Mixing Properties of Emulsified Fuel Oil from Mixing Marine Bunker-C Fuel Oil and Water
Abstract
:1. Introduction
2. Materials and Methods
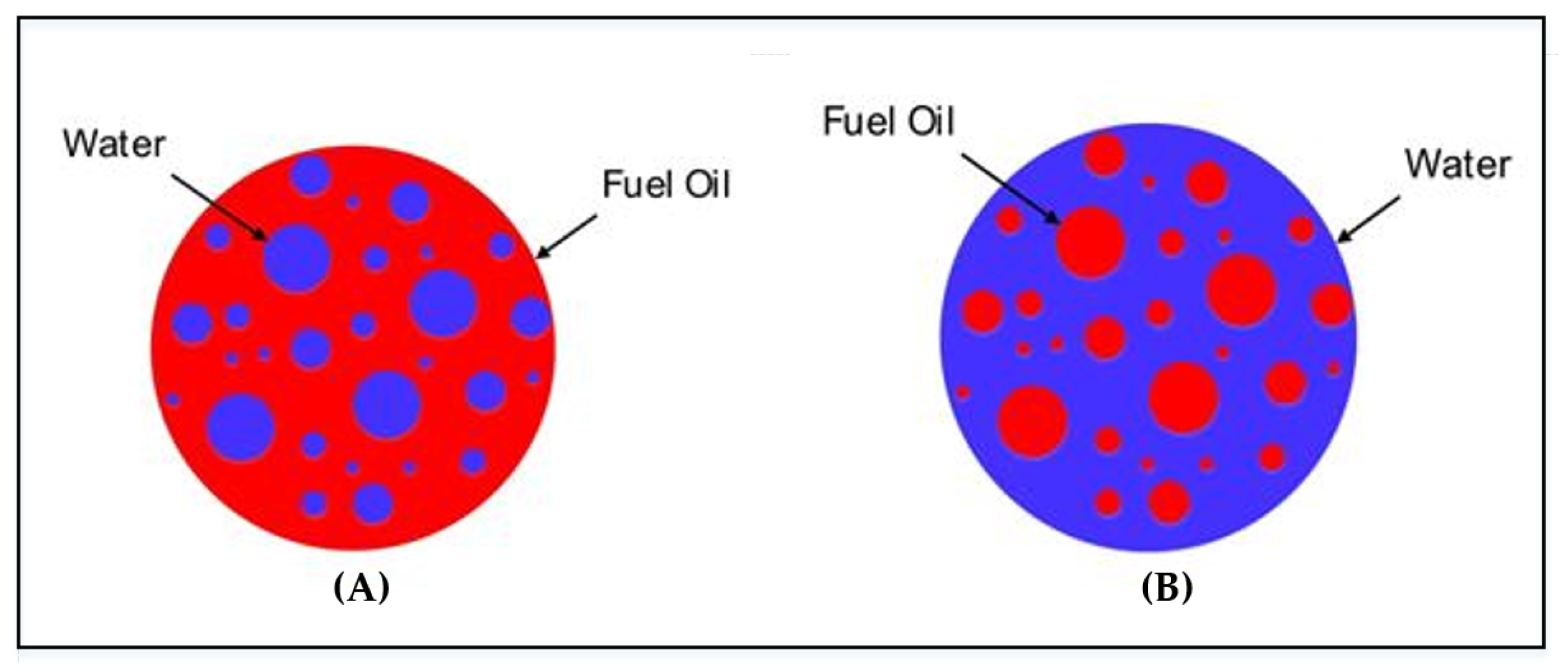
2.1. Definition of Emulsified Fuel Oil
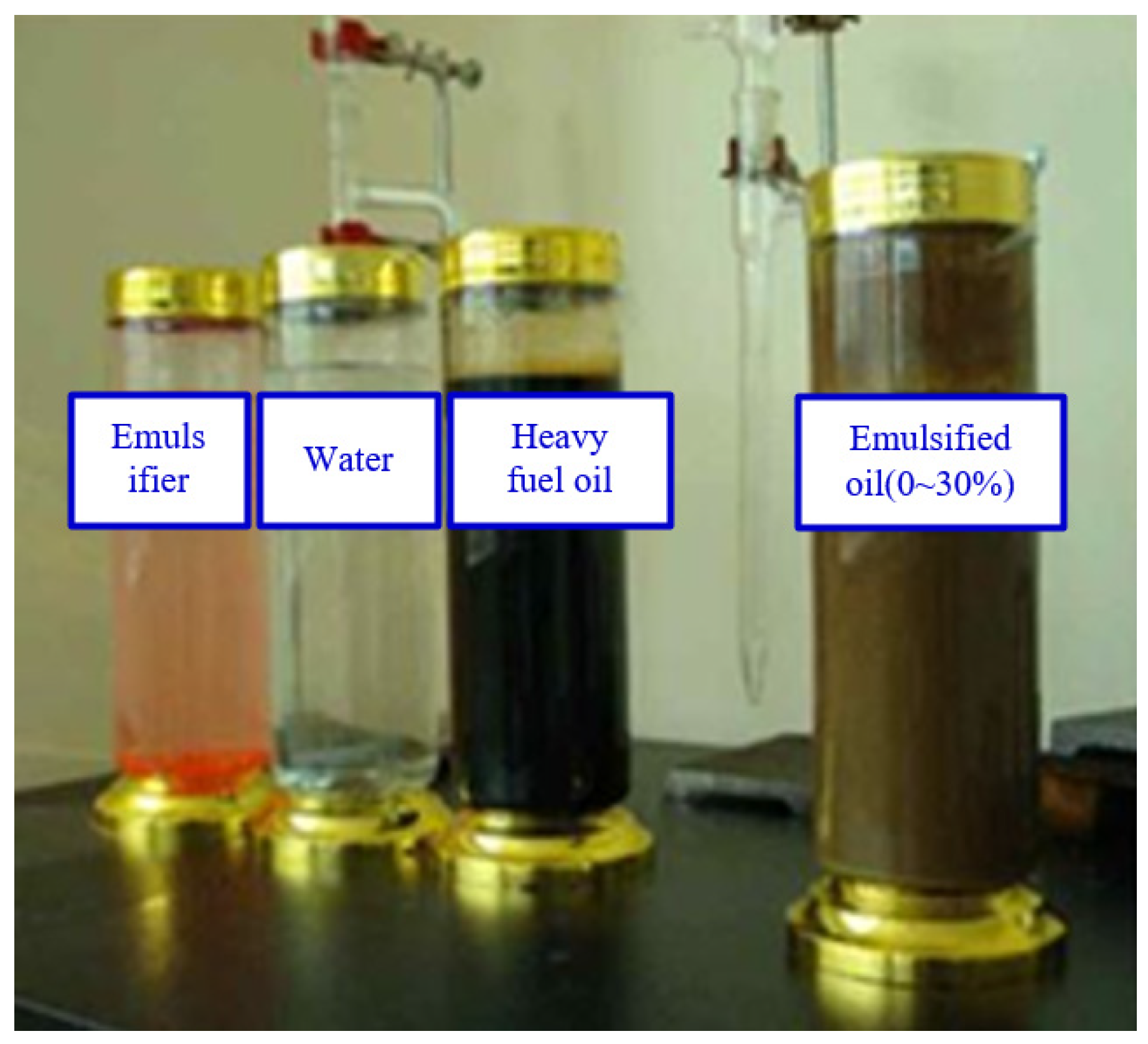
2.2. Production of Emulsified Fuel Oil
2.3. Analytical Methods
3. Results
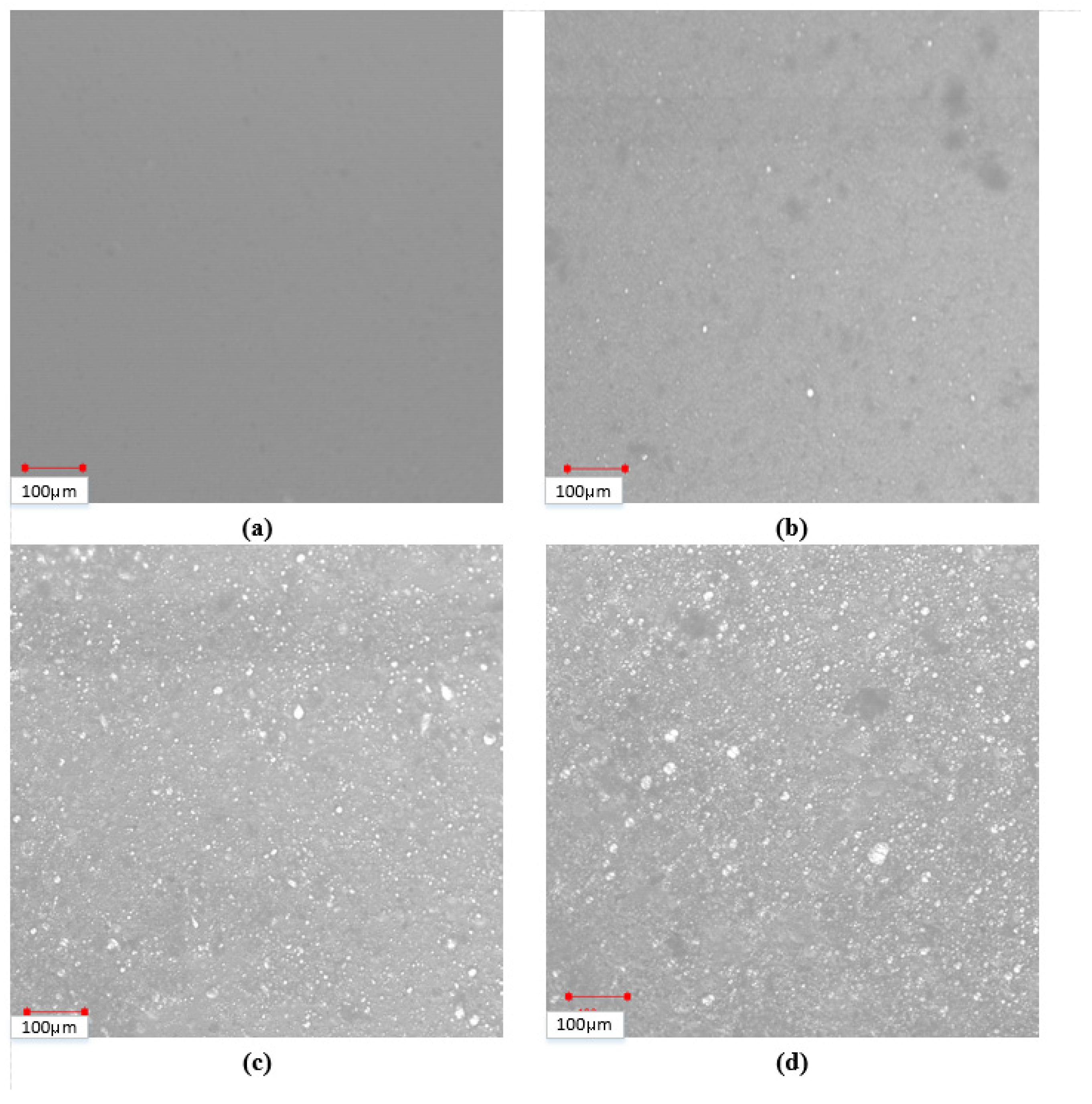
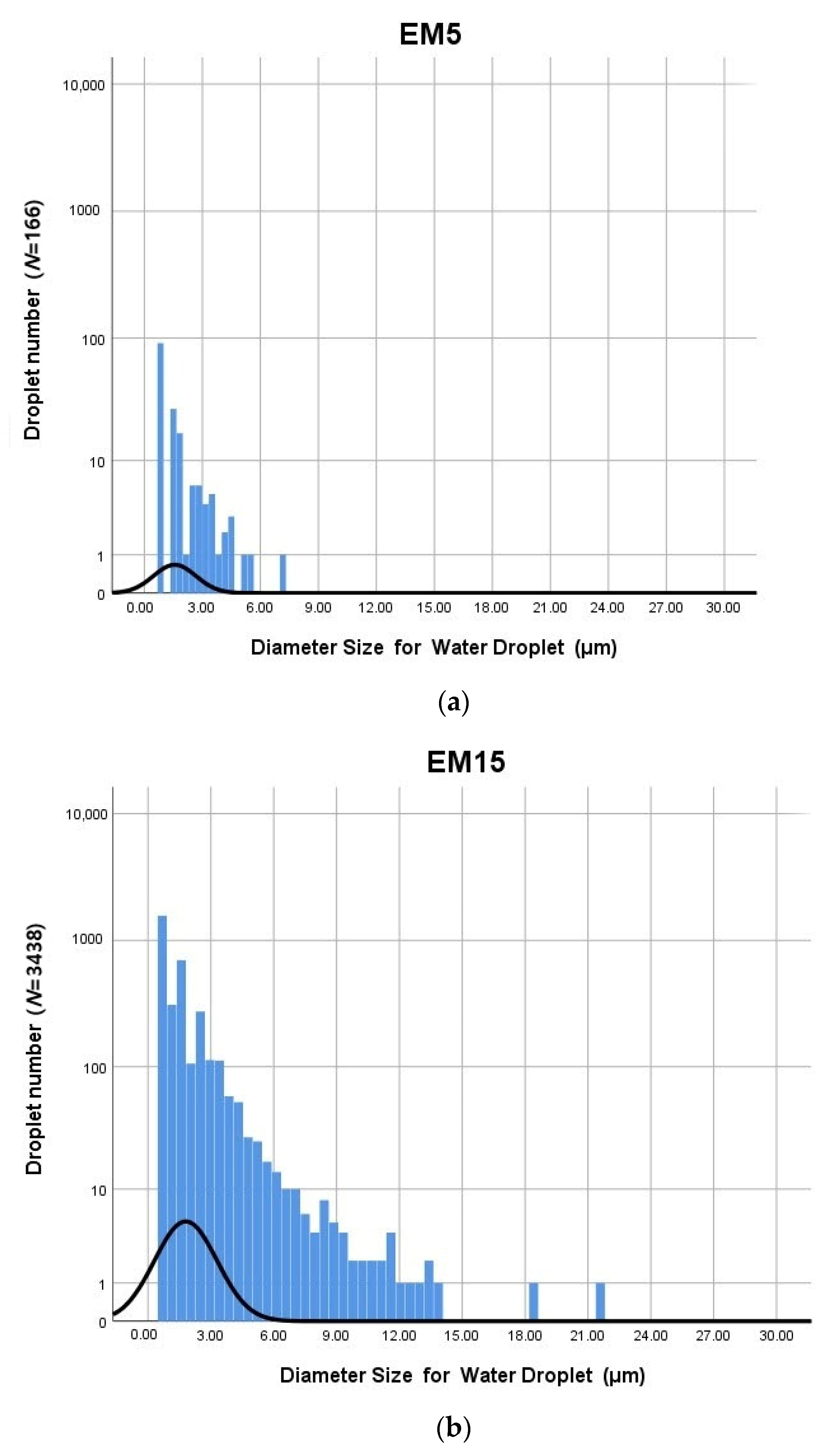
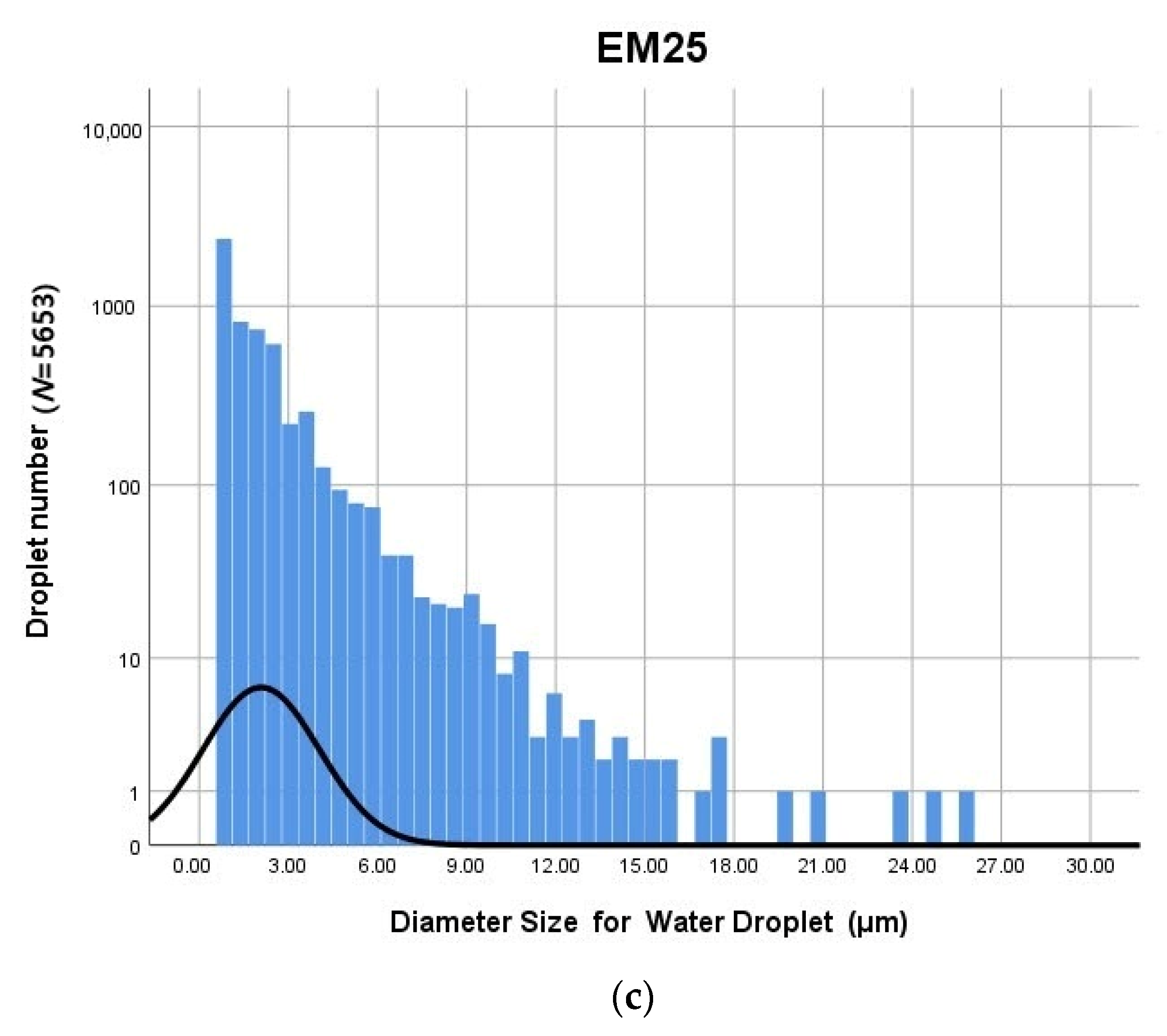
3.1. Distribution of Water Droplets
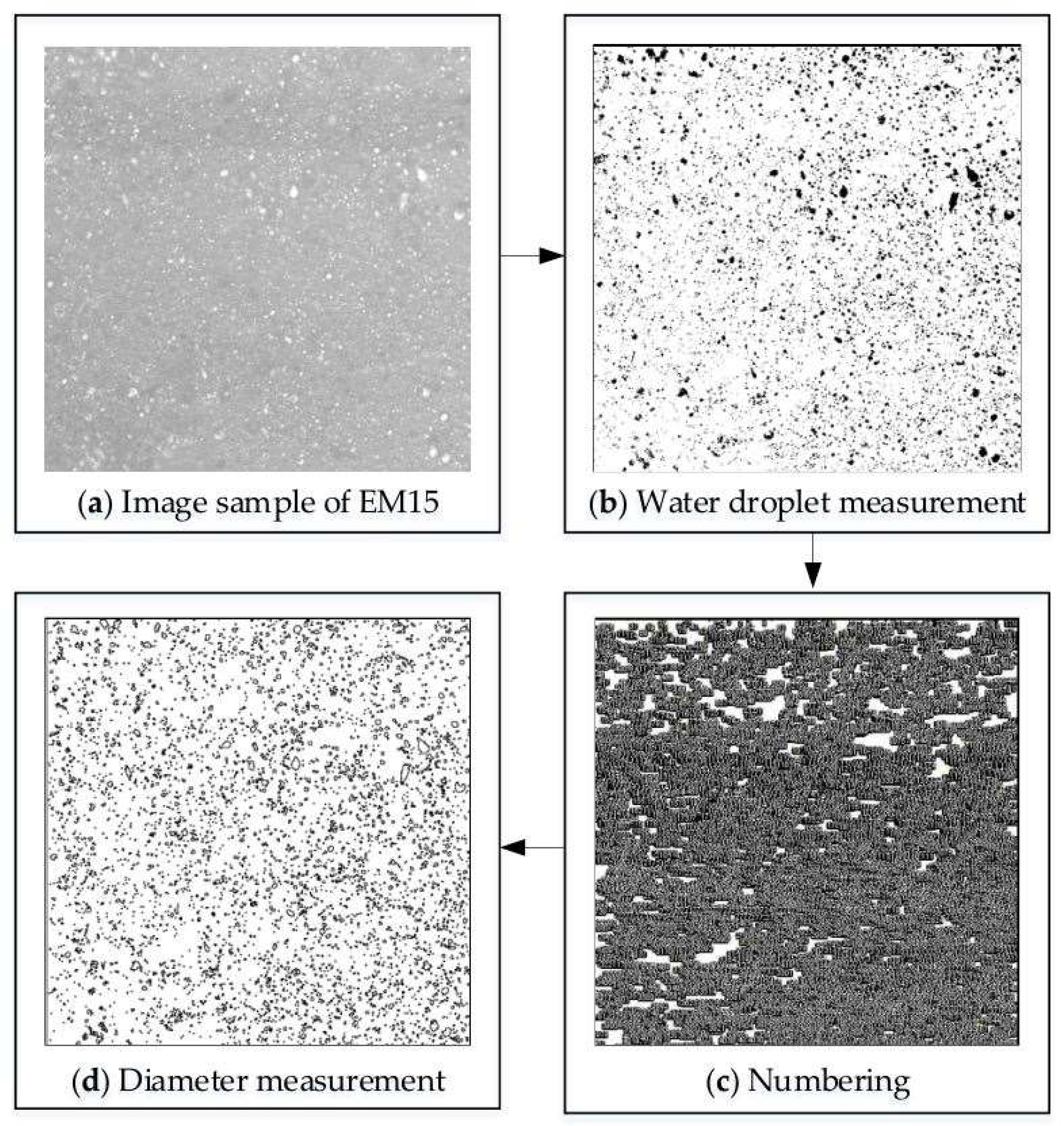
3.2. Analysis Using Image-J Open-Source Software
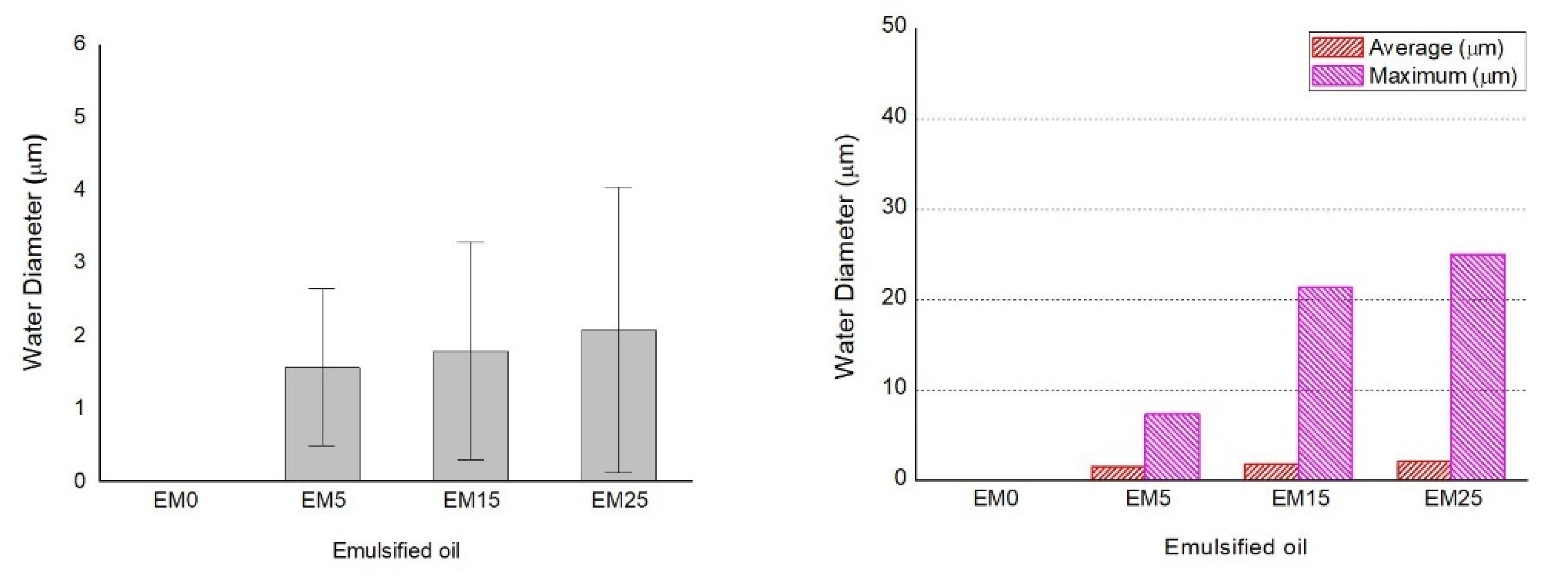
3.3. Characteristics of Droplet Micro-Particles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, T.H.; Lee, J.K. An Experimental Study on the NOx Reduction of Emulsified Oil Applied to Boiler-Burner Using Bunker C Oil. J. Korean Soc. Mech. Technol. 2019, 21, 1071–1076. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel: A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008. [Google Scholar]
- Lee, T.H. A Study on the Characteristics of Exhaust Emissions in a Marine Boiler Using Emulsified Bunker-C Oil; Jeonbuk National University: Jeonju, Korea, 2020. [Google Scholar]
- OPEC. OPEC Annual Statistical Bulletin; Organization of the Petroleum Exporting Countries: Vienna, Austria, 2017. [Google Scholar]
- Lee, T.H.; Lee, S.H.; Lee, J.K. Exhaust Gas Emission Improvements of Water/Bunker C Oil-Emulsified Fuel Applied to Marine Boiler. J. Mar. Sci. Eng. 2021, 9, 477. [Google Scholar] [CrossRef]
- Doh, H.J.; Lee, S.Y. A Study on Floating Offshore LNG Bunkering System and Its Economic Analysis. J. Korea Port Econ. Assoc. 2021, 49, 73–97. [Google Scholar]
- IMO. Amendments to the ANNEX of the Protocol of 1997 to Amend the International Convention for the Prevention of Pollution from Ships 1973 as Modified by the Protocol of 1978 Relating Thereto; IMO MEPC 62/24/Add.1; MEPC: London, UK, 2011. [Google Scholar]
- Lee, T.H.; Ryu, Y.H. A Study on Mixing Properties of Coffee Ground-Fuel for Improvement of Air Pollution from Ships. J. Korean Soc. Mech. Technol. 2021, 23, 181–186. [Google Scholar] [CrossRef]
- Lee, J.T.; Son, J.H.; Kim, J.H.; Jung, S.W.; Yoo, H.M.; Hong, H.K.; Mun, S.H.; Choi, K.H.; Lee, J.T.; Kim, J.S. Characteristics of Air Pollutants Emission from Medium-duty Trucks Equipped EGR and SCR in Korea. J. ILASS-Korea 2016, 21, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.H.; Dan, T. Investigation on the Effects of Dimethyl ether Blending to Bunker Oil for Marine Diesel Engine Use. SAE Tech. Pap. 2013. [Google Scholar] [CrossRef]
- Yoon, J.H.; Yeom, J.K. Numerical Analysis on Behavior Characteristics of Evaporative Spray of Emulsified Fuel Impinging on a Heated Flat Plate. Trans. KSME-B 2019, 43, 471–477. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, J.K. Manufacture of Water-Heavy Fuel Oil Hybrid Emulsion Oil and Component Characteristics. J. Korean Soc. Mech. Technol. 2020, 22, 884–890. [Google Scholar] [CrossRef]
- Cho, S.G. Effects on Characteristics of Combustion and Exhaust Emission by Using Emulsified Fuel in Diesel Engine; Kunsan National University Graduate School: Gunsan, Korea, 2007. [Google Scholar]
- No, J.M. Combustion Characteristics of Steam Boiler Using Heavy Oil/Emulsified Fuel; Ajou University: Suwon, Korea, 2008. [Google Scholar]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Povarov, V.G.; Efimov, I.; Smyshlyaeva, K.I.; Rudko, V.A. Application of the UNIFAC Model for the Low-Sulfur Residue Marine Fuel Asphaltenes Solubility Calculation. J. Mar. Sci. Eng. 2022, 10, 1017. [Google Scholar] [CrossRef]
- Lee, T.H.; Ryu, Y.H. Viscosity Characteristic Analysis of Coffee Ground Oil for Marine Fuel Applications. J. Korean Soc. Mech. Technol. 2021, 23, 892–897. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, J.K. An Experimental Study on the SO2 Reduction of Water 10% Mixed Emulsified Oil Applied to Boiler-Burner Using Bunker-C Oil. J. Fish. Mar. Sci. Educ. 2019, 31, 1801–1807. [Google Scholar] [CrossRef]
- Greens-Tek. Business Report of Alternative Emulsified Fuel Oil; Greens-Tek Co., Ltd.: Jeongeup, Korea, 2019. [Google Scholar]
- IMO. Prevention of Air Pollution from Ships: Reducing Shipping Emissions of Air Pollution-Feasible and Cost-Effective Options; MEPC: London, UK, 2005. [Google Scholar]
- Korea Institute of Petroleum Quality. The Test Report of Emulsified Fuel Oil; Product No. 12; Korea Institute of Petroleum Quality: Seongnam, Korea, 2008. [Google Scholar]
- Jung, G.B. Confocal microscopy and 3-d image analysis. Bric Biowave 2003, 5, 1–8. [Google Scholar]
- Seo, M.H.; Kim, J.B.; Kwon, N.I. Measurement of metal materials structure by using the manufactured Scanning Confocal Microscopy. J. Korean Soc. Precis. Eng. 2008, 25, 52–57. [Google Scholar]
- Ferreira, T.; Rasband, W. Image J User Guide Revised Edition IJ 1.46r; ImageJ/FIJI. Co., Ltd.; National Institutes of Health: Bethesda, MD, USA, 2012.
- Jung, H.M.; Won, D.Y.; Jung, J.E. Prediction of Bone Aging by Adapting Image J. Korean J. Digit. Imaging Med. 2012, 14, 63–67. [Google Scholar]
- Seo, W.J.; Seo, J.B.; Lee, J.W. Using Image J program, compared of focusing distance and grid rate. Korean J. Digit. Imaging Med. 2012, 14, 37–42. [Google Scholar]
- Lee, W.S. Combustion Characteristics of Water Emulsified Fuel and the Effect on Burner and Engine Performance; Korea Maritime University Graduate School: Busan, Korea, 2004. [Google Scholar]


| Specification | Test Method | EM0 | EM5 | EM15 | EM25 |
|---|---|---|---|---|---|
| Water content (Vol, %) | KS M ISO 3733:2008 | 0.6 | 5.0 | 15.0 | 25.0 |
| Sulfur content (m/m, %) | KS M ISO 2414:2011 | 0.28 | 0.26 | 0.23 | 0.20 |
| Flashpoint (°C) | KS M ISO 2592:2007 | 172 | 103 | 103 | 101 |
| Viscosity (@50 °C, mm2/s) | KS M ISO 3104:2008 | 60.61 | 73.32 | 97.32 | 144.80 |
| Specific Gravity @15/4 °C | KS M ISO 12185:2003 | 0.9197 | 0.9190 | 0.9270 | 0.9382 |
| List | Specification | Unit |
|---|---|---|
| Set scale range | 0–2400 | % |
| Image size | 512:512 | pixel |
| Known distance | 100 | μm |
| Pixel aspect ratio | 1.0 | - |
| Unit of length | - | μm |
| Threshold | 200:255 | - |
| Droplet size | 0.1–infinity | μm |
| Item | EM0 | EM5 | EM15 | EM25 | |
|---|---|---|---|---|---|
| Measurement | |||||
| Number (Z) | Effective | - | 166 | 3438 | 5636 |
| Missing | - | 5470 | 2198 | 0 | |
| Minimum (μm) | - | 0.90 | 0.90 | 0.90 | |
| Maximum (μm) | - | 7.31 | 21.41 | 25.01 | |
| Median (μm) | - | 0.90 | 1.35 | 1.35 | |
| SD | - | 1.07838 | 1.49292 | 1.96007 | |
| Average (μm) | - | 1.57 | 1.79 | 2.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Cho, J.; Lee, J. Mixing Properties of Emulsified Fuel Oil from Mixing Marine Bunker-C Fuel Oil and Water. J. Mar. Sci. Eng. 2022, 10, 1610. https://doi.org/10.3390/jmse10111610
Lee T, Cho J, Lee J. Mixing Properties of Emulsified Fuel Oil from Mixing Marine Bunker-C Fuel Oil and Water. Journal of Marine Science and Engineering. 2022; 10(11):1610. https://doi.org/10.3390/jmse10111610
Chicago/Turabian StyleLee, Taeho, Jinho Cho, and Jeekeun Lee. 2022. "Mixing Properties of Emulsified Fuel Oil from Mixing Marine Bunker-C Fuel Oil and Water" Journal of Marine Science and Engineering 10, no. 11: 1610. https://doi.org/10.3390/jmse10111610







