Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flat and Micro-Patterned PA TFC Membranes
2.2.1. Casting of Flat Isotropic Porous PES Support Membranes
2.2.2. Preparation of Micro-Patterned Isotropic Porous PES Supports via Micro-Imprinting Lithography
2.2.3. Preparation of Flat and Micro-Patterned PA TFC Membranes
2.2.4. Surface Modification of Flat and Micro-Patterned PA TFC Membranes
2.3. Characterization of Flat and Micro-Patterned Membranes
2.4. Measurement of Membrane Separation Performance
2.5. Colloidal Silica Fouling Experiments
2.5.1. Colloidal Fouling Experiments in Unstirred Conditions
2.5.2. Colloidal Fouling Experiments under Influence of Added Stimulus
3. Results and Discussion
3.1. Characterization of Flat and Micro-Patterned Membranes
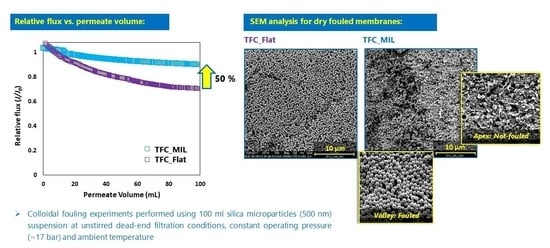
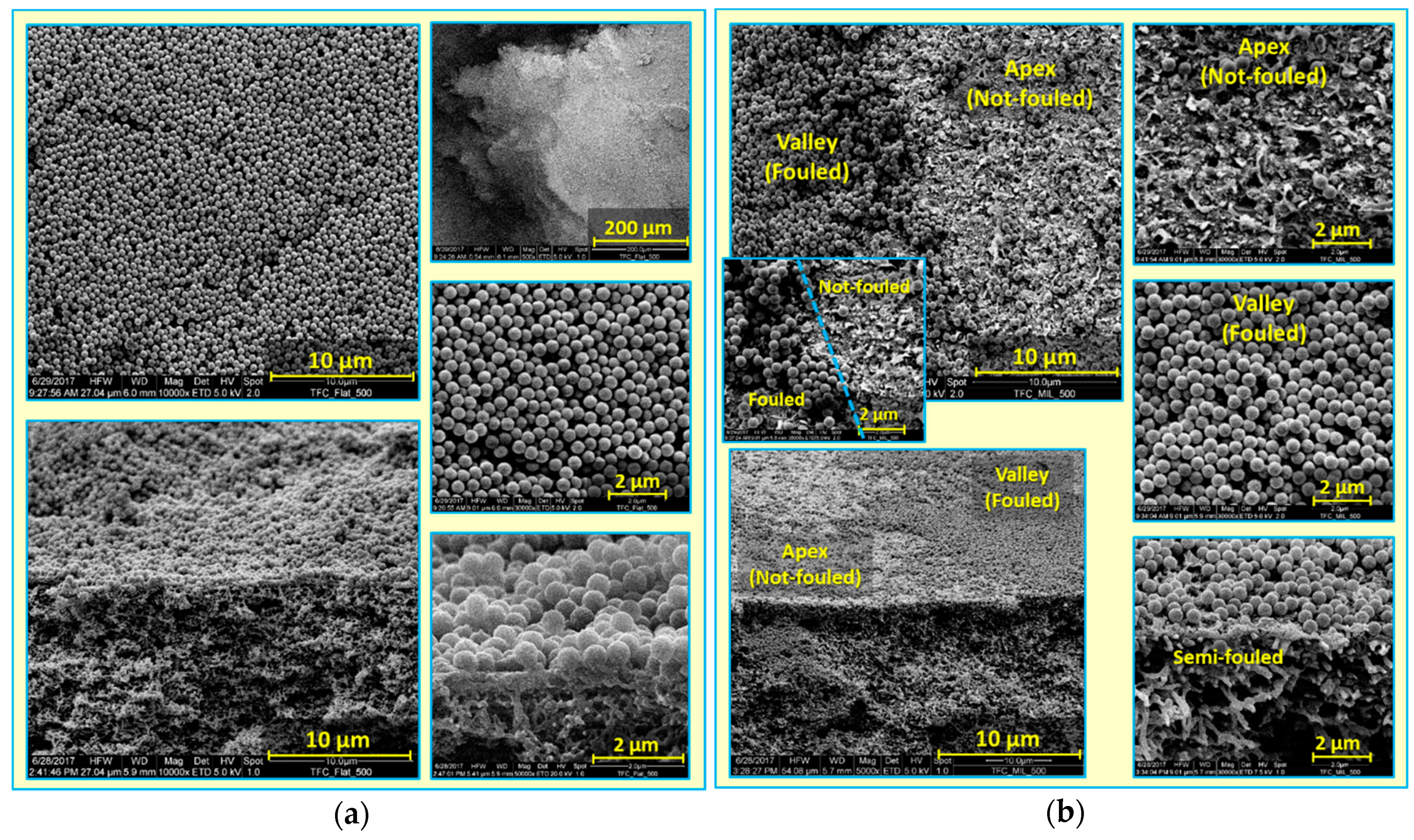
3.2. Colloidal Fouling of Flat vs. Micro-Patterned Membranes by Silica Microparticles
- Stage (i)
- When the fouling experiment starts, silica microparticles are forced to the membrane surface by permeation drag, caused by high particle concentration in the bulk, as well as high initial flux of micro-patterned PA TFC membranes [8]. Interestingly, more silica particles are directed to the surface pattern’s valleys rather than to apex regions as a result of unequal feed flow distribution near the micro-patterned membrane surface (so-called “local flux”, see Figure 5) and altered surface hydrodynamics, leading to a spatially selective deposition. In general, higher local fluxes are expected in the valleys in comparison with apex regions (Figure 5b). This may be explained by higher specific membrane active surface area, in addition to the higher local permeance because of different PA thicknesses over the surface micro-structures (i.e., much lower PA thickness over valley walls). Furthermore, the surface pattern’s valleys are assumed to act as favorable sites for the deposition of silica microparticles as a result of extremely low shear stress [23]. The valleys in micro-patterned surfaces were simulated in literature to exhibit low DLVO potential energy, called “low-energy pockets” [26,29].
- Stage (ii)
- Deposited silica microparticles are implied to act as seeds for precipitation of more silica microparticles in a physical process known as “artificial epitaxy”. This scenario, named “orientation by topographic relief”, was previously introduced as a possible mechanism under similar conditions by Givargizov [44,45]. This oriented crystallization of silica microparticles (again favoring spatially selective deposition) is suggested to dominate this stage.
- Stage (iii)
- At the end, most silica microparticles are deposited within the surface pattern’s valleys, while apex regions are not fouled (at the experiment conditions), which is believed to explain the distinguished antifouling performance of micro-patterned PA TFC membranes.
3.3. Colloidal Fouling of Flat vs. Micro-Patterned Membranes by Silica Nanoparticles
3.4. Colloidal Fouling of Flat vs. Micro-Patterned Membranes by Silica Nanoparticles under Influence of Stirring as an Added Stimulus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voutchkov, N. Energy use for membrane seawater desalination–current status and trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Allred, J.; Knoell, T.; Jeong, B.-H. Modeling the effects of fouling on fullscale reverse osmosis processes. J. Membr. Sci. 2008, 314, 33–49. [Google Scholar] [CrossRef]
- Zhu, X.; Elimelech, M. Colloidal Fouling of Reverse Osmosis Membranes: Measurements and Fouling Mechanisms. Environ. Sci. Technol. 1997, 31, 3654–3662. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Elimelech, M. Cake-Enhanced Concentration Polarization: A New Fouling Mechanism for Salt-Rejecting Membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Kim, A.S.; Elimelech, M. Influence of Crossflow Membrane Filter Geometry and Shear Rate on Colloidal Fouling in Reverse Osmosis and. Environ. Sci. Technol. 2002, 19, 357–372. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Cohen, R.D.; Probstein, R.F. Colloidal Fouling of Reverse Osmosis Membranes. J. Colloid Interface Sci. 1986, 114, 194–207. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Elimelech, M.; Zhu, X.; Childress, A.E.; Hong, S. Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J. Membr. Sci. 1997, 127, 101–109. [Google Scholar] [CrossRef]
- Litton, G.M.; Olson, T.M. Colloid deposition kinetics with surface-active agents: Evidence for discrete surface charge effects. J. Colloid Interface Sci. 1994, 165, 522–525. [Google Scholar] [CrossRef]
- Li, Q.; Song, J.; Yu, H.; Li, Z.; Pan, X.; Yang, B. Investigating the microstructures and surface features of seawater RO membranes and the dependencies of fouling resistance performances. Desalination 2014, 352, 109–117. [Google Scholar] [CrossRef]
- Boussu, K.; Belpaire, A.; Volodin, A.; Haesendonck, C.V.; Meeren, P.V.d.; Vandecasteele, C.; Bruggen, B.V.d. Influence of membrane and colloid characteristics on fouling of nanofiltration membranes. J. Membr. Sci. 2007, 289, 220–230. [Google Scholar] [CrossRef]
- Hermia, J. Constant pressure blocking filtration laws. Application to power-law non-Newtonian fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Wang, F.; Tarabara, V.V. Pore blocking mechanisms during early stages of membrane fouling by colloids. J. Colloid Interface Sci. 2008, 328, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Eaton, C.L.; Kim, J.H.; Colvin, C.K.; Lozier, J.C.; Mariñas, B.J. Removal of biological and non-biological viral surrogates by spiral-wound reverse osmosis membrane elements with intact and compromised integrity. Water Res. 2004, 38, 3821–3832. [Google Scholar] [CrossRef]
- Fimbres-Weihs, G.A.; Wiley, D.E.; Fletcher, D.F. Unsteady flows with mass transfer in narrow zigzag spacer-filled channels: A numerical study. Ind. Eng. Chem. Res. 2006, 45, 6594–6603. [Google Scholar] [CrossRef]
- Picioreanu, C.; Vrouwenvelder, J.S.; van Loosdrecht, M.C.M. Three-dimensional modeling of biofouling and fluid dynamics in feed spacer channels of membrane devices. J. Membr. Sci. 2009, 345, 340–354. [Google Scholar] [CrossRef]
- Radu, A.I.; van Steen, M.S.H.; Vrouwenvelder, J.S.; van Loosdrecht, M.C.M.; Picioreanu, C. Spacer geometry and particle deposition in spiral wound membrane feed channels. Water Res. 2014, 64, 160–176. [Google Scholar] [CrossRef]
- Ngene, I.S.; Lammertink, R.G.H.; Wessling, M.; Van der Meer, W.G.J. Particle deposition and biofilm formation on microstructured membranes. J. Membr. Sci. 2010, 364, 43–51. [Google Scholar] [CrossRef]
- Lee, Y.K.; Won, Y.J.; Yoo, J.H.; Ahn, K.H.; Lee, C.H. Flow analysis and fouling on the patterned membrane surface. J. Membr. Sci. 2013, 427, 320–325. [Google Scholar] [CrossRef]
- Jung, S.Y.; Won, Y.-J.; Jang, J.H.; Yoo, J.H.; Ahn, K.H.; Lee, C.-H. Particle deposition on the patterned membrane surface: Simulation and experiments. Desalination 2015, 370, 17–24. [Google Scholar] [CrossRef]
- Won, Y.-J.; Jung, S.-Y.; Jang, J.-H.; Lee, J.-W.; Chae, H.-R.; Choi, D.-C.; Ahn, K.H.; Lee, C.-H.; Park, P.-K. Correlation of membrane fouling with topography of patterned membranes for water treatment. J. Membr. Sci. 2016, 498, 14–19. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Bhattacharjee, S.; Elimelech, M. Effect of Membrane Surface Roughness on Colloid-Membrane DLVO Interactions. Langmuir 2003, 19, 4836–4847. [Google Scholar] [CrossRef]
- Drechsler, A.; Petong, N.; Zhang, J.; Kwok, D.Y.; Grundke, K. Force measurements between Teflon AF and colloidal silica particles in electrolyte. Colloids Surf. A 2004, 250, 357–366. [Google Scholar] [CrossRef]
- Martines, E.; Csaderova, L.; Morgan, H.; Curtis, A.S.G.; Riehle, M.O. DLVO interaction energy between a sphere and a nano-patterned plate. Colloids Surf. A 2008, 318, 45–52. [Google Scholar] [CrossRef]
- Bendersky, M.; Davis, J.M. DLVO interaction of colloidal particles with topographically and chemically heterogeneous surfaces. J. Colloid Interface Sci. 2011, 353, 87–97. [Google Scholar] [CrossRef]
- Ding, Y.; Maruf, S.; Aghajani, M.; Greenberg, A.R. Surface patterning of polymeric membranes and its effect on antifouling characteristics. Sep. Sci. Technol. 2017, 52, 240–257. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Critical flux of surface-patterned ultrafiltration membranes during cross-flow filtration of colloidal particles. J. Membr. Sci. 2014, 471, 65–71. [Google Scholar] [CrossRef]
- Maruf, S.H.; Wang, L.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Use of nanoimprinted surface patterns to mitigate colloidal deposition on ultrafiltration membranes. J. Membr. Sci. 2013, 428, 598–607. [Google Scholar] [CrossRef]
- Culfaz, P.Z.; Buetehorn, S.; Utiu, L.; Kueppers, M.; Bluemich, B.; Melin, T.; Wessling, M.; Lammertink, R.G.H. Fouling behavior of microstructured hollow fiber membranes in dead-end filtrations: Critical flux determination and NMR imaging of particle deposition. Langmuir 2011, 27, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Çulfaz, P.Z.; Rolevink, E.; van Rijn, C.; Lammertink, R.G.H.; Wessling, M. Microstructured hollow fibers for ultrafiltration. J. Membr. Sci. 2010, 347, 32–41. [Google Scholar] [CrossRef]
- Lin, K.-h.; Crocker, J.C.; Prasad, V.; Schofield, A.; Weitz, D.A.; Lubensky, T.C.; Yodh, A.G. Entropically Driven Colloidal Crystallization on Patterned Surfaces. Phys. Rev. Lett. 2000, 85, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Fabrication and characterization of a surface-patterned thin film composite membrane. J. Membr. Sci. 2014, 452, 11–19. [Google Scholar] [CrossRef]
- Weinman, S.T.; Husson, S.M. Influence of chemical coating combined with nanopatterning on alginate fouling during nanofiltration. J. Membr. Sci. 2016, 513, 146–154. [Google Scholar] [CrossRef] [Green Version]
- ElSherbiny, I.M.A.; Khalil, A.S.G.; Ulbricht, M. Tailoring Surface Characteristics of Polyamide Thin-Film Composite Membranes toward Pronounced Switchable Wettability. Adv. Mater. Interfaces 2019, 6, 1801408. [Google Scholar] [CrossRef]
- ElSherbiny, I.M.A.; Khalil, A.S.G.; Ulbricht, M. Surface micro-patterning as a promising platform towards novel polyamide thin-film composite membranes of superior performance. J. Membr. Sci. 2017, 529, 11–22. [Google Scholar] [CrossRef]
- ElSherbiny, I.M.A.; Ghannam, R.; Khalil, A.S.G.; Ulbricht, M. Isotropic macroporous polyethersulfone membranes as competitive supports for high performance polyamide desalination membranes. J. Membr. Sci. 2015, 493, 782–793. [Google Scholar] [CrossRef]
- ElSherbiny, I.M.A. Novel micro- and nano-patterned water desalination membranes. Ph.D. Thesis, University of Duisburg-Essen, Essen, Germany, 2018. [Google Scholar]
- Yu, S.; Lü, Z.; Chen, Z.; Liu, X.; Liu, M.; Gao, C. Surface modification of thin-film composite polyamide reverse osmosis membranes by coating N-isopropylacrylamide-co-acrylic acid copolymers for improved membrane properties. J. Membr. Sci. 2011, 371, 293–306. [Google Scholar] [CrossRef]
- Wu, D.; Liu, X.; Yu, S.; Liu, M.; Gao, C. Modification of aromatic polyamide thin-film composite reverse osmosis membranes by surface coating of thermo-responsive copolymers P(NIPAM-co-Am). I: Preparation and characterization. J. Membr. Sci. 2010, 352, 76–85. [Google Scholar] [CrossRef]
- Givargizov, E.I. Graphoepitaxy as an approach to oriented crystallization on amorphous substrates. J. Cryst. Growth 2008, 310, 1686–1690. [Google Scholar] [CrossRef]
- Givargizov, E.I. Mechanisms of oriented crystallization in artificial epitaxy (graphoepitaxy). Thin Solid Films 1990, 189, 389–396. [Google Scholar] [CrossRef]








| Characteristics | Silica Nanoparticles (50 wt.%) | Silica Microparticles (5 wt.%) |
|---|---|---|
| Concentration (mg/L) | 200 | 200 |
| pH | 7.4 | 6.7 |
| dn (nm) | 45 | 530 |
| PDI | 0.11 | 0.17 |
| Zeta potential (mV) | −49.2 | −50 |
| Membrane Sample 1 | Zeta Potential Measurement | Water Contact Angle (°) | Surface Roughness Analysis 2 | Pure water permeability (L/h∙m2∙bar) | Separation Performance | |||
|---|---|---|---|---|---|---|---|---|
| IEP | Zeta Potential at pH 7 (mV) | Sa (nm) | Sq (nm) | Salt (NaCl) Rejection (%) | Solution Permeability (L/h·m2·bar) | |||
| TFC_Flat | 3.8 | −80 | 43.0 ± 5 | 55 | 69 | 1.5 ± 0.1 | 96.0 ± 0.2 | 0.90 ± 0.02 |
| CP_TFC_Flat | 3.4 | −70 | 58.2 ± 8 | 58 | 72 | 1.1 ± 0.1 | 97.7 ± 0.2 | 0.78 ± 0.05 |
| TFC_MIL | 3.4 | −33 | 31.0 ± 3 | 298 | 366 | 2.3 ± 0.1 | 97.0 ± 0.2 | 1.35 ± 0.10 |
| CP_TFC_MIL | 3.8 | −26 | 38.3 ± 6 | 245 | 276 | 2.1 ± 0.1 | 97.8 ± 0.3 | 1.26 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElSherbiny, I.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes. Membranes 2019, 9, 67. https://doi.org/10.3390/membranes9060067
ElSherbiny IMA, Khalil ASG, Ulbricht M. Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes. Membranes. 2019; 9(6):67. https://doi.org/10.3390/membranes9060067
Chicago/Turabian StyleElSherbiny, Ibrahim M.A., Ahmed S.G. Khalil, and Mathias Ulbricht. 2019. "Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes" Membranes 9, no. 6: 67. https://doi.org/10.3390/membranes9060067
APA StyleElSherbiny, I. M. A., Khalil, A. S. G., & Ulbricht, M. (2019). Influence of Surface Micro-Patterning and Hydrogel Coating on Colloidal Silica Fouling of Polyamide Thin-Film Composite Membranes. Membranes, 9(6), 67. https://doi.org/10.3390/membranes9060067