hammarstedt, A.; Yore, M.M.; Kant, S.; Aryal, P.; Kiernan, U.A.; Phillips, D.A.; Maruyama, H.; Kraus, B.J.; et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-jun n-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell Biol. 2012, 32, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Farjo, K.M.; Farjo, R.A.; Halsey, S.; Moiseyev, G.; Ma, J.X. Retinol-binding protein 4 induces inflammation in human endothelial cells by a NADPH oxidase- and nuclear factor kappa b-dependent and retinol-independent mechanism. Mol. Cell Biol. 2012, 32, 5103–5115. [Google Scholar] [CrossRef] [PubMed]
- Muenzner, M.; Tuvia, N.; Deutschmann, C.; Witte, N.; Tolkachov, A.; Valai, A.; Henze, A.; Sander, L.E.; Raila, J.; Schupp, M. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor alpha activity. Mol. Cell Biol. 2013, 33, 4068–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients 2012, 4, 2069–2096. [Google Scholar] [CrossRef] [PubMed]
- Skazik, C.; Amann, P.M.; Heise, R.; Marquardt, Y.; Czaja, K.; Kim, A.; Ruhl, R.; Kurschat, P.; Merk, H.F.; Bickers, D.R.; et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J. Invest. Dermatol. 2014, 134, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.B.; Gudas, L.J. Crossing the barrier: STRA6 in epidermal differentiation. J. Invest. Dermatol. 2014, 134, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ross, A.C. Inflammation induced by lipopolysaccharide does not prevent the vitamin A and retinoic acid-induced increase in retinyl ester formation in neonatal rat lungs. Br. J. Nutr. 2013, 109, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zolfaghari, R.; Ross, A.C. Acidic retinoids in small amounts promote retinyl ester formation in neonatal lung, with transient increases in retinoid homeostatic gene expression. Nutr. Metab. (Lond.) 2013, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.B.; Kashyap, V.; Scandura, J.; Gudas, L.J. An alternative retinoic acid-responsive STRA6 promoter regulated in response to retinol deficiency. J. Biol. Chem. 2015, 290, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Jones, T.A.; Aqvist, J.; Sundelin, J.; Eriksson, U.; Rask, L.; Peterson, P.A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984, 3, 1451–1454. [Google Scholar] [PubMed]
- Breen, C.J.; Martin, D.S.; Ma, H.; McQuaid, K.; O'Kennedy, R.; Findlay, J.B. Production of functional human vitamin a transporter/RBP receptor (STRA6) for structure determination. PLoS One 2015, 10, e0122293. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6. Membranes 2015, 5, 425-453. https://doi.org/10.3390/membranes5030425
Kawaguchi R, Zhong M, Kassai M, Ter-Stepanian M, Sun H. Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6. Membranes. 2015; 5(3):425-453. https://doi.org/10.3390/membranes5030425
Chicago/Turabian StyleKawaguchi, Riki, Ming Zhong, Miki Kassai, Mariam Ter-Stepanian, and Hui Sun. 2015. "Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6" Membranes 5, no. 3: 425-453. https://doi.org/10.3390/membranes5030425
APA StyleKawaguchi, R., Zhong, M., Kassai, M., Ter-Stepanian, M., & Sun, H. (2015). Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6. Membranes, 5(3), 425-453. https://doi.org/10.3390/membranes5030425




