Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook
Abstract
:1. Introduction
2. Recent Experimental Findings
2.1. High Temperature Studies
2.2. Low Temperature Studies
3. Discussion
4. The Outlook
- (a)
- The proton conductivity of the cells. Regardless of the hydrogen source, nitrogen must react with H+, which in turn, must be supplied electrochemically. The highest proton fluxes reported until the end of 2015 were of the order of 10−7 gram atoms of H+·s−1·cm−2 [4].
- (b)
- The catalytic activity of the cathodic electrode. The reaction of hydrogen evolution competes with the reaction of NH3 synthesis and this results in a significant decrease in FE. Although FEs as high as 90% had been reported, in general the FE values are mostly lower than 10%. At temperatures <100 °C, most of the reported FE values are of the order 1% [1,4].
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| AEM | Anion exchange membrane |
| AT | Ambient temperature |
| CC | Carbon cloth |
| CNT | Carbon nanotubes |
| CP | Carbon paper |
| DFT | Density functional theory |
| EDA | Ethylene diamine |
| FE | Faradaic efficiency |
| PEBCD | Poly(N-ethyl-benzene-1,2,4,5-tetracarboxylic diimide) |
| GC | Glassy carbon |
| H-B | Haber-Bosch process |
| HNC | Hollow nanocages |
| MOF | Metal-organic-framework |
| NCM | N-doped nanoporous graphitic carbon membrane |
| NPC | Ν-doped porous carbon |
| NRR | Nitrogen reduction reaction |
| OCV | Open circuit voltage |
| PBS | Phosphate buffer solution |
| RGO | Reduced graphite oxide |
| RT | Room temperature |
| SSAS | Solid state ammonia synthesis |
| THF | Tetrahydrofuran solution |
| ZIF | Zeolitic imidazolate framework |
References
- Garagounis, I.; Kyriakou, V.; Skodra, A.; Vasileiou, E.; Stoukides, M. Electrochemical Synthesis of Ammonia in Solid Electrolyte Cells. Front. Energy Res. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Liu, H. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge. Cuihua Xuebao/Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E.; Vourros, A.; Stoukides, M. Progress in the Electrochemical Synthesis of Ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-state electrochemical synthesis of ammonia: A review. J. Solid State Electrochem. 2011, 15, 1845–1860. [Google Scholar] [CrossRef]
- Stoukides, M. Proton Conducting Materials Electrocatalyst in Solid State Ammonia Synthesis. In Proton-Conducting Ceramics: From Fundamentals to Applied Research; Marrony, M., Ed.; Pan Stanford Publishing: Singapore, 2016; pp. 377–405. ISBN 978-981-4613-84-2. [Google Scholar]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Garagounis, I.; Kyriakou, V.; Anagnostou, C.; Bourganis, V.; Papachristou, I.; Stoukides, M. Solid electrolytes: Applications in heterogeneous catalysis and chemical cogeneration. Ind. Eng. Chem. Res. 2011, 50, 431–472. [Google Scholar] [CrossRef]
- Liu, H. Proton Conducting Materials Electrocatalyst in Solid State Ammonia Synthesis. In Ammonia Synthesis Catalysts. Innovation and Practice; World Scientific Publishing: Singapore, 2013; pp. 813–831. ISBN 978-981-4355-77-3. [Google Scholar]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Marnellos, G. Ammonia Synthesis at Atmospheric Pressure. Science 1998, 282, 98–100. [Google Scholar] [CrossRef]
- Marnellos, G.; Sanopoulou, O.; Rizou, A.; Stoukides, M. The use of proton conducting solid electrolytes for improved performance of hydro- and dehydrogenation reactors. Solid State Ionics 1997, 97, 375–383. [Google Scholar] [CrossRef]
- Kishira, S.; Qing, G.; Suzu, S.; Kikuchi, R.; Takagaki, A.; Oyama, S.T. Ammonia synthesis at intermediate temperatures in solid-state electrochemical cells using cesium hydrogen phosphate based electrolytes and noble metal catalysts. Int. J. Hydrog. Energy 2017, 42, 26843–26854. [Google Scholar] [CrossRef]
- Imamura, K.; Kubota, J. Electrochemical membrane cell for NH 3 synthesis from N 2 and H 2 O by electrolysis at 200 to 250 °C using a Ru catalyst, hydrogen-permeable Pd membrane and phosphate-based electrolyte. Sustain. Energy Fuels 2018, 2, 1278–1286. [Google Scholar] [CrossRef]
- Qing, G.; Kikuchi, R.; Kishira, S.; Takagaki, A.; Sugawara, T.; Oyama, S.T. Ammonia Synthesis by N 2 and Steam Electrolysis in Solid-State Cells at 220 °C and Atmospheric Pressure. J. Electrochem. Soc. 2016, 163, E282–E287. [Google Scholar] [CrossRef]
- Kosaka, F.; Nakamura, T.; Oikawa, A.; Otomo, J. Electrochemical Acceleration of Ammonia Synthesis on Fe-Based Alkali-Promoted Electrocatalyst with Proton Conducting Solid Electrolyte. ACS Sustain. Chem. Eng. 2017, 5, 10439–10446. [Google Scholar] [CrossRef]
- Kosaka, F.; Nakamura, T.; Otomo, J. Electrochemical Ammonia Synthesis Using Mixed Protonic-Electronic Conducting Cathodes with Exsolved Ru-Nanoparticles in Proton Conducting Electrolysis Cells. J. Electrochem. Soc. 2017, 164, F1323–F1330. [Google Scholar] [CrossRef]
- Kyriakou, I.; Garagounis, A.; Vourros, E.; Vasileiou, M.S. Combination of Methane Steam Reforming and Ammonia Synthesis in an Electrochemical Membrane Reactor. In Proceedings of the PPCC 2017 International Workshop on Protonic Ceramic Fuel Cells: Status & Prospects, Bordeaux, France, 16–18 October 2017. [Google Scholar]
- Cui, B.; Zhang, J.; Liu, S.; Liu, X.; Xiang, W.; Liu, L.; Xin, H.; Lefler, M.J.; Licht, S. Electrochemical synthesis of ammonia directly from N2and water over iron-based catalysts supported on activated carbon. Green Chem. 2017, 19, 298–304. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Electrochemical Synthesis of Ammonia in Molten Salt Electrolyte Using Hydrogen and Nitrogen at Ambient Pressure. J. Electrochem. Soc. 2017, 164, H5036–H5042. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, C.Y.; Kim, J.N.; Yoon, H.C.; Han, J.I. Electrochemical synthesis of ammonia from water and nitrogen catalyzed by nano-Fe2O3and CoFe2O4suspended in a molten LiCl-KCl-CsCl electrolyte. Korean J. Chem. Eng. 2016, 33, 1777–1780. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Sánchez, P.; Dorado, F.; Stoukides, M. Enhancement of Ammonia Synthesis on a Co 3 Mo 3 N-Ag Electrocatalyst in a K-βAl 2 O 3 Solid Electrolyte Cell. ACS Sustain. Chem. Eng. 2017, 5, 8844–8851. [Google Scholar] [CrossRef]
- Licht, S.; Cui, B.; Wang, B.; Li, F.F.; Lau, J.; Liu, S. Ammonia synthesis by N2and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 2014, 345, 637–640. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Pliangos, C.; Brosda, S. Electrochemical Activation of Catalysis; Kluwer Academic/Plenum: New York, NY, USA, 2001; ISBN 978-1-4757-8234-9. [Google Scholar]
- Vayenas, C.G.; Koutsodontis, C.G. Non-Faradaic electrochemical activation of catalysis. J. Chem. Phys. 2008, 128, 182506. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, Z.; Shi, X.; Fan, M.; Sun, X. Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions by Fe2O3 Nanorods. ChemCatChem 2018, 10, 4530–4535. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS 2 Catalyst: Theoretical and Experimental Studies. Adv. Mater. 2018, 30, 2–7. [Google Scholar]
- Cheng, H.; Ding, L.X.; Chen, G.F.; Zhang, L.; Xue, J.; Wang, H. Molybdenum Carbide Nanodots Enable Efficient Electrocatalytic Nitrogen Fixation under Ambient Conditions. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Cao, X.; Wu, S.; Zeng, X.; Ding, L.X.; Zhu, M.; Wang, H. Ammonia Electrosynthesis with High Selectivity under Ambient Conditions via a Li + Incorporation Strategy. J. Am. Chem. Soc. 2017, 139, 9771–9774. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Room-Temperature Electrocatalytic Synthesis of NH3 from H2O and N2 in a Gas-Liquid-Solid Three-Phase Reactor. ACS Sustain. Chem. Eng. 2017, 5, 7393–7400. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Quan, X.; Fan, X.; Chen, S.; Yu, H.; Zhao, H.; Zhang, Y.; Zhao, J. Facile Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions on N-Doped Porous Carbon. ACS Catal. 2018, 8, 1186–1191. [Google Scholar] [CrossRef]
- Yang, D.; Chen, T.; Wang, Z. Electrochemical reduction of aqueous nitrogen (N 2) at a low overpotential on (110)-oriented Mo nanofilm. J. Mater. Chem. A 2017, 5, 18967–18971. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Hu, L.; Chen, G.; Xin, H.; Feng, X. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 2018, 9, 1795. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Qiao, M.; Wu, G.; Chen, W.; Yuan, T.; Xu, Q.; Chen, M.; Zhang, Y.; Wang, X.; et al. Atomically dispersed Au 1 catalyst towards efficient electrochemical synthesis of ammonia. Sci. Bull. 2018, 63, 1246–1253. [Google Scholar] [CrossRef]
- Katayama, A.; Inomata, T.; Ozawa, T.; Masuda, H. Electrochemical conversion of dinitrogen to ammonia induced by a metal complex-supported ionic liquid. Electrochem. Commun. 2016, 67, 6–10. [Google Scholar] [CrossRef]
- Chen, S.; Perathoner, S.; Ampelli, C.; Mebrahtu, C.; Su, D.; Centi, G. Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon-Nanotube-Based Electrocatalyst. Angew. Chem. Int. Ed. 2017, 56, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, R.M.; Du, H.; Xia, L.; Qu, F. Highly efficient electrochemical ammonia synthesis: Via nitrogen reduction reactions on a VN nanowire array under ambient conditions. Chem. Commun. 2018, 54, 5323–5325. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017, 29, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Bao, D.; Shi, M.M.; Wulan, B.R.; Yan, J.M.; Jiang, Q. Amorphizing of Au Nanoparticles by CeOx–RGO Hybrid Support towards Highly Efficient Electrocatalyst for N2 Reduction under Ambient Conditions. Adv. Mater. 2017, 29, 1–6. [Google Scholar]
- Zhang, R.; Zhang, Y.; Ren, X.; Cui, G.; Asiri, A.M.; Zheng, B.; Sun, X. High-Efficiency Electrosynthesis of Ammonia with High Selectivity under Ambient Conditions Enabled by VN Nanosheet Array. ACS Sustain. Chem. Eng. 2018, 6, 9545–9549. [Google Scholar] [CrossRef]
- Qiu, W.; Xie, X.Y.; Qiu, J.; Fang, W.H.; Liang, R.; Ren, X.; Ji, X.; Cui, G.; Asiri, A.M.; Cui, G.; et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Z.; Ma, Y.; Cui, G.; Xie, F.; Wang, F.; Wu, Y.; Gao, S.; Xu, Y.; Sun, X. Ambient N 2 fixation to NH 3 at ambient conditions: Using Nb 2 O 5 nanofiber as a high-performance electrocatalyst. Nano Energy 2018, 52, 264–270. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, Q.; Ye, S.; Sun, W.; Shao, Y.; Jiang, Z.; Qiao, Q.; Zhu, Y.; Song, P.; et al. Ambient Electrosynthesis of Ammonia: Electrode Porosity and Composition Engineering. Angew. Chem. Int. Ed. 2018, 57, 12360–12364. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liang, Z.; Zhao, J.; Zhu, B.; Cai, K.; Zou, R.; Xu, Q. Hierarchical Cobalt Phosphide Hollow Nanocages toward Electrocatalytic Ammonia Synthesis under Ambient Pressure and Room Temperature. Small Methods 2018, 2, 1800204. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Liu, X.M.; Wang, C.; Ma, W.; Zhang, Q. Highly Selective Electrochemical Reduction of Dinitrogen to Ammonia at Ambient Temperature and Pressure over Iron Oxide Catalysts. Chem. A Eur. J. 2018, 24, 18494–18501. [Google Scholar] [CrossRef] [PubMed]
- Shipman, M.A.; Symes, M.D. A re-evaluation of Sn(II) phthalocyanine as a catalyst for the electrosynthesis of ammonia. Electrochim. Acta 2017, 258, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Bao, D.; Zhang, Q.; Meng, F.-L.; Zhong, H.-X.; Shi, M.-M.; Zhang, Y.; Yan, J.-M.; Jiang, Q.; Zhang, X.-B. Electrochemical Reduction of N 2 under Ambient Conditions for Artificial N 2 Fixation and Renewable Energy Storage Using N 2/NH 3 Cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lim, A.; Yoon, C.; Jang, J.H.; Ham, H.C.; Han, J.; Nam, S.; Kim, D.; Sung, Y.E.; Choi, J.; et al. Electrochemical Synthesis of NH3 at Low Temperature and Atmospheric Pressure Using a γ-Fe2O3 Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10986–10995. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, F.; Liu, N.; Li, G.; Fan, T.; Chen, B. Highly efficient metal–organic-framework catalysts for electrochemical synthesis of ammonia from N2 (air) and water at low temperature and ambient pressure. J. Mater. Sci. 2017, 52, 10175–10185. [Google Scholar] [CrossRef]
- Liu, H.M.; Han, S.H.; Zhao, Y.; Zhu, Y.Y.; Tian, X.L.; Zeng, J.H.; Jiang, J.X.; Xia, B.Y.; Chen, Y. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction. J. Mater. Chem. A 2018, 6, 3211–3217. [Google Scholar] [CrossRef]
- Song, Y.; Johnson, D.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Huang, J.; Yang, F.; Zhang, F.; Qiao, R.; et al. A physical catalyst for the electrolysis of nitrogen to ammonia. Sci. Adv. 2018, 4, e1700336. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, N.; Yoo, C.-Y.; Kim, J.-N.; Yoon, H.C.; Han, J.-I. Communication—Electrochemical Reduction of Nitrogen to Ammonia in 2-Propanol under Ambient Temperature and Pressure. J. Electrochem. Soc. 2016, 163, F610–F612. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, C.-Y.; Kim, J.-N.; Yoon, H.C.; Han, J.-I. Electrochemical Synthesis of Ammonia from Water and Nitrogen in Ethylenediamine under Ambient Temperature and Pressure. J. Electrochem. Soc. 2016, 163, F1523–F1526. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Kang, C.S.M.; Wang, D.; Xiao, C.; Zhou, F.; Azofra, L.M.; Cavallo, L.; Zhang, X.; Macfarlane, D.R. Rational Electrode-Electrolyte Design for Efficient Ammonia Electrosynthesis under Ambient Conditions. ACS Energy Lett. 2018, 3, 1219–1224. [Google Scholar] [CrossRef]
- Zhou, F.; Azofra, L.M.; Ali, M.; Kar, M.; Simonov, A.N.; McDonnell-Worth, C.; Sun, C.; Zhang, X.; Macfarlane, D.R. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci. 2017, 10, 2516–2520. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.; Lee, H.K.; Tsung, C.-K.; Ling, X.Y.; Phang, I.Y.; Koh, C.S.L.; Lee, Y.H. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv. 2018, 4, eaar3208. [Google Scholar] [Green Version]
- Park, J.H.; Yoon, H.C.; Kim, J.N.; Jeong, C.H.; Jeong, E.Y.; Yun, D.S.; Yoon, H.; Park, S.H.; Han, M.H.; Yoo, C.Y. Anion-exchange-membrane-based electrochemical synthesis of ammonia as a carrier of hydrogen energy. Korean J. Chem. Eng. 2018, 35, 1620–1625. [Google Scholar] [CrossRef]
- Abghoui, Y.; Garden, A.L.; Howalt, J.G.; Vegge, T.; Skúlason, E. Electroreduction of N2 to Ammonia at Ambient Conditions on Mononitrides of Zr, Nb, Cr, and V: A DFT Guide for Experiments. ACS Catal. 2016, 6, 635–646. [Google Scholar] [CrossRef]
- Abghoui, Y.; Garden, A.L.; Hlynsson, V.F.; Björgvinsdóttir, S.; Ólafsdóttir, H.; Skúlason, E. Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design. Phys. Chem. Chem. Phys. 2015, 17, 4909–4918. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, S.; Wang, H.; Li, H.; Shao, M. A Spectroscopic Study on the Nitrogen Electrochemical Reduction Reaction on Gold and Platinum Surfaces. J. Am. Chem. Soc. 2018, 140, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Irvine, J.T.S.; Tao, S. Synthesis of ammonia directly from air and water at ambient temperature and pressure. Sci. Rep. 2013, 3, 1145. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Influence of the Supporting Matrix on the Electrochemical Properties of CsH 5 (PO 4) 2 Composites at Intermediate Temperatures. J. Phys. Chem. C 2008, 112, 15532–15536. [Google Scholar] [CrossRef]
- Kim, G.; Griffin, J.M.; Blanc, F.; Haile, S.M.; Grey, C.P. Characterization of the Dynamics in the Protonic Conductor CsH 2 PO 4 by 17 O Solid-State NMR Spectroscopy and First-Principles Calculations: Correlating Phosphate and Protonic Motion. J. Am. Chem. Soc. 2015, 137, 3867–3876. [Google Scholar] [CrossRef]
- Martsinkevich, V.V.; Ponomareva, V.G. Double salts Cs1−xMxH2PO4 (M = Na, K, Rb) as proton conductors. Solid State Ionics 2012, 225, 236–240. [Google Scholar] [CrossRef]
- Muroyama, H.; Kudo, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Electrochemical properties of MH2PO4/SiP2O7-based electrolytes (M=alkaline metal) for use in intermediate-temperature fuel cells. Solid State Ionics 2007, 178, 1512–1516. [Google Scholar] [CrossRef]



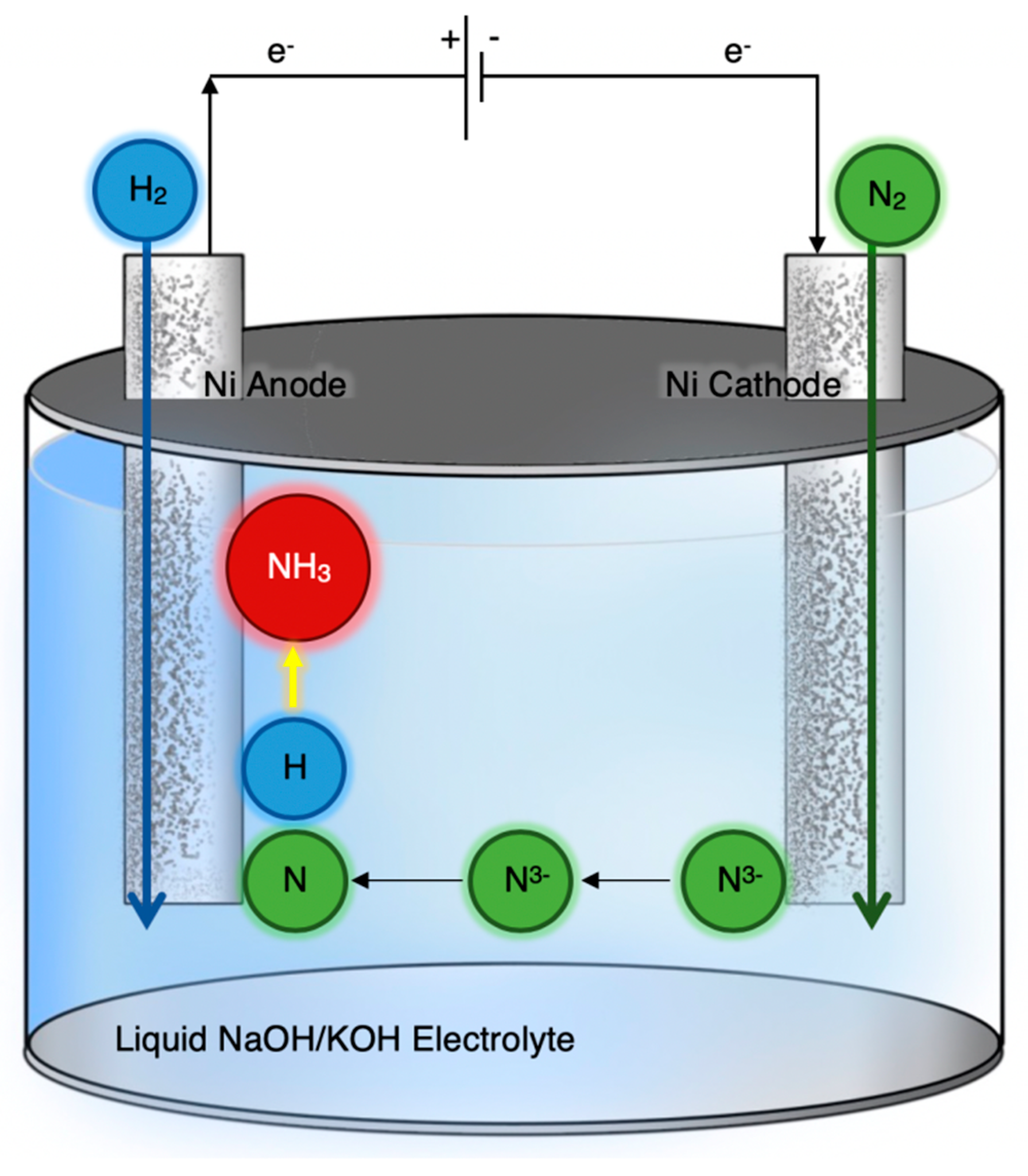
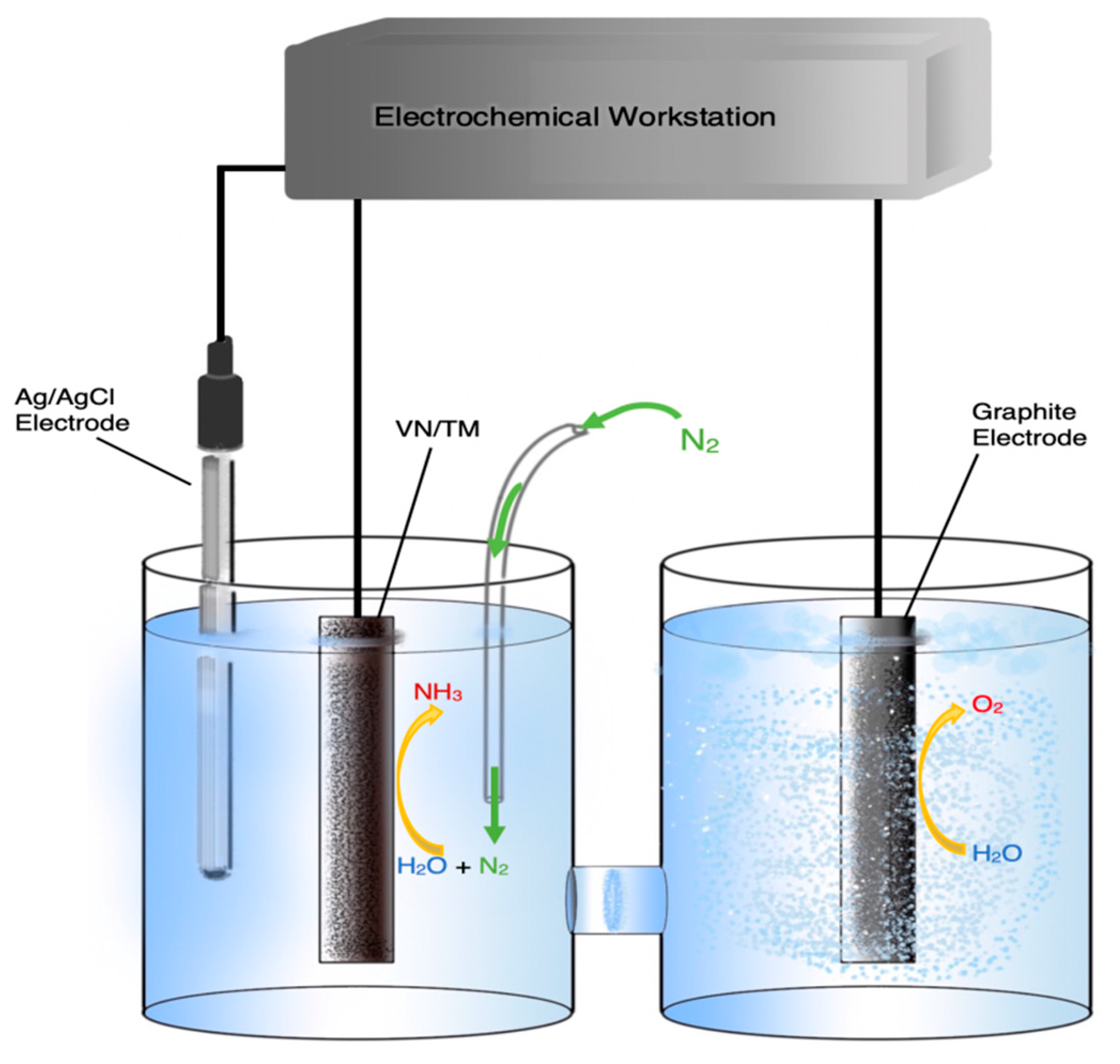


| Temp. (°C) | Cathode | Anode | Electrolyte | Reactants (Cathode/Anode) | rNH3 (mol∙s−1∙cm−2) | FE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 220 | Ru/C | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2 | 8.5 × 10−11 | 0.075 | [13] |
| 220 | Pt/C | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2 | 2.3 × 10−10 | 0.05 | [13] |
| 220 | Ru | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2 | 1.7 × 10−10 | 0.12 | [13] |
| 220 | Ag-Pd | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2 | 8.5 × 10−11 | 0.1 | [13] |
| 220 | Pt/C | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2O | 6.5 × 10−12 | 0.025 | [13] |
| 220 | Pt-Ru/C | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2O | 1.3 × 10−11 | 0.04 | [13] |
| 220 | Ru/C | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2O | 1.9 × 10−11 | 0.14 | [13] |
| 220 | Ru | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2O | 1.25 × 10−11 | 0.055 | [13] |
| 220 | Ag-Pd | Pt/C | CsH2PO4/SiP2O7 composite | N2/H2O | 0.9 × 10−11 | 0.06 | [13] |
| 200–250 | Ru/Cs+/MgO |Pd-Ag* | Pt | CsH2PO4/SiP2O7 | N2/H2O | 9 × 10−10 | 2.6 | [14] |
| 220 | Pt/TiO2|C* | Pt/C | CsH5(PO4)2/SiO2 | N2/H2O | 2 × 10−10 | 2.1 | [15] |
| 500–650 | K, Al modified Fe-BCY | Pt | BaCe0.9Y0.1O3 (BCY) | N2/H2 | 2.4 × 10−11 | 0.005 | [16] |
| 500–650 | K, Al modified Fe-BCY | Pt | BaCe0.9Y0.1O3 (BCY) | N2-H2(15%)/H2 | 6.7 × 10−10 | 0.5 | [16] |
| 500 | Ni-(BCYR) BaCe0.8Y0.1Ru0.1O3 | Pt | BaCe0.9Y0.1O3 (BCY) | N2/H2O(2%)-H2(20%) | 1.1 × 10−11 | 0.22 | [17] |
| 500 | LST (La0.3Sr0.6TiO3)-BCYR | Pt | BaCe0.9Y0.1O3 (BCY) | N2/H2O(2%)-H2(20%) | 1.1 × 10−11 | 2.1 | [17] |
| 500–650 | VN-Fe | Ni-BZCY72 | BZCY81 | N2/CH4-H2O | 1.89 × 10−9 | 14 | [18] |
| 250 | Stainless steel (Fe2O3/AC) | Ni | NaOH-KOH molten salt with | N2/H2O | 8.27 × 10−9 | 13.7 | [19] |
| 200–255 | Ni (Fe3O4) | Ni | KOH-NaOH molten salt | N2/H2 | 6.54 × 10−10 | 9.46 | [20] |
| 327 | Ni (Fe2O3) | Li-Al alloy | LiCl/KCl/CsCl | N2/H2O | 3 × 10−10 | N/A | [21] |
| 327 | Ni (CoFe2O4) | Li-Al alloy | LiCl/KCl/CsCl | N2/H2O | 1.78 × 10−10 | N/A | [21] |
| 400–550 | Co3Mo3N-Ag | Au | K-β″-Al2O3 | N2/H2 | 2.7 × 10−9 | Λ = 300 | [22] |
| Temp. (°C) | Cathode | Anode | Electrolyte | Reactants (Cathode/Anode) | rNH3 (mol∙s−1∙cm−2) | FE (%) | Ref |
|---|---|---|---|---|---|---|---|
| AT | Fe2O3/CP | Graphite rod | Nafion 211/0.1 M Na2SO4 | N2/H2O | 1.03 × 10−10 | 0.94 | [26] |
| RT | MoS2/CC | Graphite rod | Nafion/0.1 M Na2SO4 | N2/H2O | 8.08 × 10−11 | 1.17 | [27] |
| AT | Mo2C/C | Pt | Nafion 211/0.5 M Li2SO4 | N2/H2O | N/A | 7.8 | [28] |
| 25 | PEBCD/CC | Pt | Nafion 211/0.5 M Li2SO4 | N2/H2O | 3.28 × 10−11 | 2.91 | [29] |
| 20 | 30% Fe2O3/CNT | Pt | Nafion 115/0.25 M K2SO4 | N2/H2O | 1 × 10−11 | 0.125 | [30] |
| 20 | 30% Fe2O3/CNT | Pt | Nafion 115/0.25 M KHSO4 | N2/H2O | 7.87 × 10−12 | 0.07 | [30] |
| RT | NPC-750 | Pt | Nafion 117/0.05 M H2SO4 | N2/H2O | 2.33 × 10−10 | 1.42 | [31] |
| RT | Mo-D-R-5h | Pt | Membrane/0.01 M H2SO4 | N2/H2O | 3.09 × 10−11 | 0.72 | [32] |
| AT | Pd/C | Pt | Nafion 115/0.05 M H2SO4 | N2/H2O | 1.2 × 10−11 | 0.03 | [33] |
| RT | Au NPs/C3N4/CP | Pt | Nafion 115/0.5 M H2SO4 | N2/H2O | N/A | 6 | [34] |
| RT | Au1/C3N4/CP | Pt | Nafion 115/0.5 M H2SO4 | N2/H2O | N/A | 11.1 | [34] |
| RT | CP (Cp2TiCl2/[C9H20N]+ [(C2F5)3PF3]-) | Pt | Nafion 212/0.2 M H2SO4 | N2/H2O | N/A | 0.2 | [35] |
| 20 | 30% Fe2O3/CNT | Pt | Nafion 115/0.5 M KHCO3 | N2/H2O | 8.5 × 10−12 | 0.125 | [30] |
| 20 | Fe2O3/CNT | Pt | Nafion/KHCO3 | N2/H2O | 3.59 × 10−12 | 0.15 | [36] |
| RT | MoS2/CC | Graphite rod | Nafion/0.1 M HCl | N2/H2O | 8.48 × 10−11 | 0.096 | [27] |
| AT | VN/CC | Graphite rod | Membrane/0.1 M HCl | N2/H2O | 2.48 × 10−10 | 3.58 | [37] |
| 60 | Au/TiO2 | Pt | Nafion 211/0.1 M HCl | N2/H2O | 5 × 10−10 | 13.5 | [38] |
| RT | Au/TiO2 | Pt | Nafion 211/0.1 M HCl | N2/H2O | 3.5 × 10−10 | 8.11 | [38] |
| RT | Amorphous Au/CeOx-RGO | Pt | Nafion 211/0.1 M HCl | N2/H2O | 2.7 × 10−8 | 10.1 | [39] |
| RT | VN/(Titanium Mesh) | Graphite rod | Nafion/0.1 M HCl | N2/H2O | 8.4 × 10−11 | 2.25 | [40] |
| AT | B4C/CP | Graphite rod | Nafion 211/0.1 M HCl | N2/H2O | 4.34 × 10−11 | 15.95 | [41] |
| RT | Nb2O5/CP | Graphite rod | Membrane/0.1 M HCl | N2/H2O | 6.8 × 10−10 | 9.26 | [42] |
| AT | NCM | Pt | Membrane/0.1 M HCl | N2/H2O | 1.3 × 10−10 | 5.2 | [43] |
| AT | NCM-AuNPs | Pt | Membrane/0.1 M HCl | N2/H2O | 5.88 × 10−10 | 22 | [43] |
| AT | Pd/C | Pt | Nafion 115/0.1 M PBS | N2/H2O | 2.2 × 10−11 | 8.2 | [33] |
| AT | Au/C | Pt | Nafion 115/0.1 M PBS | N2/H2O | 2.4 × 10−12 | 1.2 | [33] |
| AT | Pt/C | Pt | Nafion 115/0.1 M PBS | N2/H2O | 2.4 × 10−12 | 0.2 | [33] |
| AT | Pd/C | Pt | Nafion 115/0.1 M NaOH | N2/H2O | 1.07 × 10−11 | 0.075 | [33] |
| AT | CoP (hollow nano-cages) | Pt | Nafion 117/1 M KOH | N2/H2O | 8.8 × 10−11 | 7.36 | [44] |
| AT | o-Fe2O3-CNT/CP | Graphite rod | Nafion/ 0.1 M KOH | N2/H2O | 2.37 × 10−11 | 8.28 | [45] |
| RT | Carbon foil (Sn(II) phthalocyanine) | Pt | 1 M KOH | N2/H2O | 1.4 × 10−11* | 2* | [46] |
| RT | Tetrahexahedral Au/CP | Graphite plate | Nafion 211/0.1 M KOH | N2/H2O | 2.7 × 10−11 | 3.9 | [47] |
| 65 | Tetrahexahedral Au/CP | Graphite plate | Nafion 211/0.1 M KOH | N2/H2O | 2.2 × 10−10 | 6.8 | [47] |
| 20 | 30% Fe2O3/CNT | Pt | Nafion 115/0.5 M KOH | N2/H2O | 1.06 × 10−11 | 0.164 | [30] |
| 20 | o-CNT | Pt | Nafion 115/0.5 M KOH | N2/H2O | 3.44 × 10−12 | - | [30] |
| 65 | Fe2O3/CP | Ti/IrO2 | Membrane/0.1 M KOH | N2/H2O | 3.47 × 10−12 | 1.96 | [48] |
| 20 | Nano-Fe2O3 | Pt | Nafion 115/0.5 M KOH | N2/H2O | 1.49 × 10−12 | - | [30] |
| 90 | MOF (Fe) | Pt | Nafion 117/2 M KOH | N2/H2O | 2.12 × 10−9 | 1.43 | [49] |
| 90 | MOF (Co) | Pt | Nafion 117/2 M KOH | N2/H2O | 1.64 × 10−9 | 1.06 | [49] |
| 90 | MOF (Cu) | Pt | Nafion 117/2 M KOH | N2/H2O | 1.24 × 10−9 | 0.96 | [49] |
| 90 | MOF (Fe) | Pt | Nafion 117/2 M KOH | N2(Air)/H2O | 1.52 × 10−9 | 0.88 | [49] |
| AT | Rh NNs | Carbon rod | Nafion211/0.1 M KOH | N2/H2O | 6.24 × 10−9 | 0.7 | [50] |
| AT | Carbon nanospikes | Pt | Membrane/0.25 M LiClO4 | N2/H2O | 1.59 × 10−9 | 11.56 | [51] |
| AT | Ni | Pt | 2-propanol: 0.01 M H2SO4 (9:1v/v) | N2/H2O | 1.54 × 10−11 | 0.89 | [52] |
| 25 | Ni | GC | CMX/0.1 M LiCl in EDA | N2/H2O (0.05 M H2SO4) | 3.58 × 10−11 | 17.2 | [53] |
| AT | α-Fe/Fe3O4 | Pt | [C4mpyr][eFAP] FPEE mix | N2/H2O | 2.35 × 10−11 | 32 | [54] |
| AT | Fe-Stainless Steel mesh | Pt | [P6,6,6,14][eFAP] ionic liquid | N2/H2O | 2.04 × 10−11 | 46 | [55] |
| AT | Fe-Stainless Steel mesh | Pt | [C4mpyr][eFAP] ionic liquid | N2/H2O | 2.2 × 10−11 | 35 | [55] |
| AT | Fe-Fluorine doped tin oxide glass | Pt | [C4mpyr][eFAP] ionic liquid | N2/H2O | 6.5 × 10−12 | 38 | [55] |
| AT | Fe-Fluorine doped tin oxide glass | Pt | [P6,6,6,14][eFAP] ionic liquid | N2/H2O | 6.5 × 10−12 | 60 | [55] |
| AT | Fe-Nickel foam | Pt | [P6,6,6,14][eFAP] ionic liquid | N2/H2O | 1.88 × 10−11 | 21 | [55] |
| RT | Ag-Au/ZIF | Pt | THF-based electrolyte | N2/H2O | 1 × 10−11 | 18 ± 4 | [56] |
| AT | Pt/C | Pt/C | AEM | N2-H2O | 1.96 × 10−11 | 1.73 | [57] |
| 65 | Fe2O3/CP | Ti/IrO2 | FAA-3 Fumatech (AEM) | N2-H2O | 1.91 × 10−13 | 0.044 | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garagounis, I.; Vourros, A.; Stoukides, D.; Dasopoulos, D.; Stoukides, M. Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook. Membranes 2019, 9, 112. https://doi.org/10.3390/membranes9090112
Garagounis I, Vourros A, Stoukides D, Dasopoulos D, Stoukides M. Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook. Membranes. 2019; 9(9):112. https://doi.org/10.3390/membranes9090112
Chicago/Turabian StyleGaragounis, Ioannis, Anastasios Vourros, Demetrios Stoukides, Dionisios Dasopoulos, and Michael Stoukides. 2019. "Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook" Membranes 9, no. 9: 112. https://doi.org/10.3390/membranes9090112
APA StyleGaragounis, I., Vourros, A., Stoukides, D., Dasopoulos, D., & Stoukides, M. (2019). Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook. Membranes, 9(9), 112. https://doi.org/10.3390/membranes9090112







