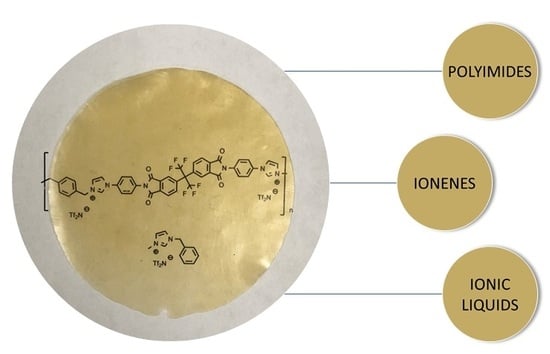
Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Imidazole-Aniline Precursors
2.3.1. Synthesis of 4-(1H-imidazol-1-yl)aniline “I4A”
2.3.2. Synthesis of 3-(1H-imidazol-1-yl)aniline “I3A”
2.3.3. Synthesis of 2-(1H-imidazol-1-yl)aniline (“I2A”)
2.4. Synthesis of Aromatic Polyimide Monomers
2.4.1. Synthesis of 5,5′-(perfluoropropane-2,2-diyl)bis(2-(4-(1H-imidazol-1yl)phenyl)isoindoline-1,3-dione) (“6FDA-I4A”)
2.4.2. Synthesis of 5,5′-(perfluoropropane-2,2-diyl)bis(2-(3-(1H-imidazol-1-l)phenyl)isoindoline-1,3-dione) (“6FDA-I3A”)
2.4.3. Synthesis of 5,5′-(perfluoropropane-2,2-diyl)bis(2-(2-(1H-imidazol-1yl)phenyl)isoindoline-1,3-dione) (“6FDA I2A”)
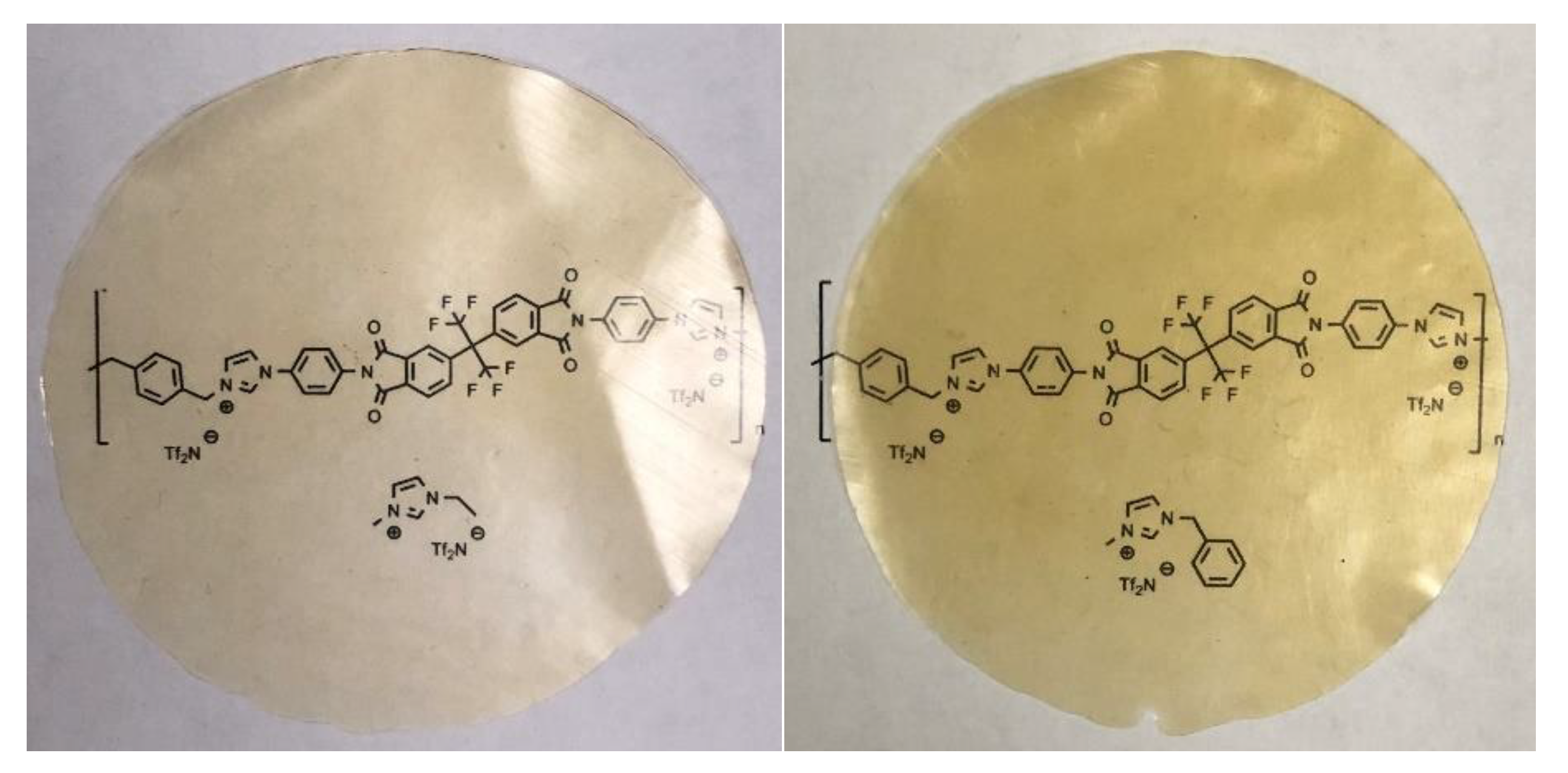
2.5. Formation of Polyimide-Ionenes
2.5.1. Synthesis of [6FDA I4A pXy][Tf2N]
2.5.2. Synthesis of [6FDA I3A mXy][Tf2N]
2.5.3. Synthesis of [6FDA I2A oXy][Tf2N]
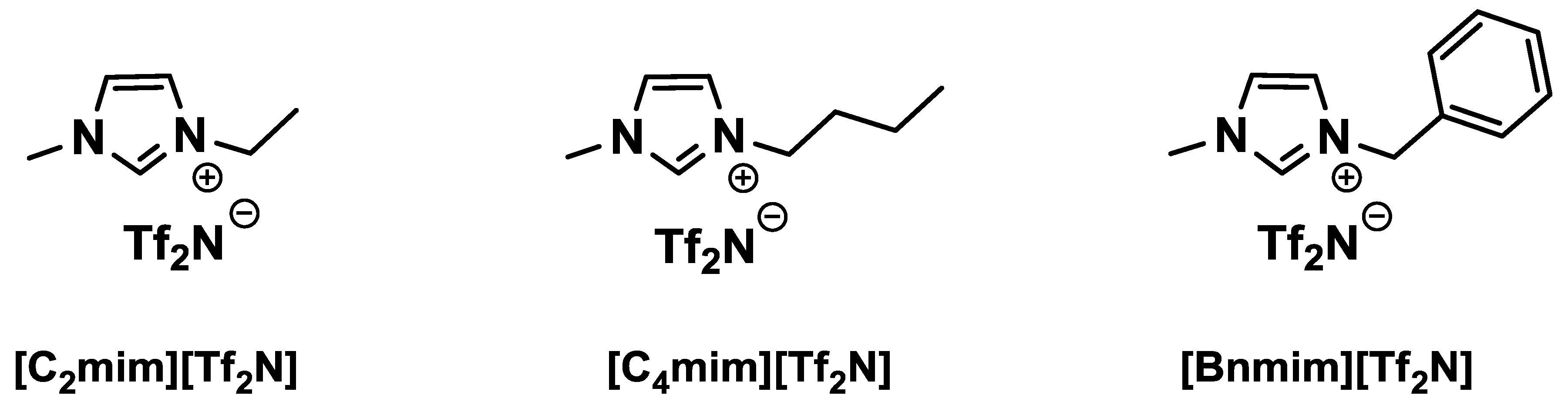
2.6. Preparation of Ionic Liquids
2.7. Membrane Preparation
2.8. Gas Separation Measurements
3. Results and Discussion
3.1. Characterization
3.2. Thermal Characterization
3.3. Structural Characterization
3.4. Gas Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Rozhanskiia, I.; Okuyama, K.; Gotoa, K. Synthesis and properties of polyimides derived from isomeric biphenyltetracarboxylic dianhydrides. Polymer 2000, 41, 7057–7065. [Google Scholar] [CrossRef]
- Mi, Z.; Liu, Z.; Yao, J.; Wang, C.; Zhou, C.; Wang, D.; Zhao, X.; Zhou, H.; Zhang, Y.; Chen, C. Transparent and soluble polyimide films from 1,4:3,6-dianhydro-D-mannitol based dianhydride and diamines containing aromatic and semiaromatic units: Preparation, characterization, thermal and mechanical properties. Polym. Degrad. Stab. 2018, 151, 80–89. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yang, C.-P.; Hsiao, S.-H. Soluble and Colorless Poly(ether-imide)s Based on a Benzonorbornane Bis(ether anhydride) and Trifluoromethyl-Substituted Aromatic Bis(ether-amine)s. Macromol. Chem. Phys. 2006, 207, 1888–1898. [Google Scholar] [CrossRef]
- Calle, M.; Lozano, A.n.E.; de La Campa, J.G.; de Abajo, J. Novel Aromatic Polyimides Derived from 5′-t-Butyl-2′-pivaloylimino-3,4,3′′,4′′-m-terphenyltetracarboxylic Dianhydride with Potential Application on Gas Separation Processes. Macromolecules 2010, 43, 2268–2275. [Google Scholar] [CrossRef]
- Kammakakam, I.; Nam, S.; Kim, T.-H. PEG–imidazolium-functionalized 6FDA–durene polyimide as a novel polymeric membrane for enhanced CO2 separation. RSC Adv. 2016, 6, 31083–31091. [Google Scholar] [CrossRef]
- Wiegand, J.R.; Smith, Z.P.; Liu, Q.; Patterson, C.T.; Freeman, B.D.; Guo, R. Synthesis and characterization of triptycene-based polyimides with tunable high fractional free volume for gas separation membranes. J. Mater. Chem. A 2014, 2, 13309–13320. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, H.B. High Performance Polyimide with High Internal Free Volume Elements. Macromol. Rapid Commun. 2011, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, C.-C.; Kincer, M.R.; Koros, W.J. Thermal analysis and its application in evaluation of fluorinated polyimide membranes for gas separation. Polymer 2011, 52, 4073–4082. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, L.; Xiang, D.; Cao, B.; Hosseini, S.; Li, P. Approaches to Suppress CO2-Induced Plasticization of Polyimide Membranes in Gas Separation Applications. Processes 2019, 7. [Google Scholar] [CrossRef]
- Kammakakam, I.; Nam, S.; Kim, T.-H. Ionic group-mediated crosslinked polyimide membranes for enhanced CO2 separation. RSC Adv. 2015, 5, 69907–69914. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Q.; Anderson, J.L.; Varanasi, S.; Coleman, M.R. Synthesis of copolyimides based on room temperature ionic liquid diamines. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 4036–4046. [Google Scholar] [CrossRef]
- Li, P.; Coleman, M.R. Synthesis of room temperature ionic liquids based random copolyimides for gas separation applications. Eur. Polym. J. 2013, 49, 482–491. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, F.; Jin, J. Tröger’s Base-Based Microporous Polyimide Membranes for High-Performance Gas Separation. ACS Macro Lett. 2014, 3, 597–601. [Google Scholar] [CrossRef]
- Yoshino, M.; Ito, K.; KITA, H.; Okamoto, K.-I. Effects of Hard-Segment Polymers on CO2/N2 GasSeparation Properties of Poly(ethylene oxide)-Segmented Copolymers. J. Polym. Sci. Part. B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Raeissi, S.; Peters, C.J. A potential ionic liquid for CO2-separating gas membranes: Selection and gas solubility studies. Green Chem. 2009, 11, 185–192. [Google Scholar] [CrossRef]
- Cowan, M.G.; Masuda, M.; McDanel, W.M.; Kohno, Y.; Gin, D.L.; Noble, R.D. Phosphonium-based poly(Ionic liquid) membranes: The effect of cation alkyl chain length on light gas separation properties and Ionic conductivity. J. Membr. Sci. 2016, 498, 408–413. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Królikowski, M. Separation of aromatic hydrocarbons from alkanes using ammonium ionic liquid C2NTf2 at T=298.15K. Fluid Phase Equilib. 2007, 259, 173–179. [Google Scholar] [CrossRef]
- Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T.; Kallitsis, J. New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO(2) Separation. Polymers (Basel) 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2Separations in Imidazolium-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Shao, Z.-G.; Zhang, G.; Zhao, Y.; Li, J.; Yi, B. Preparation and characterization of imidazolium-functionalized poly (ether sulfone) as anion exchange membrane and ionomer for fuel cell application. Int. J. Hydrogen Energy 2013, 38, 9285–9296. [Google Scholar] [CrossRef]
- Kammakakam, I.; Kim, H.W.; Nam, S.; Park, H.B.; Kim, T.-H. Alkyl imidazolium-functionalized cardo-based poly(ether ketone)s as novel polymer membranes for O2/N2 and CO2/N2 separations. Polymer 2013, 54, 3534–3541. [Google Scholar] [CrossRef]
- Gye, B.; Kammakakam, I.; You, H.; Nam, S.; Kim, T.-H. PEG-imidazolium-incorporated polyimides as high-performance CO2-selective polymer membranes: The effects of PEG-imidazolium content. Sep. Purif. Technol. 2017, 179, 283–290. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Ye, N.; Teng, M.; He, R. Modification of poly(aryl ether ketone) using imidazolium groups as both pendants and bridging joints for anion exchange membranes. Eur. Polym. J. 2015, 73, 116–126. [Google Scholar] [CrossRef]
- Ohno, H. Design of Ion Conductive Polymers Based on Ionic Liquids. Macromol. Symp. 2007, 249–250, 551–556. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Chu, H.; Lim, S.; Yim, T.; Kim, Y.-J.; Kim, T.-H. Preparation and characterization of imidazolium-PEO-based Ionene/PVDF(HFP)/LiTFSI as a novel Gel polymer electrolyte. Macromol. Res. 2014, 23, 38–44. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E. Recent Advances in the Design of Ionenes: Toward Convergence with High-Performance Polymers. Macromol. Chem. Phys. 2019. [Google Scholar] [CrossRef]
- O’Harra, K.E.; Kammakakam, I.; Bara, J.E.; Jackson, E.M. Understanding the effects of backbone chemistry and anion type on the structure and thermal behaviors of imidazolium polyimide-ionenes. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Self-healing imidazolium-based ionene-polyamide membranes: An experimental study on physical and gas transport properties. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Bara, J.E.; Jackson, E.M. Design and Synthesis of Imidazolium-Mediated Tröger’s Base-Containing Ionene Polymers for Advanced CO2 Separation Membranes. ACS Omega 2019, 4, 3439–3448. [Google Scholar] [CrossRef]
- Mittenthal, M.S.; Flowers, B.S.; Bara, J.E.; Whitley, J.W.; Spear, S.K.; Roveda, J.D.; Wallace, D.A.; Shannon, M.S.; Holler, R.; Martens, R.; et al. Ionic Polyimides: Hybrid Polymer Architectures and Composites with Ionic Liquids for Advanced Gas Separation Membranes. Ind. Eng. Chem. Res. 2017, 56, 5055–5069. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.-S. High performance membranes based on ionic liquid polymers for CO2 separation from the flue gas. Green Chem. 2012, 14. [Google Scholar] [CrossRef]
- Paschoal, V.H.; Faria, L.F.O.; Ribeiro, M.C.C. Vibrational Spectroscopy of Ionic Liquids. Chem. Rev. 2017, 117, 7053–7112. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, B.; Coleman, M.R.; Li, P. Gas transport properties in (6FDA-RTIL)-(6FDA-MDA) block copolyimides. J. Appl. Polym. Sci. 2016, 133, 43077. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Kamath, M.G.; Fu, S.; Itta, A.K.; Qiu, W.; Liu, G.; Swaidan, R.; Koros, W.J. 6FDA-DETDA: DABE polyimide-derived carbon molecular sieve hollow fiber membranes: Circumventing unusual aging phenomena. J. Membr. Sci. 2018, 546, 197–205. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Hou, R.; Lau, C.H.; Konstas, K.; Kitchin, M.; Dong, G.; Lee, J.; Lee, W.H.; Seong, J.G.; Lee, Y.M.; et al. Highly permeable Thermally Rearranged Mixed Matrix Membranes (TR-MMM). J. Membr. Sci. 2019, 585, 260–270. [Google Scholar] [CrossRef]
- Halasa, A.F.; Wathen, G.D.; Hsu, W.L.; Matrana, B.A.; Massie, J.M. Relationship between interchain spacing of amorphous polymers and blend miscibility as determined by wide-angle X-ray scattering. J. Appl. Polym. Sci. 1991, 43, 183–190. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]





| Materials | Mass/Ratios | ||||
|---|---|---|---|---|---|
| ID | Polyimide-Ionene | IL (Equivalents) | Polyimide-Ionene [g] | IL [g] | Vtotal w/Solvent [mL] |
| 1 | [6FDA I4A pXy][Tf2N] | Neat | 0.75 | - | 7.5 |
| 2 | [C2mim][Tf2N] (1) | 0.50 | 0.141 | 9 | |
| 3 | [C2mim][Tf2N] (2) | 0.50 | 0.281 | 9 | |
| 4 | [C4mim][Tf2N] (1) | 0.50 | 0.151 | 9 | |
| 5 | [C4mim][Tf2N] (2) | 0.50 | 0.301 | 9 | |
| 6 | [Bnmim][Tf2N] (2) | 0.50 | 0.326 | 9 | |
| 7 | [6FDA I3A mXy][Tf2N] | Neat | 0.75 | - | 7.5 |
| 8 | [C2mim][Tf2N] (2) | 0.50 | 0.281 | 9 | |
| 9 | [C4mim][Tf2N] (2) | 0.50 | 0.301 | 9 | |
| 10 | [Bnmim][Tf2N] (2) | 0.50 | 0.326 | 9 | |
| 11 | [6FDA I2A oXy][Tf2N] | Neat | 0.75 | - | 7.5 |
| 12 | [C2mim][Tf2N] (2) | 0.50 | 0.281 | 9 | |
| 13 | [C4mim][Tf2N] (2) | 0.50 | 0.301 | 9 | |
| 14 | [Bnmim][Tf2N] (2) | 0.50 | 0.326 | 9 | |
| ID | Polyimide-Ionene | IL (Equivalents) | Tg [°C] | d-spacing [Å] |
|---|---|---|---|---|
| 1 | [6FDA I4A pXy][Tf2N] | Neat | 205.9 | 4.61 |
| 2 | [C2mim][Tf2N] (1) | 199.4 | 4.55 | |
| 3 | [C2mim][Tf2N] (2) | 209.9 | 3.92 | |
| 4 | [C4mim][Tf2N] (1) | 182.4 | 4.83 | |
| 5 | [C4mim][Tf2N] (2) | 185.2 | 4.33 | |
| 6 | [Bnmim][Tf2N] (2) | 218.9 | 4.73 | |
| 7 | [6FDA I3A mXy][Tf2N] | Neat | 236.2 | 4.66 |
| 8 | [C2mim][Tf2N] (2) | 219.7 | 4.12 | |
| 9 | [C4mim][Tf2N] (2) | 199.2 | 4.24 | |
| 10 | [Bnmim][Tf2N] (2) | 185.5 | 4.11 | |
| 11 | [6FDA I2A oXy][Tf2N] | Neat | 221.8 | 4.61 |
| 12 | [C2mim][Tf2N] (2) | 221.4 | 4.55 | |
| 13 | [C4mim][Tf2N] (2) | 243.3 | 4.35 | |
| 14 | [Bnmim][Tf2N] (2) | 236.3 | 4.26 |
| [6FDA I4A pXy][Tf2N] | PCO2 | PN2 | PCH4 | αCO2/N2 | αCO2/CH4 |
|---|---|---|---|---|---|
| Neat | 2.15 ± 0.16 | 0.103 ± 0.01 | 0.161 ± 0.02 | 20.9 ± 2 | 13.4 ± 1 |
| [C4mim][Tf2N] (2 equiv.) | 4.57 ± 0.30 | 0.239 ± 0.02 | 0.189 ± 0.02 | 19.1 ± 1 | 24.8 ± 2 |
| [Bnmim][Tf2N] (2 equiv.) | 6.32 ± 0.15 | 0.282 ± 0.02 | 0.294 ± 0.02 | 22.4 ± 1 | 21.5 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Harra, K.E.; Kammakakam, I.; Devriese, E.M.; Noll, D.M.; Bara, J.E.; Jackson, E.M. Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes. Membranes 2019, 9, 79. https://doi.org/10.3390/membranes9070079
O’Harra KE, Kammakakam I, Devriese EM, Noll DM, Bara JE, Jackson EM. Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes. Membranes. 2019; 9(7):79. https://doi.org/10.3390/membranes9070079
Chicago/Turabian StyleO’Harra, Kathryn E., Irshad Kammakakam, Emily M. Devriese, Danielle M. Noll, Jason E. Bara, and Enrique M. Jackson. 2019. "Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes" Membranes 9, no. 7: 79. https://doi.org/10.3390/membranes9070079
APA StyleO’Harra, K. E., Kammakakam, I., Devriese, E. M., Noll, D. M., Bara, J. E., & Jackson, E. M. (2019). Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes. Membranes, 9(7), 79. https://doi.org/10.3390/membranes9070079