Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices
Abstract
:1. Introduction
2. Experimental
3. Results
3.1. Structural Characterization
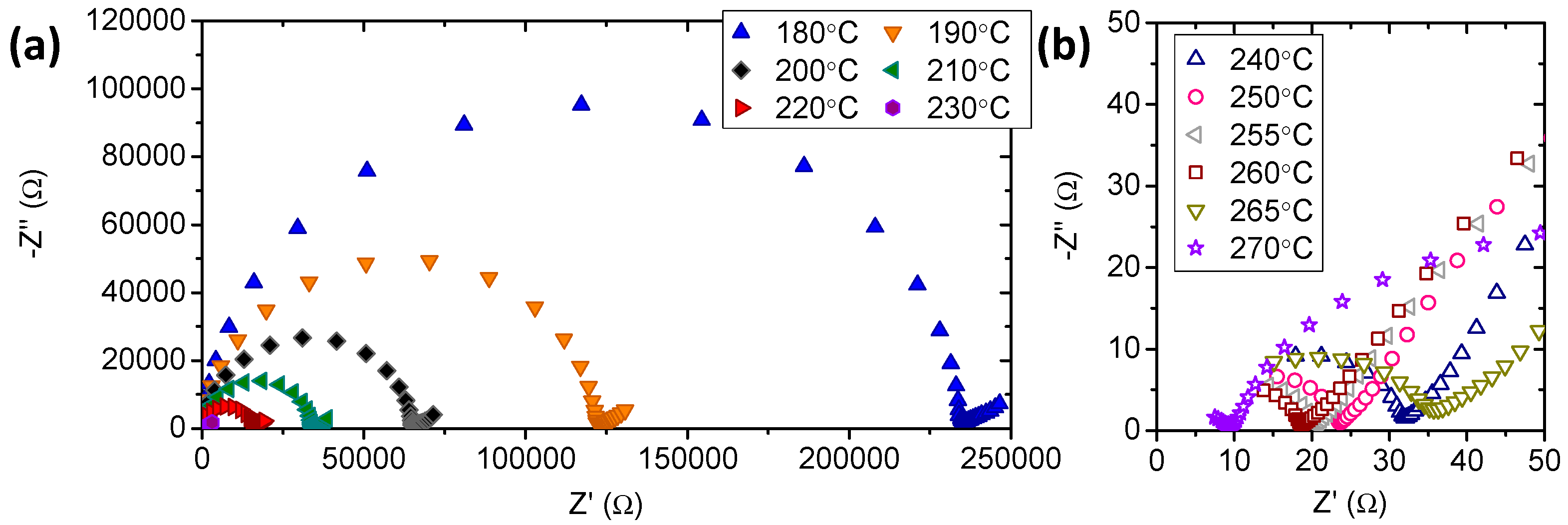
3.2. Electrochemical Characterization
3.3. Fuel Cell Measurements
3.4. Electrolysis Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Minh, N.Q. CERAMIC FUEL-CELLS. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Morejudo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martínez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merie, R.B. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef]
- Otomo, J.; Minagawa, N.; Wen, C.-J.; Eguchi, K.; Takahashi, H. Protonic conduction of CsH2PO4 and its composite with silica in dry and humid atmospheres. Solid State Ion. 2003, 156, 357–369. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Louie, M.W.; Kislitsyn, M.; Bhattacharya, K.; Haile, S.M. Phase transformation and hysteresis behavior in Cs1−xRbxH2PO4. Solid State Ion. 2010, 181, 173–179. [Google Scholar] [CrossRef]
- Chisholm, C.R.I.; Boysen, D.A.; Papandrew, A.B.; Zecevic, S.; Cha, S.; Sasaki, K.A.; Varga, A.; Giapis, K.P.; Haile, S.M. From laboratory breakthrough to technological realization: The development path for solid acid fuel cells. Electrochem. Soc. Interface 2009, 18, 53–59. [Google Scholar]
- Taninouchi, Y.K.; Uda, T.; Awakura, Y.; Ikeda, A.; Haile, S.M. Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260 °C. J. Mater. Chem. 2007, 17, 3182–3189. [Google Scholar] [CrossRef]
- Taninouchi, Y.k.; Uda, T.; Awakura, Y. Dehydration of CsH2PO4 at temperatures higher than 260 °C and the ionic conductivity of liquid product. Solid State Ion. 2008, 178, 1648–1653. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Bagryantseva, I.N. Superprotonic CsH2PO4-CsHSO4 solid solutions. Inorg. Mater. 2012, 48, 187–194. [Google Scholar] [CrossRef]
- Uda, T.; Boysen, D.A.; Chisholm, C.R.I.; Haile, S.M. Alcohol fuel cells at optimal temperatures. Electrochem. Solid-State Lett. 2006, 9, A261–A264. [Google Scholar] [CrossRef]
- Bartley, G.J.J.; Burch, R. Support and morphological effects in the synthesis of methanol over Cu/ZnO, Cu/ZrO2 and Cu/SiO2 catalysts. Appl. Catal. 1988, 43, 141–153. [Google Scholar] [CrossRef]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Hallinder, J. Electrolytes and Electrodes for Electrochemical Cells Operating at 200–300 °C; Technical University of Denmark: Lyngby, Denmark, 2013. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chisholm, C.R.I.; Haile, S.M. X-ray structure refinement of CsHSO4 in phase II. Mater. Res. Bull. 2000, 35, 999–1005. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Wang, H.J.; Boukamp, B.A. Conductivity study of dense BaZr0.9Y0.1O(3−δ) obtained by spark plasma sintering. Solid State Ion. 2012, 213, 36–41. [Google Scholar] [CrossRef]
- Ikeda, A.; Kitchaev, D.A.; Haile, S.M. Phase behavior and superprotonic conductivity in the Cs1−xRbxH2PO4 and Cs1−xKxH2PO4 systems. J. Mater. Chem. A 2014, 2, 204–214. [Google Scholar] [CrossRef]
- Hallinder, J.; Holtappels, P.; Mogensen, M. Materials and Manufacturing of Electrochemical Cells for Reduction of CO2 into Liquid Fuels. Meet. Abstr. 2011, MA2011-02, 1507. [Google Scholar]
- Lee, H.-S.; Tuckerman, M.E. The Structure and Proton Transport Mechanisms in the Superprotonic Phase of CsH2PO4: An Ab Initio Molecular Dynamics Study. J. Phys. Chem. C 2008, 112, 9917–9930. [Google Scholar] [CrossRef]
- Bronowska, W. Comment on “Does the structural superionic phase transition at 231 °C in CsH2PO4 really not exist?” [J. Chem. Phys. 110, 4847 (1999)]. J. Chem. Phys. 2001, 114, 611–612. [Google Scholar] [CrossRef]
- Romain, F.; Novak, A. Raman study of the high-temperature phase transition in CsH2PO4. J. Mol. Struct. 1991, 263, 69–74. [Google Scholar] [CrossRef]
- Papandrew, A.B.; Chisholm, C.R.I.; Elgammal, R.A.; Özer, M.M.; Zecevic, S.K. Advanced electrodes for solid acid fuel cells by platinum deposition on CsH2PO4. Chem. Mater. 2011, 23, 1659–1667. [Google Scholar] [CrossRef]
- Boysen, D.A.; Uda, T.; Chisholm, C.R.I.; Haile, S.M. High-Performance Solid Acid Fuel Cells Through Humidity Stabilization. Science 2004, 303, 68–70. [Google Scholar] [CrossRef]
- Yoshimi, S.; Matsui, T.; Kikuchi, R.; Eguchi, K. Temperature and humidity dependence of the electrode polarization in intermediate-temperature fuel cells employing CsH2PO4/SiP2O7-based composite electrolytes. J. Power Sources 2008, 179, 497–503. [Google Scholar] [CrossRef]
- Perez, J.; Gonzalez, E.R.; Ticianelli, E.A. Oxygen electrocatalysis on thin porous coating rotating platinum electrodes. Electrochim. Acta 1998, 44, 1329–1339. [Google Scholar] [CrossRef]
- Prag, C.B. Intermediate Temperature Steam Electrolysis with Phosphate-Based Electrolytes; Technical University of Denmark: Lyngby, Denmark, 2014. [Google Scholar]
- Schiffer, Z.J.; Manthiram, K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. [Google Scholar] [CrossRef]
- Serra, J.M. Electrifying chemistry with protonic cells. Nat. Energy 2019, 4, 178–179. [Google Scholar] [CrossRef]








| Compound | Nomenclature |
|---|---|
| CsH2PO4 | Cs |
| (CsH2PO4)0.8(BaHPO4)0.2 | Ba |
| (CsH2PO4)0.8(RbH2PO4)0.2 | Rb |
| (CsH2PO4)0.8(HSO4)0.2 | S |
| (CsH2PO4)0.8(HMoO4)0.2 | Mo |
| (CsH2PO4)0.8(HWO4)0.2 | W |
| Compound | Eact (T < 230 °C) (eV) | Eact (T > 230 °C) (eV) |
|---|---|---|
| Cs | 1.26 | 0.65 |
| Mo | 0.61 | 0.19 |
| S | 0.42 | 0.42 |
| W | 0.93 | 0.25 |
| Rb | 1.44 | 0.92 |
| Ba | 0.69 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete, L.; Andrio, A.; Escolástico, S.; Moya, S.; Compañ, V.; Serra, J.M. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes 2019, 9, 49. https://doi.org/10.3390/membranes9040049
Navarrete L, Andrio A, Escolástico S, Moya S, Compañ V, Serra JM. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes. 2019; 9(4):49. https://doi.org/10.3390/membranes9040049
Chicago/Turabian StyleNavarrete, Laura, Andreu Andrio, Sonia Escolástico, Sergio Moya, Vicente Compañ, and José M. Serra. 2019. "Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices" Membranes 9, no. 4: 49. https://doi.org/10.3390/membranes9040049
APA StyleNavarrete, L., Andrio, A., Escolástico, S., Moya, S., Compañ, V., & Serra, J. M. (2019). Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes, 9(4), 49. https://doi.org/10.3390/membranes9040049







