Effect of Short-Term Contact with C1–C4 Monohydric Alcohols on the Water Permeance of MPD-TMC Thin-Film Composite Reverse Osmosis Membranes
Abstract
:1. Introduction
2. Theory
3. Experimental
3.1. Materials
3.2. TFC Membrane Characterization
3.3. TFC Membrane Performance Testing
4. Results and Discussion
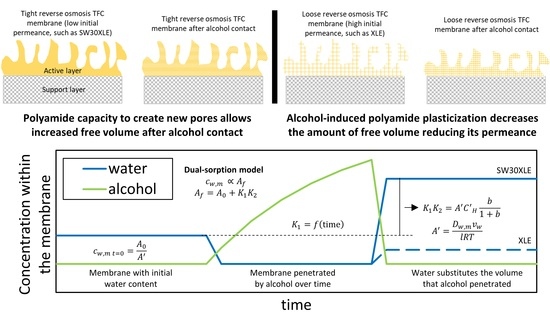
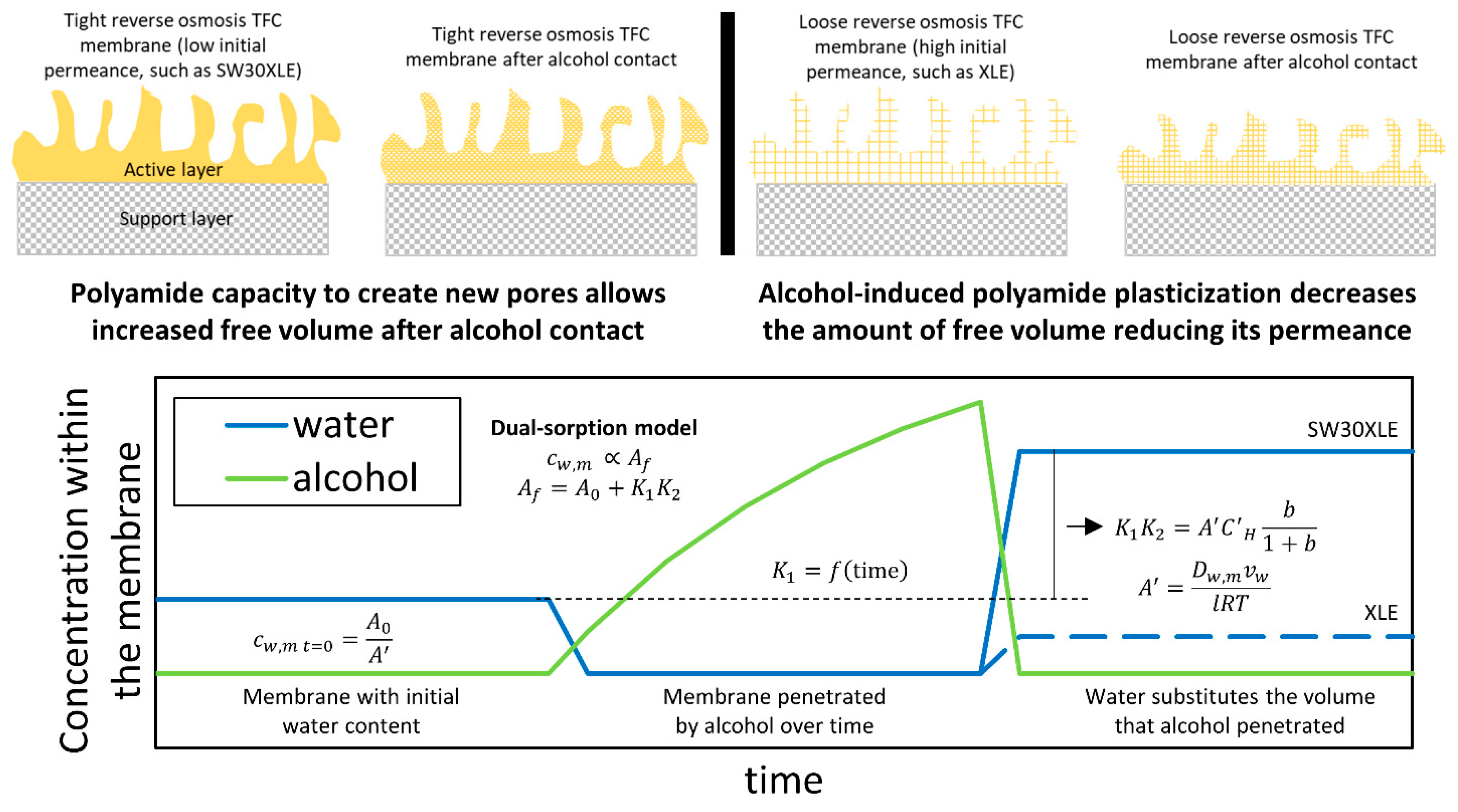
4.1. Active Layer Characteristics in Contact with Short-Chain Alcohols and Water
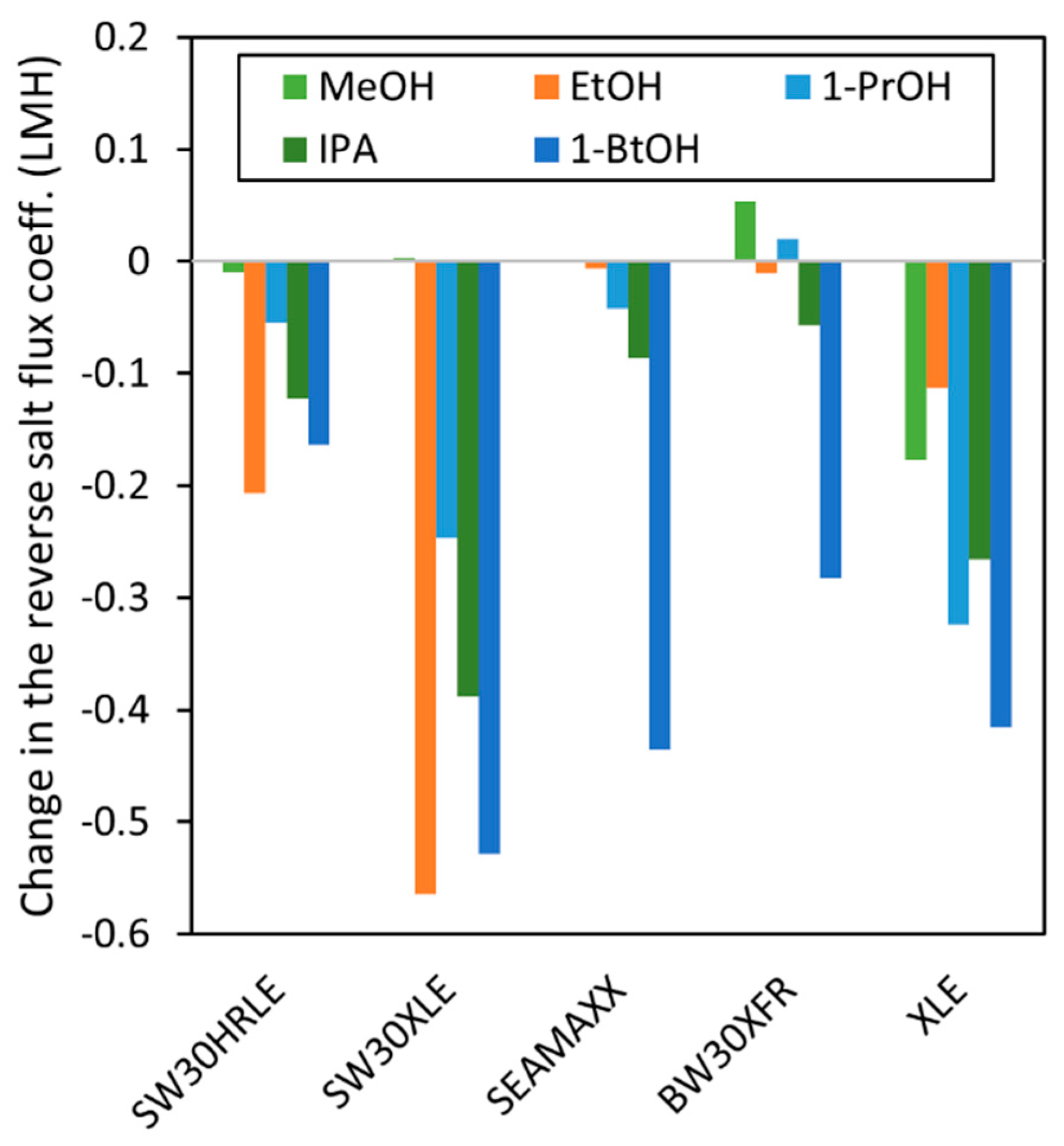
4.2. Effect of Short-Chain Alcohol Contact on TFC Membrane Transport Properties
5. Conclusions
Supplementary materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Bartholomew, T.V.; Mey, L.; Arena, J.T.; Siefert, N.S.; Mauter, M.S. Osmotically assisted reverse osmosis for high salinity brine treatment. Desalination 2017, 421, 3–11. [Google Scholar] [CrossRef]
- Shaffer, D.L.; LaManna, J.M.; Jacobson, D.L.; Hussey, D.S.; Elimelech, M.; Chan, E.P. Studying water and solute transport through desalination membranes via neutron radiography. J. Membr. Sci. 2017, 548, 667–675. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Cath, T.Y.; Elimelech, M.; McCutcheon, J.R.; McGinnis, R.L.; Achilli, A.; Anastasio, D.; Brady, A.R.; Childress, A.E.; Farr, I.V.; Hancock, N.T.; et al. Standard Methodology for Evaluating Membrane Performance in Osmotically Driven Membrane Processes. Desalination 2013, 312, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Idarraga-Mora, J.A.; Ladner, D.A.; Husson, S.M. Thin-film composite membranes on polyester woven mesh with variable opening size for pressure-retarded osmosis. J. Membr. Sci. 2018, 549, 251–259. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Cao, Z.; Wang, J.; Guo, C. Enhanced Performance of Thin Film Composite Forward Osmosis Membrane by Chemical Post-Treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022023. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.; Mukherjee, D.; Gill, W.N. Flux enhancement by hydrophilization of thin film composite reverse osmosis membranes. J. Membr. Sci. 1996, 114, 39–50. [Google Scholar] [CrossRef]
- Wang, X.; Duitsman, E.; Rajagopalan, N.; Namboodiri, V.V. Chemical treatment of commercial reverse osmosis membranes for use in FO. Desalination 2013, 319, 66–72. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, F.; Chung, T.S. Substrate modifications and alcohol treatment on thin film composite membranes for osmotic power. Chem. Eng. Sci. 2013, 87, 40–50. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.; Hill, A.J. Advanced Fabrication of Carbon Molecular Sieve Membranes by Nonsolvent Pretreatment of Precursor Polymers. Ind. Eng. Chem. Res. 2004, 43, 6476–6483. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, Y.; Sun, S.P.; Chung, T.S. Molecular design of thin film composite (TFC) hollow fiber membranes for isopropanol dehydration via pervaporation. J. Membr. Sci. 2012, 405–406, 123–133. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Reinhard, M. Effects of surface coating process conditions on the water permeation and salt rejection properties of composite polyamide reverse osmosis membranes. J. Membr. Sci. 2011, 367, 249–255. [Google Scholar] [CrossRef]
- Lang, K.; Sourirajan, S.; Matsuura, T.; Chowdhury, G. A study on the preparation of polyvinyl alcohol thin-film composite membranes and reverse osmosis testing. Desalination 1996, 104, 185–196. [Google Scholar] [CrossRef]
- Shin, M.G.; Park, S.; Kwon, S.J.; Kwon, H.; Park, J.B.; Lee, J. Facile performance enhancement of reverse osmosis membranes via solvent activation with benzyl alcohol. J. Membr. Sci. 2019, 578, 220–229. [Google Scholar] [CrossRef]
- Rezac, M.E.; Le Roux, J.D.; Paul, D.R.; Koros, W.J. Effect of mild solvent post-treatments on the gas transport properties of glassy polymer membranes. J. Membr. Sci. 1994, 90, 213–229. [Google Scholar] [CrossRef]
- Aharoni, S.M. The solubility parameters of aromatic polyamides. J. Appl. Polym. Sci. 1992, 45, 813–817. [Google Scholar] [CrossRef]
- Ismail, A.F.; Matsuura, T. Progress in transport theory and characterization method of Reverse Osmosis (RO) membrane in past fifty years. Desalination 2018, 434, 2–11. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Suzuki, T. Positron Annihilation Spectroscopic Evidence to Demonstrate the Flux-Enhancement Mechanism in Morphology-Controlled Thin-Film-Composite (TFC) Membrane. Environ. Sci. Technol. 2005, 39, 1764–1770. [Google Scholar] [CrossRef]
- Aharoni, S.M.; Edwards, S.F. Gels of rigid polyamide networks. Macromolecules 1989, 22, 3361–3374. [Google Scholar] [CrossRef]
- Guo, J.; Barbari, T.A. A dual mode, local equilibrium relaxation model for small molecule diffusion in a glassy polymer. Macromolecules 2008, 41, 238–245. [Google Scholar] [CrossRef]
- Guo, J.; Barbari, T.A. A dual mode interpretation of the kinetics of penetrant-induced swelling and deswelling in a glassy polymer. Polymer (Guildf) 2010, 51, 5145–5150. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd: Chichester, UK, 2004. [Google Scholar]
- Freger, V. Swelling and Morphology of the Skin Layer of Polyamide Composite Membranes: An Atomic Force Microscopy Study. Environ. Sci. Technol. 2004, 38, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Feng, C.; Lopez, R.; Coronell, O. Identifying facile and accurate methods to measure the thickness of the active layers of thin-film composite membranes—A comparison of seven characterization techniques. J. Membr. Sci. 2016, 498, 167–179. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Lin, L.; Lopez, R.; Ramon, G.Z.; Coronell, O. Investigating the void structure of the polyamide active layers of thin-film composite membranes. J. Membr. Sci. 2016, 497, 365–376. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes II. Membrane physiochemical properties and their dependence on polyamide and coating layers. Desalination 2009, 242, 168–182. [Google Scholar] [CrossRef]
- Xu, J.; Yan, H.; Zhang, Y.; Pan, G.; Liu, Y. The morphology of fully-aromatic polyamide separation layer and its relationship with separation performance of TFC membranes. J. Membr. Sci. 2017, 541, 174–188. [Google Scholar] [CrossRef]
- Khorshidi, B.; Soltannia, B.; Thundat, T.; Sadrzadeh, M. Synthesis of thin film composite polyamide membranes: Effect of monohydric and polyhydric alcohol additives in aqueous solution. J. Membr. Sci. 2017, 523, 336–345. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Weinman, S.T.; Fierce, E.M.; Husson, S.M. Nanopatterning commercial nanofiltration and reverse osmosis membranes. Sep. Purif. Technol. 2019, 209, 646–657. [Google Scholar] [CrossRef]
- DuPont FILMTEC (TM), Reverse Osmosis & Nanofiltration/Water Solutions. Available online: https://www.dupont.com/water/reverse-osmosis.html (accessed on 6 August 2019).
- Hofs, B.; Schurer, R.; Harmsen, D.J.H.; Ceccarelli, C.; Beerendonk, E.F.; Cornelissen, E.R. Characterization and performance of a commercial thin film nanocomposite seawater reverse osmosis membrane and comparison with a thin film composite. J. Membr. Sci. 2013, 446, 68–78. [Google Scholar] [CrossRef]
- Rowe, B.W.; Pas, S.J.; Hill, A.J.; Suzuki, R.; Freeman, B.D.; Paul, D.R. A variable energy positron annihilation lifetime spectroscopy study of physical aging in thin glassy polymer films. Polymer (Guildf) 2009, 50, 6149–6156. [Google Scholar] [CrossRef]
- Pendergast, M.T.M.; Nygaard, J.M.; Ghosh, A.K.; Hoek, E.M.V. Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 2010, 261, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Idarraga-Mora, J.A.; Childress, A.S.; Friedel, P.S.; Ladner, D.A.; Rao, A.; Husson, S. Role of Nanocomposite Support Stiffness on TFC Membrane Water Permeance. Membranes (Basel) 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, M.T.M.; Ghosh, A.K.; Hoek, E.M.V. Separation performance and interfacial properties of nanocomposite reverse osmosis membranes. Desalination 2013, 308, 180–185. [Google Scholar] [CrossRef]
- Park, S.; Hwang, I.; Kwak, H.-Y. Binary Liquid−Liquid Equilibrium (LLE) for Dibutyl Ether (DBE) + Water from (288.15 to 318.15) K and Ternary LLE for Systems of DBE + C1 ∼ C4 Alcohols + Water at 298.15 K. J. Chem. Eng. Data 2008, 53, 2089–2094. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Nelson, R.D.; Lide, D.R.; Maryott, A.A. Selected Values of Electric Dipole Moments for Molecules in the Gas Phase; National Standard Reference Data Series: Washington, DC, USA, 1967. [Google Scholar]
- Reid, R.; Prausnitz, J.; Sherwood, T. The Peoperties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 1977. [Google Scholar]
- Van Der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Infuence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]








| TFC Membrane | Water Permeance (LMH/bar) | NaCl Rejection (%) |
|---|---|---|
| SW30HRLE | 1.37 ± 0.09 | 94.8% ± 1.7% |
| SW30XLE | 0.74 ± 0.11 | 90.9% ± 3.2% |
| SEAMAXX | 3.44 ± 0.21 | 95.7% ± 1.1% |
| BW30XFR | 2.67 ± 0.14 | 96.7% ± 1.4% |
| XLE | 5.36 ± 0.35 | 87.6% ± 1.9% |
| Alcohol | Solubility Parameter (MPa)1/2 |
|---|---|
| Methanol | 29.41 |
| Ethanol | 26.52 |
| n-propanol | 24.60 |
| IPA | 23.58 |
| n-butanol | 23.20 |
| Water | 47.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idarraga-Mora, J.A.; Lemelin, M.A.; Weinman, S.T.; Husson, S.M. Effect of Short-Term Contact with C1–C4 Monohydric Alcohols on the Water Permeance of MPD-TMC Thin-Film Composite Reverse Osmosis Membranes. Membranes 2019, 9, 92. https://doi.org/10.3390/membranes9080092
Idarraga-Mora JA, Lemelin MA, Weinman ST, Husson SM. Effect of Short-Term Contact with C1–C4 Monohydric Alcohols on the Water Permeance of MPD-TMC Thin-Film Composite Reverse Osmosis Membranes. Membranes. 2019; 9(8):92. https://doi.org/10.3390/membranes9080092
Chicago/Turabian StyleIdarraga-Mora, Jaime A., Michael A. Lemelin, Steven T. Weinman, and Scott M. Husson. 2019. "Effect of Short-Term Contact with C1–C4 Monohydric Alcohols on the Water Permeance of MPD-TMC Thin-Film Composite Reverse Osmosis Membranes" Membranes 9, no. 8: 92. https://doi.org/10.3390/membranes9080092