3.1.2. Total Recirculation Tests
Figure 3 shows the TMP variation in the different 24 h assays conducted with the chosen permeate flux (67 L·m
−2·h
−1) and different flux maintenance strategies (detailed in
Table 3).
The effectiveness of the different flux maintenance strategies was calculated using Equation (1), where ∆
TMPT1 is the total variation of the TMP in the 24 h of test without flux maintenance strategy (test 1) and ∆
TMPtest refers to the variation of TMP in each test.
In tests 2 and 3 a positive effect of backpulse (test 2) and backpulse combined with backwashing each 2 h (test 3) was observed compared with test 1 (no flux maintenance strategies and a TMP variation of 0.53 bar in the 24 h of assay). A transmembrane pressure variation of 0.48 and 0.43 bar in the 24 h was observed in tests 2 and 3, respectively. When the filtration assay was performed with backpulses each 10 min and backwashing each hour (test 4), a transmembrane pressure variation of 0.28 bar was obtained, nearly half the variation of transmembrane pressure observed when no flux maintenance strategies were applied, indicating that these strategies are rather efficient for fouling mitigation.
The higher effectiveness (𝝶) value presented in
Table 4 indicates a lower fouling potential when the combined flux maintenance strategies were applied in test 4. The trend observed was expected: as the flux maintenance strategies are intensified, the effectiveness increases.
After test 4, in order to improve the permeate production, a new test was performed increasing 50% of the controlled permeate flux (100 L·m−2·h−1). However, under these conditions, a flux decrease of 55% was observed in the 24 hour assay which indicated that, for the oily wastewater tested, it was not possible to maintain this higher flux even when the flux maintenance systems are applied.
Using the optimal conditions (test 4), that allowed operation at a lower transmembrane pressure variation, a fouling rate was calculated using the TMP values recorded between 10 and 24 h. The fouling rate obtained (6 × 10−4 bar/h) was used to estimate the time needed to achieve 0.64 bar (the TMP obtained without flux maintenance strategies). The result obtained estimates an operation of 19 days using the optimal conditions proposed and shows that a long term continuous operation (without the need to stop the process and perform chemical cleanings) can be expected using these conditions.
Table 5 summarizes the percent rejection and adsorption/deposition related with the different parameters—total solids, total suspended solids, chemical oxygen demand, total organic carbon and oil and grease. The apparent rejection of the different parameters was calculated according to the following equation:
where
Cf is the concentration of the different parameters in the feed water,
Cp is the concentration of the different parameters in the permeate stream (
Table 6). The percent adsorption or deposition of the different parameters in the total recirculation tests was calculated according to Equation (3).
where
Cf0 and
Cf24 are the concentrations of the parameters in the feed tank at t = 0 and 24, respectively and
Vf0 and
Vf24 are the volumes of feed at t = 0 and 24 h, respectively.
Extremely high percent removals of total suspended solids (>99%) and oil and grease (>97%) were observed in tests 1–4.
Table 6 shows that membrane filtration ensures removals of these parameters until values lower than the legislation discharge limits. Removal of oil and grease is significantly due to adsorption/deposition on the membrane surface. The high adsorption/deposition of oil and grease was minimized by 48% using the optimized flux maintenance strategy (test 4).
Yang et al. [
35] prepared a ZrO
2/α-Al
2O
3 microfiltration membrane to treat oil-in-water emulsions, obtaining removals higher than 99% of oil. However, the hydraulic permeability of the microfiltration membranes were much lower than the hydraulic permeability of the silicon carbide membranes used in this work. Cui et al. [
23] also reported removals higher than 99% of oil when using NaA/α-Al
2O
3 membranes to treat oil-in-water emulsions. In this case, the permeate fluxes were only 5 and 18 L·m
−2·h
−1, with a filtration time of 600 min. Regarding polymeric membranes, good oil and grease removals were also reported but with higher transmembrane pressures [
36,
37]. Ochando-Pulido et al. [
38] achieved extremely high removals of total suspended solids from olive mill wastewaters by an ultrafiltration process using polymeric membranes but, once again, with fluxes not higher than 10 L·m
−2·h
−1. The silicon carbide membranes tested in this study ensure extremely high removals of oil and grease and total suspended solids allowing high permeate fluxes with low transmembrane pressure.
Lower removals of total solids, chemical oxygen demand and total organic carbon were observed, achieving up to 69% of chemical oxygen demand rejection in test 4 and 68% of total organic carbon rejection in test 3. Using the optimized conditions, higher values of rejection of all the tested parameters, except TOC, were obtained by ultrafiltration (this study,
Table 5, test 4) compared to the removal values obtained by dissolved air flotation reported in a previous study (Total solids: 27%, total suspended solids: 98%, COD: 67%, TOC: 72%, Oil and grease: 77%; [
39]). Ultrafiltration can therefore be applied instead of flotation for the treatment of olive mill wastewaters. COD removal was not enough to achieve values under the limit legislated. However, good percent removals were achieved when compared with other studied membrane processes: Coskun et al. [
40] achieved the same range of removals combining ultrafiltration and nanofiltration to treat olive mill wastewaters. A previous study [
41] obtained a maximum removal of 15% of COD from an olive mill wastewater using a regenerated cellulose membrane in dead-end configuration. The results obtained in this study are extremely promising since tests were performed using robust ceramic membranes and in conditions closer to reality in terms of flow dynamics. The membranes tested can achieve good removals with only one membrane step, maintaining a high permeate flux, during prolonged operation periods, with a low transmembrane pressure increase.
Higher percent adsorption/deposition values were reported in the assay without flux maintenance strategies (test 1) compared to the assays conducted with backpulse (test 2) and the tests conducted with backpulse and backwash (tests 3 and 4). These results were expected since backpulse and backwash are used to release the fouling components from the membrane surface.
The total membrane resistance (
Rt), corresponding to the sum of the membrane resistance (
Rm) and the resistance due to fouling (
Rf) in tests 1 to 4 was calculated at t = 24 h using Equation (4):
where
TMP refers to the transmembrane pressure,
J to the permeate flux and µ
t to the fluid viscosity corrected to the working temperature, according to Equation (5) [
42]:
The value of the membrane resistance was determined (
Rm = 1.58 × 10
11 m
−1) using the value of the hydraulic permeability determined during clean water flux measurements. In order to analyse the effect of the cleaning strategies in the fouling formation, the values of resistance due to fouling of tests 1–4 were calculated and results clearly show the effect of backpulse and backwash strategies in the total resistance of the membrane. In test 1, conducted without flux maintenance strategies, the resistance of the membrane due to fouling at the end of the test was 4.14 × 10
12 m
−1. The use of backpulses each 10 min resulted in a decrease of the resistance of the membrane due to fouling to 3.75 × 10
12 m
−1. With backwashes each two hours in addition to the backpulses (
Rf = 3.54 × 10
12 m
−1) the difference was minor but when backwashes were performed each hour an accentuated decrease in membrane resistance due to fouling was observed (2.29 × 10
12 m
−1). The considerable reduction in the resistance due to fouling, observed in test 4, may be interpreted taking into consideration the results presented in
Table 5. The only parameter that could justify this difference taking into account the deposition/adsorption results is oil and grease. Therefore, it can be concluded that the reduction of the fouling resistance can be due to an effective release of oil and grease from the surface of the membrane when backpulses each 10 min are combined with backwashes every hour. The conditions employed in test 4 were therefore applied in a final concentration study, that better simulates real filtration conditions, conducted with total recirculation of the retentate and total recovery of the permeate.
3.1.3. Optimization of Membrane Cleaning
In order to optimize the cleaning protocol of the membrane, different cleaning solutions were tested and analysed in terms of total suspended solids and oil and grease—the contaminants considered to be the most important in fouling formation. In all tests, the first cleaning step was a rinsing step with hot water (60 ± 5 °C). Alkaline and acid solutions were tested after the rinsing step, and the effect of the temperature of the cleaning solutions was studied.
The first approach included the use of a 4% NaOH solution, recommended by the membrane manufacturer since it has a low cost, is easily available and can efficiently remove the oil and grease adsorbed on the surface of the membrane [
43].
Figure 4a shows the permeability of the membrane recovered after each cleaning step in test 1. Results show that rinsing and using NaOH at controlled temperature (60 ± 5 °C) was not enough to recover the permeability of the membrane. A solution of 2% citric acid was therefore employed. The results obtained show that the permeability was totally restored. It was thus concluded that the use of an acid solution may also be important to recover the permeability of the membrane with this wastewater.
After test 2 (
Figure 4b), the strategy to clean the membrane was therefore the use of both acid and alkaline solutions at 60 ± 5 °C after an initial rinsing step. In this protocol, the sequence of steps was inverted, with the acid cleaning performed before the alkaline cleaning. The acid solution by itself was not enough to recover the permeability and only 6.5% of the adsorbed total suspended solids were recovered in this step. Even though total suspended solids and oil and grease were not detected after the acid cleaning step, a quick recovery of permeability was obtained using the consecutive acid and basic cleaning agents.
To understand if the use of a high temperature was really needed, cleaning after test 3 was performed with acid and alkaline solutions at room temperature—25 °C after rinsing with hot water (
Figure 7c). The cleanings performed at 25 °C were not enough to restore the membrane permeability. After cleaning with NaOH at 60 ± 5 °C, the permeability was totally restored, so it was concluded that it is necessary to increase the temperature at least in one step of the chemical cleaning. A total of 100% of the suspended solids and 23% of the oil and grease adsorbed on the membrane were detected after analysing the cleaning solutions. Again, rinsing with hot water proved to be the most important step in the removal of total suspended solids (57%) and oil and grease (100%); 4% of the adsorbed total suspended solids was detected in the acid solution.
To study if the sequence of the chemical cleanings was important, in test 4 (
Figure 4d) this procedure was performed after rinsing with an alkaline cleaning followed by an acid cleaning both at 60 ± 5 °C. It was observed that the permeability was totally restored after a sequence of basic and acid cleaning steps, indicating that the sequence does not seem to be important (compared with
Figure 7b). Nevertheless, alternating alkaline and acid cleanings seems to be important in addition to the temperature: results indicate that the first chemical cleaning contributes to the destructuring of the existent fouling facilitating the subsequent cleaning. Furthermore, 75% of the total suspended solids removed using this cleaning protocol were recovered in the rinsing step, 21% in the alkaline cleaning and 4% in the acid cleaning. In sum, 82% of the total suspended solids adsorbed on the membrane surface were recovered in the cleaning procedure. All the adsorbed oil and grease were recovered in the rinsing step. Since the permeate flux was totally restored after the proposed cleaning procedure, the results indicate that total suspended solids, oil and grease and inorganic matter are important agents involved in fouling formation during the filtration of these wastewaters.
3.1.4. Concentration Test
Figure 5 shows the TMP variation during the concentration test. During the first hour, the flux was set at 67 L·m
−2·h
−1 and backpulses every 10 min were performed in addition to backwashes every hour; the optimized conditions were determined in the total recirculation tests. The transmembrane pressure variation during the first hour was only 0.02 bar, very low compared to 0.15 bar variation obtained in the same period in the total recirculation assay—
Figure 3d. In order to increase the process efficiency, the controlled permeate flux was therefore increased 50% in relation to the initial permeate flux, to 100 L·m
−2·h
−1, while keeping the flux maintenance strategies previously optimized.
The lower transmembrane pressure variation in this assay was due to a better quality of the large volume of oily wastewater received for the concentration study (
Table 7), that was much less concentrated in terms of the water quality parameters analysed.
In these conditions, a final concentration factor (Equation (6)) of 5.2 was achieved, corresponding to a permeate recovery of 81%.
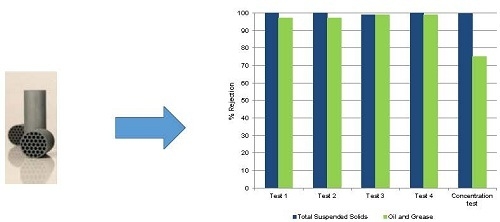
Figure 6 presents the percent rejection of the different parameters obtained in samples collected during the concentration assay.
Results show that the rejections of the different parameters monitored were maintained during the 7 h concentration study, evidencing that the quality of the permeate over time was not deteriorated despite the increasing concentration of the different components in the feed wastewater due to the total recirculation of the retentate. The results obtained in terms of rejection were consistent with the results previously obtained in the 24 h total recirculation test. The silicon carbide membranes used ensure high removals of total suspended solids and oil and grease. The value of membrane resistance at working temperature due to fouling at the end of this test was 2.31 × 1012 m−1.
Huang et al. [
44] adapted the Hermia’s model [
45] to describe fouling mechanisms in membrane processes performed at constant TMP and developed a similar one for membrane processes conducted at controlled permeate flux. This model was applied to the results obtained in the concentration test in order to identify the different fouling mechanisms involved. The results obtained indicate that the main fouling mechanism involved in this process is cake formation, since it presents the best coefficient of determination (0.92). This result is in accordance with other published studies, where the fouling formation during the ultrafiltration of oily wastewaters is mainly attributed to cake formation [
46,
47]. This fouling mechanism is attributed to the deposit of large molecules on the membrane surface. Results are thus in accordance with the assumption that total suspended solids and oil and grease are important parameters in fouling formation [
48].
Figure 7 relates the maximum TMP achieved before backwashes with the concentration of total suspended solids present in the feed at the same time. A linear regression with a coefficient of determination (R
2) of 0.99 was obtained, indicating a strong relationship between these two variables and confirming the influence of the concentration of total suspended solids present in the feed in the cake formation.
The optimized cleaning procedure was applied after the concentration test to restore the membrane permeability. In this case, after the alkaline step, the permeability was totally restored and the acid cleaning step was therefore not needed. This may be due to the better quality of the wastewater. All the adsorbed total suspended solids and oil and grease were recovered.