PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries
Abstract
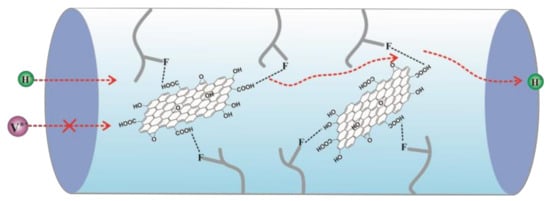
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.3.1. Membrane Morphology
2.3.2. Ion Permeability and Selectivity
2.3.3. Area Resistance and Conductivity
2.3.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.5. VFB Performance
2.3.6. Mechanical Properties
3. Results and Discussion
3.1. Characterization of PVDF and PVDF/Graphene Composite Membranes
3.2. VFB Single Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, H.; Duan, J.; Du, W.; Xue, S.; Sun, J. China’s energy storage industry: Develop status, existing problems and countermeasures. Renew. Sustain. Energy Rev. 2017, 71, 767–784. [Google Scholar] [CrossRef]
- Sum, E.; Rychcik, M.; Skyllas-Kazacos, M. Investigation of the V(V)/V(IV) System for Use in the Positive Half-Cell of a Redox Battery. J. Power Sources 1985, 16, 85–95. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, H.M.; Li, X.F.; Liu, T.; Xing, F. Vanadium Flow Battery for Energy Storage: Prospects and Challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bai, H.; Lv, Y.; Lv, Z.; Xiang, Y.; Lu, S. Enhanced membrane ion selectivity by incorporating graphene oxide nanosheet for vanadium redox flow battery application. Electrochim. Acta 2017, 248, 454–461. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: a review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Chen, H.; Cong, G.; Lu, Y.-C. Recent progress in organic redox flow batteries: Active materials, electrolytes and membranes. J. Energy Chem. 2018, 27, 1304–1325. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.N.L.; Hoang, T.K.A.; Chen, P. Recent development of polymer membranes as separators for all-vanadium redox flow batteries. RSC Adv. 2015, 5, 72805–72815. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane Development for Vanadium Redox Flow Batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, H.M.; Mai, Z.S.; Zhang, H.Z.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Kim, S.; Tighe, T.B.; Schwenzer, B.; Yan, J.; Zhang, J.; Liu, J.; Yang, Z.; Hickner, M.A. Chemical and mechanical degradation of sulfonated poly(sulfone) membranes in vanadium redox flow batteries. J. Appl. Electrochem. 2011, 41, 1201–1213. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Xiao, C.; Qiao, L.; Fu, Q.; Li, X. Highly selective charged porous membranes with improved ion conductivity. Nano Energy 2018, 48, 353–360. [Google Scholar] [CrossRef]
- Yu, L.; Lin, F.; Xu, L.; Xi, J. A recast Nafion/graphene oxide composite membrane for advanced vanadium redox flow batteries. RSC Adv. 2016, 6, 3756–3763. [Google Scholar] [CrossRef]
- Xi, J.Y.; Wu, Z.H.; Qiu, X.P.; Chen, L.Q. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Mai, Z.S.; Zhang, H.M.; Li, X.F.; Xiao, S.H.; Zhang, H.Z. Nafion/polyvinylidene fluoride blend membranes with improved ion selectivity for vanadium redox flow battery application. J. Power Sources 2011, 196, 5737–5741. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Qiao, L.; Zhang, H.; Li, X. Advanced porous PBI membranes with tunable performance induced by the polymer-solvent interaction for flow battery application. Energy Storage Mater. 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Ding, L.; Song, X.; Wang, L.; Zhao, Z.; He, G. Preparation of dense polybenzimidazole proton exchange membranes with different basicity and flexibility for vanadium redox flow battery applications. Electrochim. Acta 2018, 292, 10–19. [Google Scholar] [CrossRef]
- Jang, J.-K.; Kim, T.-H.; Yoon, S.J.; Lee, J.Y.; Lee, J.-C.; Hong, Y.T. Highly proton conductive, dense polybenzimidazole membranes with low permeability to vanadium and enhanced H2SO4 absorption capability for use in vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 14342–14355. [Google Scholar] [CrossRef]
- Jia, C.; Cheng, Y.; Ling, X.; Wei, G.; Liu, J.; Yan, C. Sulfonated Poly(Ether Ether Ketone)/Functionalized Carbon Nanotube Composite Membrane for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2015, 153, 44–48. [Google Scholar] [CrossRef]
- Yun, S.; Heo, Y.; Im, H.; Kim, J. Sulfonated multiwalled carbon nanotube/sulfonated poly(ether sulfone) composite membrane with low methanol permeability for direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 126, E513–E521. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhang, J.; Chen, J.; Zhu, S.; Liu, X.; Wang, R. Sulfonated poly(ether ether ketone)/poly(vinylidene fluoride)/graphene composite membrane for a vanadium redox flow battery. J. Solid State Electrochem. 2016, 21, 1185–1194. [Google Scholar] [CrossRef]
- Dai, W.; Shen, Y.; Li, Z.; Yu, L.; Xi, J.; Qiu, X. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- Ji, Y.; Tay, Z.Y.; Li, S.F.Y. Highly selective sulfonated poly(ether ether ketone)/titanium oxide composite membranes for vanadium redox flow batteries. J. Membr. Sci. 2017, 539, 197–205. [Google Scholar] [CrossRef]
- Liu, S.; Sang, X.X.; Wang, L.H.; Zhang, J.L.; Song, J.L.; Han, B.X. Incorporation of metal-organic framework in polymer membrane enhances vanadium flow battery performance. Electrochim. Acta 2017, 257, 243–249. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Zhang, J. Nanofiltration (NF) membranes: the next generation separators for all vanadium redox flow batteries (VRBs)? Energy Environ. Sci. 2011, 4, 1676–1679. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, Z.; Li, X.; Xu, W.; Zhang, H. Hydrophilic poly(vinylidene fluoride) porous membrane with well connected ion transport networks for vanadium flow battery. J. Power Sources 2015, 298, 228–235. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Liu, Z.; Qing, G. Synthesis of nanoporous PVDF membranes by controllable crystallization for selective proton permeation. J. Membr. Sci. 2016, 517, 111–120. [Google Scholar] [CrossRef]
- Bhunia, R.; Dey, R.; Das, S.; Hussain, S.; Bhar, R.; Kumar Pal, A. Enhanced piezo-electric property induced in graphene oxide/polyvinylidene fluoride composite flexible thin films. Polym. Compos. 2018, 39, 4205–4216. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, G.-S.; Cao, W.-Q.; Wei, Y.-Z.; Cao, M.-S.; Guo, L. Fabrication of multi-functional PVDF/RGO composites via a simple thermal reduction process and their enhanced electromagnetic wave absorption and dielectric properties. RSC Adv. 2014, 4, 19594–19601. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Gao, W.; Wu, G.; Janicke, M.T.; Cullen, D.A.; Mukundan, R.; Baldwin, J.K.; Brosha, E.L.; Galande, C.; Ajayan, P.M.; More, K.L.; et al. Ozonated graphene oxide film as a proton-exchange membrane. Angew. Chem. Int. Ed. 2014, 53, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Choi, C.; Heo, J.; Kim, D.W.; Lee, J.Y.; Hong, Y.T.; Jung, H.T.; Kim, H.T. Pore-Size-Tuned Graphene Oxide Frameworks as Ion-Selective and Protective Layers on Hydrocarbon Membranes for Vanadium Redox-Flow Batteries. Nano Lett. 2018, 18, 3962–3968. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhu, X.; Li, M.; Lu, W.; Li, X.; Zhang, H. A Highly Ion-Selective Zeolite Flake Layer on Porous Membranes for Flow Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 3058–3062. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Amine functionalized graphene oxide containing C16 chain grafted with poly(ether sulfone) by DABCO coupling: Anion exchange membrane for vanadium redox flow battery. J. Membr. Sci. 2019, 575, 109–117. [Google Scholar] [CrossRef]
- Luo, T.; David, O.; Gendel, Y.; Wessling, M. Porous poly(benzimidazole) membrane for all vanadium redox flow battery. J. Power Sources 2016, 312, 45–54. [Google Scholar] [CrossRef]










| Sample | Thickness (μm) | Permeability of Protons (×10−5 cm2/min) | Permeability of VO2+ (×10−7 cm2/min) | Selectivity | Proton Conductivity (mS/cm) |
|---|---|---|---|---|---|
| PVDF | 123 | 3.41 | 12.8 | 26.6 | 23.5 |
| PVDF/G-0.05 | 124 | 2.91 | 9.52 | 30.6 | 31.3 |
| PVDF/G-0.10 | 141 | 2.85 | 9.81 | 29.1 | 31.3 |
| PVDF/G-0.15 | 125 | 3.40 | 8.89 | 38.2 | 37.1 |
| PVDF/G-0.2 | 115 | 2.35 | 7.09 | 33.1 | 32.6 |
| PVDF/G-0.3 | 128 | 2.50 | 7.13 | 35.1 | 30.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.; Wan, L.; Wang, B. PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries. Membranes 2019, 9, 89. https://doi.org/10.3390/membranes9070089
Lai Y, Wan L, Wang B. PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries. Membranes. 2019; 9(7):89. https://doi.org/10.3390/membranes9070089
Chicago/Turabian StyleLai, Yiming, Lei Wan, and Baoguo Wang. 2019. "PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries" Membranes 9, no. 7: 89. https://doi.org/10.3390/membranes9070089
APA StyleLai, Y., Wan, L., & Wang, B. (2019). PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries. Membranes, 9(7), 89. https://doi.org/10.3390/membranes9070089