Interaction Study of Phospholipid Membranes with an N-Glucosylated ?-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence Measurements on LUVs
2.1.1. Peptide-Membrane Association

2.1.2. Membrane perturbation induced by the type I’ β-turn peptide structure CSF114

2.2. Electrochemical Measurements at Mercury-Supported Lipid SAMs and tBLMs
2.2.1. Interaction of the type I’ β-turn peptide structure CSF114 with Hg-supported DOPC and DOPS SAMs




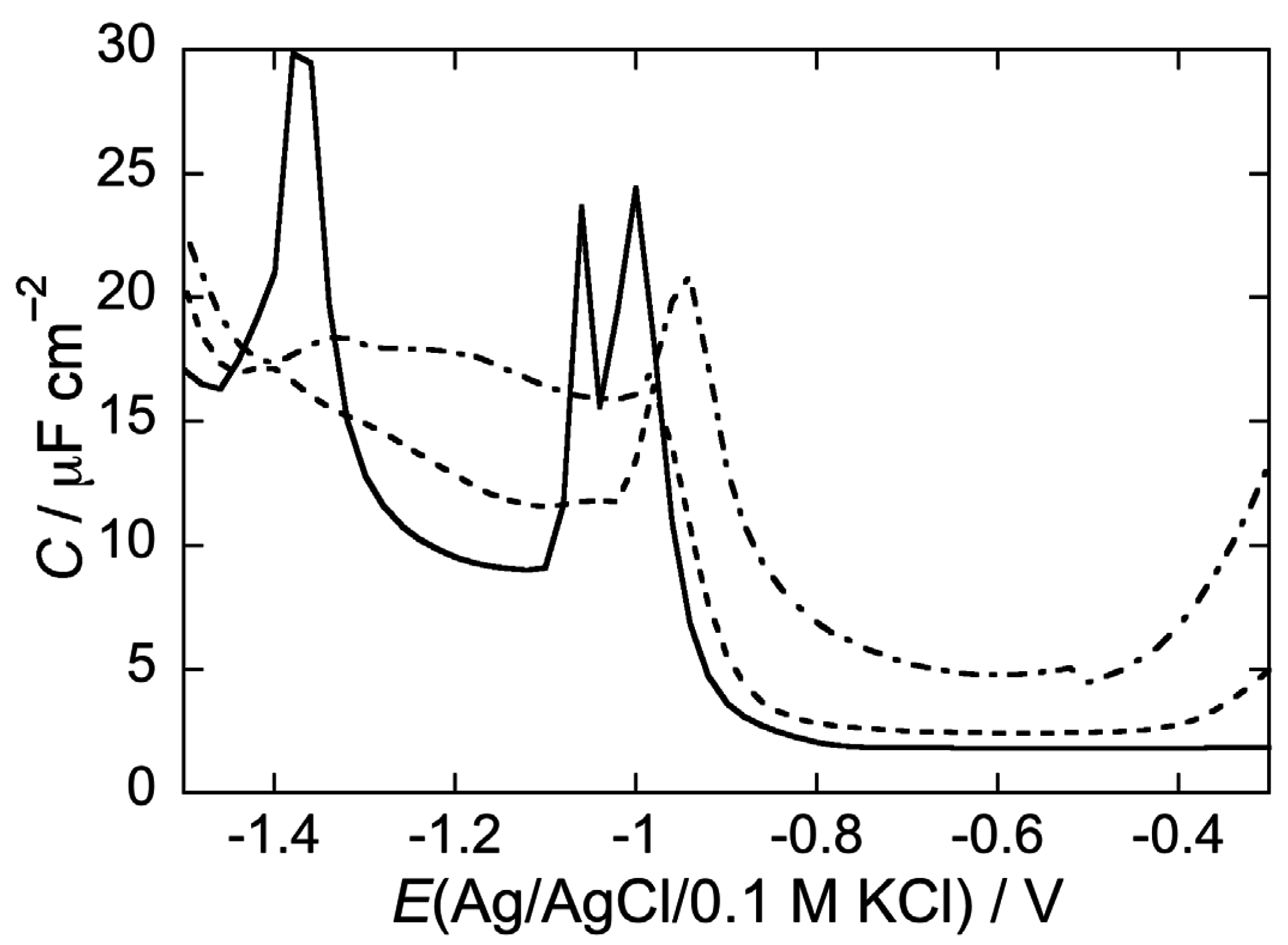


2.2.2. Interaction of the type I’ β-turn peptide CSF114 with Hg-supported DPTL/DOPC and DPTL/DOPS tBLMs
3. Experimental Section
3.1. Liposome Preparation
3.2. Fluorescence Measurements
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Mazzucco, S.; Matà, S.; Vergelli, M.; Fioresi, R.; Nardi, E.; Mazzanti, B.; Chelli, M.; Lolli, F.; Ginanneschi, M.; Pinto, F.; Massacesi, L.; Papini, A.M. A synthetic glycopeptide of human myelin oligodendrocyte glycoprotein to detect antibody responses in multiple sclerosis and other neurological diseases. Bioorg. Med. Chem. Lett. 1999, 9, 167–172. [Google Scholar] [CrossRef]
- Carotenuto, A.; D’Ursi, A.M.; Nardi, E.; Papini, A.M.; Rovero, P. Conformational analysis of a glycosylated human myelin oligodendrocyte glycoprotein peptide epitope able to detect antibody response in multiple sclerosis. J. Med. Chem. 2001, 44, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Mulinacci, B.; Carotenuto, A.; Bonetti, B.; Sabatino, G.; Mazzanti, B.; D’Ursi, A.M.; Novellino, E.; Pazzagli, M.; Lovato, L.; et al. An N-glucosylated peptide detecting disease-specific autoantibodies, biomarkers of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2005, 29, 10273–10278. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Mazzanti, B.; Pazzagli, M.; Peroni, E.; Alcaro, M.C.; Lanzillo, R.; Brescia Morra, V.; Santoro, L.; Gasperini, C.; Galgani, S.; et al. The glycopeptide CSF114(Glc) detects serum antibodies in multiple sclerosis. J. Neuroimmunol. 2005, 167, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolli, F.; Rovero, P.; Chelli, M.; Papini, A.M. Toward biomarkers in multiple sclerosis: new advances. Expert Rev. Neurother. 2006, 6, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Kunze, J.; Leitch, J.; Schwan, A.L.; Faragher, R.J.; Naumann, R.; Schiller, S.; Knoll, W.; Dutcher, J.R.; Lipkowski, J. New method to measure packing densities of self-assembled thiolipid monolayers. Langmuir 2006, 22, 5509–5519. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Guidelli, R. Equilibrium distribution of K+ ions in the hydrophilic spacer of tethered bilayer lipid membranes. Soft Matter 2009, 5, 2294–2301. [Google Scholar] [CrossRef]
- Becucci, L.; Romero Léon, R.; Moncelli, M.R.; Rovero, P.; Guidelli, R. Electrochemical investigation of melittin reconstituted into a mercury-supported lipid bilayer. Langmuir 2006, 22, 6644–6650. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Moncelli, M.R.; Guidelli, R. Impedance spectroscopy of OmpF porin reconstituted into a Hg-supported lipid bilayer. Langmuir 2006, 22, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Santucci, A.; Guidelli, R. Gramicidin conducting dimers in lipid bilayers are stabilized by single-file ionic flux along them. J. Phys. Chem. B 2007, 111, 9814–9820. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Guidelli, R. Kinetics of channel formation in bilayer lipid membranes (BLMs) and tethered BLMs: monazomycin and melittin. Langmuir 2007, 23, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Carbone, M.V.; Biagiotti, T.; D'Amico, M.; Olivotto, M.; Guidelli, R. Incorporation of the HERG potassium channel in a mercury supported lipid bilayer. J. Phys. Chem. B 2008, 112, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Papini, M.; Muller, D.; Scaloni, A.; Veglia, G.; Guidelli, R. Probing membrane permeabilization by the antimicrobial peptide distinctin in mercury-supported biomimetic membranes. Biochim. Biophys. Acta 2011, 1808, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Maran, F.; Guidelli, R. Probing membrane permeabilization by the antibiotic lipopeptaibol trichogin GA IV in a tethered bilayer lipid membrane. Biochim. Biophys. Acta 2012, 1818, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Valensin, D.; Innocenti, M.; Guidelli, R. Dermcidin, an anionic antibiotic peptide: influence of lipid charge, pH and Zn2+ on its interaction with a biomimetic membrane. Soft Matter 2014, 10, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Guidelli, R. Mercury-supported biomimetic membranes for the investigation of antimicrobial peptides. Pharmaceuticals 2014, 7, 136–168. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Guidelli, R. An Electrochemical Platform for Studying Biomebrane Processes. Mercury-Supported Biomimetic Membranes; Lambert Academic: Saarbrücken, Germany, 2014. [Google Scholar]
- Nelson, A.; Benton, A. Phospholipid monolayers at the mercury/water interface. J. Electroanal. Chem. 1986, 202, 253–270. [Google Scholar] [CrossRef]
- Nelson, A. Electrochemical studies of thallium(I) transport across gramicidin modified electrode-adsorbed phospholipid monolayers. J. Electroanal. Chem. 1991, 303, 221–236. [Google Scholar]
- Becucci, L.; Moncelli, M.R.; Guidelli, R. Thallous ion movements through gramicidin channels incorporated in lipid monolayers supported by mercury. Biophys. J. 2002, 82, 852–864. [Google Scholar] [CrossRef]
- Baldini, C.; Peggion, C.; Falletta, E.; Moncelli, M.R.; Guidelli, R.; Toniolo, C. A lipid monolayer made permeable to Tl(I) ions by the lipopeptaibol trichogin GA IV. In Understanding Biology Using Peptides; Blondelle, S.E., Ed.; American Peptide Society: New York, NY, USA, 2005; pp. 265–266. [Google Scholar]
- Stoodley, R.; Shepherd, J.; Wasan, K.M.; Bizzotto, D. Amphotericin B interactions with a DOPC monolayer. Electrochemical investigations. Biochim. Biophys. Acta 2002, 1564, 289–297. [Google Scholar] [CrossRef]
- Becucci, L.; Innocenti, M.; Bellandi, S.; Guidelli, R. Permeabilization of mercury-supported biomimetic membranes by amphotericin B and the role of calcium ions. Electrochim. Acta 2013, 112, 719–726. [Google Scholar] [CrossRef]
- Bocchinfuso, G.; Bobone, S.; Palleschi, A.; Stella, L. Fluorescence spectroscopy and molecular dynamics simulations in studies on the mechanism of membrane destabilization by antimicrobial peptides. Cell. Mol. Life Sci. 2011, 68, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Bocchinfuso, G.; Park, Y.; Palleschi, A.; Hahm, K.-S.; Stella., L. The importance of being kinked: the role of Pro residues in the selectivity of the helical antimicrobial peptide P5. J. Pept. Sci. 2013, 19, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, C.; Orioni, B.; Coletta, M.; Formaggio, F.; Toniolo, C.; Maulucci, G.; De Spirito, M.; Pispisa, B.; Venanzi, M.; Stella, L. Fluctuations and the rate-limiting step of peptide-induced membrane leakage. Biophys. J. 2010, 99, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Bizzotto, D.; Nelson, A. Continuing electrochemical studies of phospholipid monolayers of dioleoyl phosphatidylcholine at the mercury-electrolyte interface. Langmuir 1998, 14, 6269–6273. [Google Scholar] [CrossRef]
- Bizzotto, D.; Yang, Y.; Shepherd, J.L.; Stoodley, R.; Agak, J.; Stauffer, V.; Lathuillière, M.; Akhtar, A.S.; Chung, E. Electrochemical and spectroelectrochemical characterization of lipid organization in an electric field. J. Electroanal. Chem. 2004, 574, 167–184. [Google Scholar] [CrossRef]
- Nelson, A.; Auffret, N.; Borlakoglu, J. Interaction of hydrophobic organic compounds with mercury adsorbed dioleoylphosphatidylcholine monolayers. Biochim. Biophys. Acta 1990, 1021, 205–216. [Google Scholar] [CrossRef]
- Lecompte, M.-F.; Bras, A.-C.; Dousset, N.; Portas, I.; Salvayre, R.; Ayrault-Jarrier, M. Binding steps of apolipoprotein A-I with phospholipid monolayers: Adsorption and penetration. Biochemistry 1998, 37, 16165–16171. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Moncelli, M.R.; Herrero, R.; Guidelli, R. Dipole potentials of monolayers of phosphatidylcholine, phosphatidylserine and phosphatidic acid on mercury. Langmuir 2000, 16, 7694–7700. [Google Scholar] [CrossRef]
- Moncelli, M.R.; Becucci, L.; Tadini Buoninsegni, F.; Guidelli, R. Surface dipole potential at the interface between water and self-assembled monolayers of phosphatidylserine and phosphatidic acid. Biophys. J. 1998, 74, 2388–2397. [Google Scholar] [CrossRef]
- Moncelli, M.R.; Becucci, L.; Guidelli, R. The intrinsic pKa values for phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine in monolayers deposited on mercury electrodes. Biophys. J. 1994, 66, 1969–1980. [Google Scholar] [CrossRef]
- Becucci, L.; Foresti, M.L.; Schwan, A.; Guidelli, R. Can proton pumping by SERCA enhance the regulatory role of phospholamban and sarcolipin? Biochim. Biophys. Acta 2013, 1828, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, R.; Becucci, L. Estimate of the potential difference across metal/water interfaces and across the lipid bilayer moiety of biomimetic membranes: an approach. Soft Matter 2011, 7, 2195–2201. [Google Scholar] [CrossRef]
- Papini, A.M. Simple test for multiple sclerosis. Nat. Med. 2005, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Real-Fernández, F.; Passalacqua, I.; Peroni, E.; Chelli, M.; Lolli, F.; Papini, A.M.; Rovero, P. Glycopeptide-based antibody detection in multiple sclerosis by surface plasmon resonance. Sensors 2012, 12, 5596–5607. [Google Scholar] [CrossRef] [PubMed]
- Real-Fernández, F.; Rossi, G.; Lolli, F.; Papini, A.M.; Rovero, P. Label-free method for anti-glucopeptide antibody detection in multiple sclerosis. Methods X 2015, 2, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; D’Ursi, A.M.; Mulinacci, B.; Paolini, I.; Lolli, F.; Papini, A.M.; Novellino, E.; Rovero, P. Conformation-activity relationship of designed glycopeptides as synthetic probes for the detection of autoantibodies, biomarkers of multiple sclerosis. J. Med. Chem. 2006, 49, 5072–5079. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Alcaro, M.C.; Saviello, M.R.; Peroni, E.; Nuti, F.; Papini, A.M.; Novellino, E.; Rovero, P. Designed glycopeptides with different β-turn types as synthetic probes for the detection of autoantibodies as biomarkers of multiple sclerosis. J. Med. Chem. 2008, 51, 5304–5309. [Google Scholar] [CrossRef] [PubMed]
- Nuti, F.; Paolini, I.; Cardona, F.; Chelli, M.; Lolli, F.; Brandi, A.; Goti, A.; Rovero, P.; Papini, A.M. FMOC-protected iminosugar modified asparagine derivatives as building blocks for glycomimetics-containing peptides. Bioorg. Med. Chem. 2007, 15, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Nuti, F.; Peroni, E.; Real-Fernández, F.; Bonache, M.A.; Le Chavalier-Isaad, A.; Chelli, M.; Lubin-Germain, N.; Uziel, J.; Rovero, P.; Lolli, F.; Papini, A.M. Post-translationally modified peptides efficiently mimicking neo-antigens: A challenge for theragnostics of autoimmune diseases. Biopolymers (Pept. Sci.) 2010, 94, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.M. The use of post-translationally modified peptides for detection of biomarkers of immune-mediated diseases. J. Pept. Sci. 2009, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Guidelli, R.; Becucci, L. Electrochemistry of biomimetic membranes. In Applications of Electrochemistry and Nanotechnology in Biology and Medicine; Eliaz, N., Ed.; Springer: New York, NY, USA, 2011; Volume 53, pp. 147–266. [Google Scholar]
- Brown, D.A.; London, E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998, 164, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A. To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006, 1758, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Becucci, L.; Martinuzzi, S.; Monetti, E.; Mercatelli, R.; Quercioli, F.; Battistel, D.; Guidelli, R. Electrochemical impedance spectroscopy and fluorescence lifetime imaging of lipid mixtures self-assembled on mercury. Soft Matter 2010, 6, 2733–2741. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, R.F.M.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Lipid rafts have different sizes depending on membrane composition: A time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 2005, 346, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.M.; Loura, M.S.; Prieto, M. Membrane lipid domains and rafts: current applications of fluorescence lifetime spectroscopy and imaging. Chem. Phys. Lipids 2009, 157, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–72. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, F.; Sabatino, G.; Chelli, M.; Rovero, R.; Papini, A.M. A convenient microwave-enhanced solid-phase synthesis of difficult peptide sequences: Case study of gramicidin A and CSF114(Glc). Int. J. Pept. Res. Ther. 2007, 13, 203–208. [Google Scholar] [CrossRef]
- Stella, L.; Mazzuca, C.; Venanzi, M.; Palleschi, A.; Didoné, M.; Formaggio, F.; Toniolo, C.; Pispisa, B. Aggregation and water-membrane partition as major determinants of the activity of the antibiotic peptide trichogin GA IV. Biophys. J. 2004, 86, 936–945. [Google Scholar] [CrossRef]
- Moncelli, M.R.; Becucci, L. A novel model of the hanging mercury drop electrode. J. Electroanal. Chem. 1997, 433, 91–96. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becucci, L.; Benci, S.; Nuti, F.; Real-Fernandez, F.; Vaezi, Z.; Stella, L.; Venanzi, M.; Rovero, P.; Papini, A.M. Interaction Study of Phospholipid Membranes with an N-Glucosylated ?-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis. Membranes 2015, 5, 576-596. https://doi.org/10.3390/membranes5040576
Becucci L, Benci S, Nuti F, Real-Fernandez F, Vaezi Z, Stella L, Venanzi M, Rovero P, Papini AM. Interaction Study of Phospholipid Membranes with an N-Glucosylated ?-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis. Membranes. 2015; 5(4):576-596. https://doi.org/10.3390/membranes5040576
Chicago/Turabian StyleBecucci, Lucia, Stefano Benci, Francesca Nuti, Feliciana Real-Fernandez, Zahra Vaezi, Lorenzo Stella, Mariano Venanzi, Paolo Rovero, and Anna Maria Papini. 2015. "Interaction Study of Phospholipid Membranes with an N-Glucosylated ?-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis" Membranes 5, no. 4: 576-596. https://doi.org/10.3390/membranes5040576
APA StyleBecucci, L., Benci, S., Nuti, F., Real-Fernandez, F., Vaezi, Z., Stella, L., Venanzi, M., Rovero, P., & Papini, A. M. (2015). Interaction Study of Phospholipid Membranes with an N-Glucosylated ?-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis. Membranes, 5(4), 576-596. https://doi.org/10.3390/membranes5040576







