Synthesis, Transfer, and Gas Separation Characteristics of MOF-Templated Polymer Membranes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
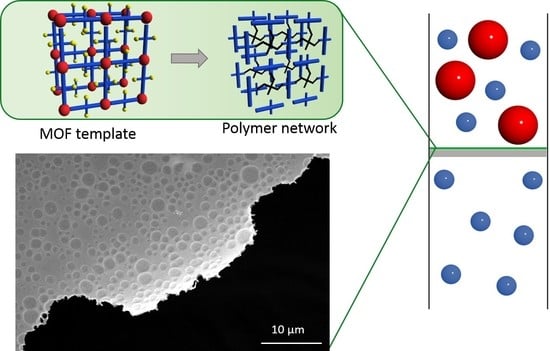
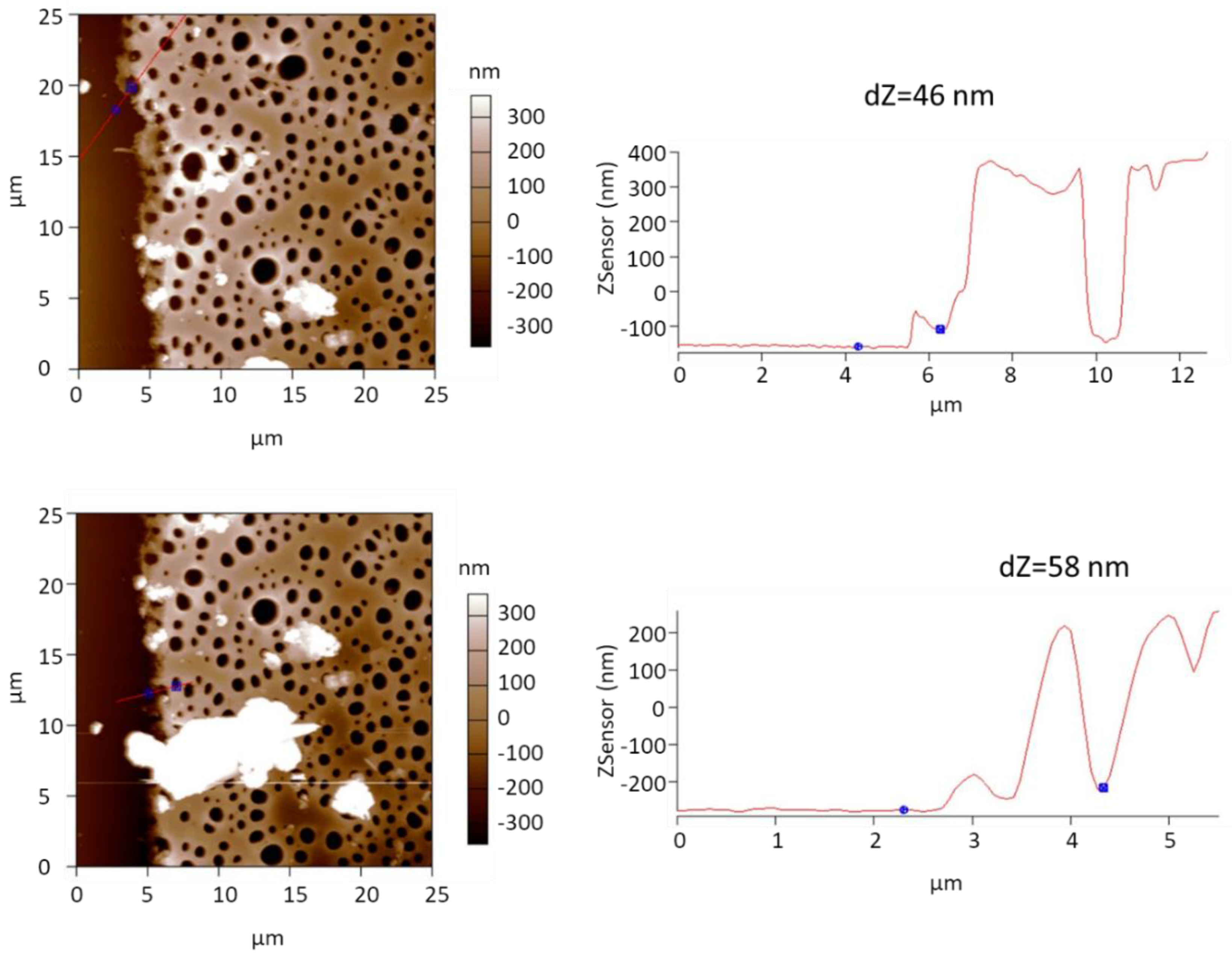
3.1. Preparation of Freestanding SURGEL Membranes
3.2. Gas Permeation Experiments
3.3. Activation Energies of the SURGEL Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Baker, R.W. Research needs in the membrane separation industry: Looking back, looking forward. J. Membr. Sci. 2010, 362, 134–136. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Membrane Preparation. In Membrane Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 9–14. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Gin, D.; Noble, R.D. Designing the Next Generation of Chemical Separation Membranes. Science 2011, 332, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, X.; Yang, X.; Bai, Y.; Shao, L. Nanoporous framework “reservoir” maximizing low-molecular-weight enhancer impregnation into CO2-philic membranes for highly-efficient CO2 capture. J. Membr. Sci. 2019, 570, 278–285. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Bai, Y.; Shao, L. Ultra-facile aqueous synthesis of nanoporous zeolitic imidazolate framework membranes for hydrogen purification and olefin/paraffin separation. J. Mater. Chem. A 2019, 7, 10898–10904. [Google Scholar] [CrossRef]
- Tsotsalas, M.; Liu, J.; Tettmann, B.; Grosjean, S.; Shahnas, A.; Wang, Z.; Azucena, C.; Addicoat, M.; Heine, T.; Lahann, J.; et al. Fabrication of Highly Uniform Gel Coatings by the Conversion of Surface-Anchored Metal–Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 8–11. [Google Scholar] [CrossRef]
- Shah, M.; McCarthy, M.C.; Sachdeva, S.; Lee, A.K.; Jeong, H.K. Current Status of Metal–Organic Framework Membranes for Gas Separations: Promises and Challenges. Ind. Eng. Chem. Res. 2012, 51, 2179–2199. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M.; O’Keeffe, M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Tee, L.; Zhao, D. Mixed matrix membranes composed of two-dimensional metal–organic framework nanosheets for pre-combustion CO2 capture: A relationship study of filler morphology versus membrane performance. J. Mater. Chem. A 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Kulprathipanja, S. Mixed Matrix Membrane Development. Ann. N. Y. Acad. Sci. 2003, 984, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S.; Chung, N.T.S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Husain, S.; Koros, W.J. Mixed matrix hollow fiber membranes made with modified HSSZ-13 zeolite in polyetherimide polymer matrix for gas separation. J. Membr. Sci. 2007, 288, 195–207. [Google Scholar] [CrossRef]
- Wuttke, S.; Medina, D.D.; Rotter, J.M.; Begum, S.; Stassin, T.; Ameloot, R.; Oschatz, M.; Tsotsalas, M. Bringing Porous Organic and Carbon-Based Materials toward Thin-Film Applications. Adv. Funct. Mater. 2018, 28, 1801545. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, R.A.; et al. Step-by-Step Route for the Synthesis of Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef]
- Ishiwata, T.; Furukawa, Y.; Sugikawa, K.; Kokado, K.; Sada, K. Transformation of Metal–Organic Framework to Polymer Gel by Cross-Linking the Organic Ligands Preorganized in Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 5427–5432. [Google Scholar] [CrossRef]
- Lindemann, P.; Tsotsalas, M.; Shishatskiy, S.; Abetz, V.; Krolla-Sidenstein, P.; Azucena, C.; Monnereau, L.; Beyer, A.; Gölzhäuser, A.; Mugnaini, V.; et al. Preparation of Freestanding Conjugated Microporous Polymer Nanomembranes for Gas Separation. Chem. Mater. 2014, 26, 7189–7193. [Google Scholar] [CrossRef]
- Schmitt, S.; Hümmer, J.; Kraus, S.; Welle, A.; Grosjean, S.; Hanke-Roos, M.; Rosenhahn, A.; Bräse, S.; Wöll, C.; Lee-Thedieck, C.; et al. Tuning the Cell Adhesion on Biofunctionalized Nanoporous Organic Frameworks. Adv. Funct. Mater. 2016, 26, 8455–8462. [Google Scholar] [CrossRef]
- Begum, S.; Hassan, Z.; Bräse, S.; Wöll, C.; Tsotsalas, M. Metal–Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019, 52, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Begum, S.; Wang, Z.; Krolla, P.; Wagner, D.; Bräse, S.; Wöll, C.; Tsotsalas, M. High Antimicrobial Activity of Metal–Organic Framework-Templated Porphyrin Polymer Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Diring, S.; Weidler, P.G.; Begum, S.; Heißler, S.; Kitagawa, S.; Wöll, C.; Furukawa, S.; Tsotsalas, M. Localized Conversion of Metal–Organic Frameworks into Polymer Gels via Light-Induced Click Chemistry. Chem. Mater. 2017, 29, 5982–5989. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Wind, J.; Peinemann, K.V. Nanometric thin film membranes manufactured on square meter scale: Ultra-thin films for CO2 capture. Nanotechnology 2010, 21, 395301. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, P.; Träutlein, Y.; Wöll, C.; Tsotsalas, M. Layer-by-layer Synthesis and Transfer of Freestanding Conjugated Microporous Polymer Nanomembranes. J. Vis. Exp. 2015, 106, e53324. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Shishatskiy, S.; Wind, J.; Zhang, X.; Nottbohm, C.T.; Mellech, N.; Winter, A.; Vieker, H.; Qiu, J.; Dietz, K.-J.; et al. Carbon Nanomembranes (CNMs) Supported by Polymer: Mechanics and Gas Permeation. Adv. Mater. 2014, 26, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Shishatskii, A.M.; Yampol’skii, Y.P.; Peinemann, K.V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation. J. Membr. Sci. 2008, 307, 88–95. [Google Scholar] [CrossRef]
- Turchanin, A.; Beyer, A.; Nottbohm, C.T.; Zhang, X.; Stosch, R.; Sologubenko, A.; Mayer, J.; Hinze, P.; Weimann, T.; Gölzhäuser, A. One Nanometer Thin Carbon Nanosheets with Tunable Conductivity and Stiffness. Adv. Mater. 2009, 21, 1233–1237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Takeoka, S. Morphological Evolution within Spin-Cast Ultrathin Polymer Blend Films Clarified by a Freestanding Method. Macromolecules 2012, 45, 4315–4321. [Google Scholar] [CrossRef]
- Behling, D.; Hattenbach, K.; Ohlrogge, K.; Peinemann, K.V.; Wind, J. Method of Removing Organic Compounds from Air/Permanent Gas Mixtures. U.S. Patent 4,994,094, 19 February 1991. [Google Scholar]
- Henis, J.M.; Tripodi, M.K. Composite hollow fiber membranes for gas separation: The resistance model approach. J. Membr. Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. A Novel Approach to Gas Separations Using Composite Hollow Fiber Membranes. Sep. Sci. Technol. 1980, 15, 1059–1068. [Google Scholar] [CrossRef]
- Bux, H.; Feldhoff, A.; Cravillon, J.; Wiebcke, M.; Li, Y.S.; Caro, J. Oriented Zeolitic Imidazolate Framework-8 Membrane with Sharp H2/C3H8Molecular Sieve Separation. Chem. Mater. 2011, 23, 2262–2269. [Google Scholar] [CrossRef]
- Huang, A.; Caro, J. Covalent Post-Functionalization of Zeolitic Imidazolate Framework ZIF-90 Membrane for Enhanced Hydrogen Selectivity. Angew. Chem. Int. Ed. 2011, 50, 4979–4982. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Gas Separation with Membranes. In Membrane Technology; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 53–90. [Google Scholar]
- Huang, A.; Dou, W.; Caro, J. Steam-Stable Zeolitic Imidazolate Framework ZIF-90 Membrane with Hydrogen Selectivity through Covalent Functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Liang, F.; Steinbach, F.; Gesing, T.M.; Caro, J. Neutral and Cation-Free LTA-Type Aluminophosphate (AlPO4) Molecular Sieve Membrane with High Hydrogen Permselectivity. J. Am. Chem. Soc. 2010, 132, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Stannett, V. Simple Gases, Diffusion in Polymers; Academic Press: London, UK; New York, NY, USA, 1968; pp. 41–75. [Google Scholar]
- Yampolskii, Y.; Shishatskii, S.; Alentiev, A.; Loza, K. Correlations with and prediction of activation energies of gas permeation and diffusion in glassy polymers. J. Membr. Sci. 1998, 148, 59–69. [Google Scholar] [CrossRef]
- Wang, Z.; Knebel, A.; Grosjean, S.; Wagner, D.; Bräse, S.; Wöll, C.; Caro, J.; Heinke, L. Tunable molecular separation by nanoporous membranes. Nat. Commun. 2016, 7, 13872. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, S.; Shishatskiy, S.; Krolla, P.; An, Q.; Begum, S.; Welle, A.; Hashem, T.; Grosjean, S.; Abetz, V.; Bräse, S.; et al. Synthesis, Transfer, and Gas Separation Characteristics of MOF-Templated Polymer Membranes. Membranes 2019, 9, 124. https://doi.org/10.3390/membranes9100124
Schmitt S, Shishatskiy S, Krolla P, An Q, Begum S, Welle A, Hashem T, Grosjean S, Abetz V, Bräse S, et al. Synthesis, Transfer, and Gas Separation Characteristics of MOF-Templated Polymer Membranes. Membranes. 2019; 9(10):124. https://doi.org/10.3390/membranes9100124
Chicago/Turabian StyleSchmitt, Sophia, Sergey Shishatskiy, Peter Krolla, Qi An, Salma Begum, Alexander Welle, Tawheed Hashem, Sylvain Grosjean, Volker Abetz, Stefan Bräse, and et al. 2019. "Synthesis, Transfer, and Gas Separation Characteristics of MOF-Templated Polymer Membranes" Membranes 9, no. 10: 124. https://doi.org/10.3390/membranes9100124
APA StyleSchmitt, S., Shishatskiy, S., Krolla, P., An, Q., Begum, S., Welle, A., Hashem, T., Grosjean, S., Abetz, V., Bräse, S., Wöll, C., & Tsotsalas, M. (2019). Synthesis, Transfer, and Gas Separation Characteristics of MOF-Templated Polymer Membranes. Membranes, 9(10), 124. https://doi.org/10.3390/membranes9100124