Water Surface Behaviour of Irrawaddy Dolphin Orcaella brevirostris (Owen in Gray, 1866) and Influencing Factors in the Bay of Brunei, Brunei Darussalam
Abstract
:1. Introduction
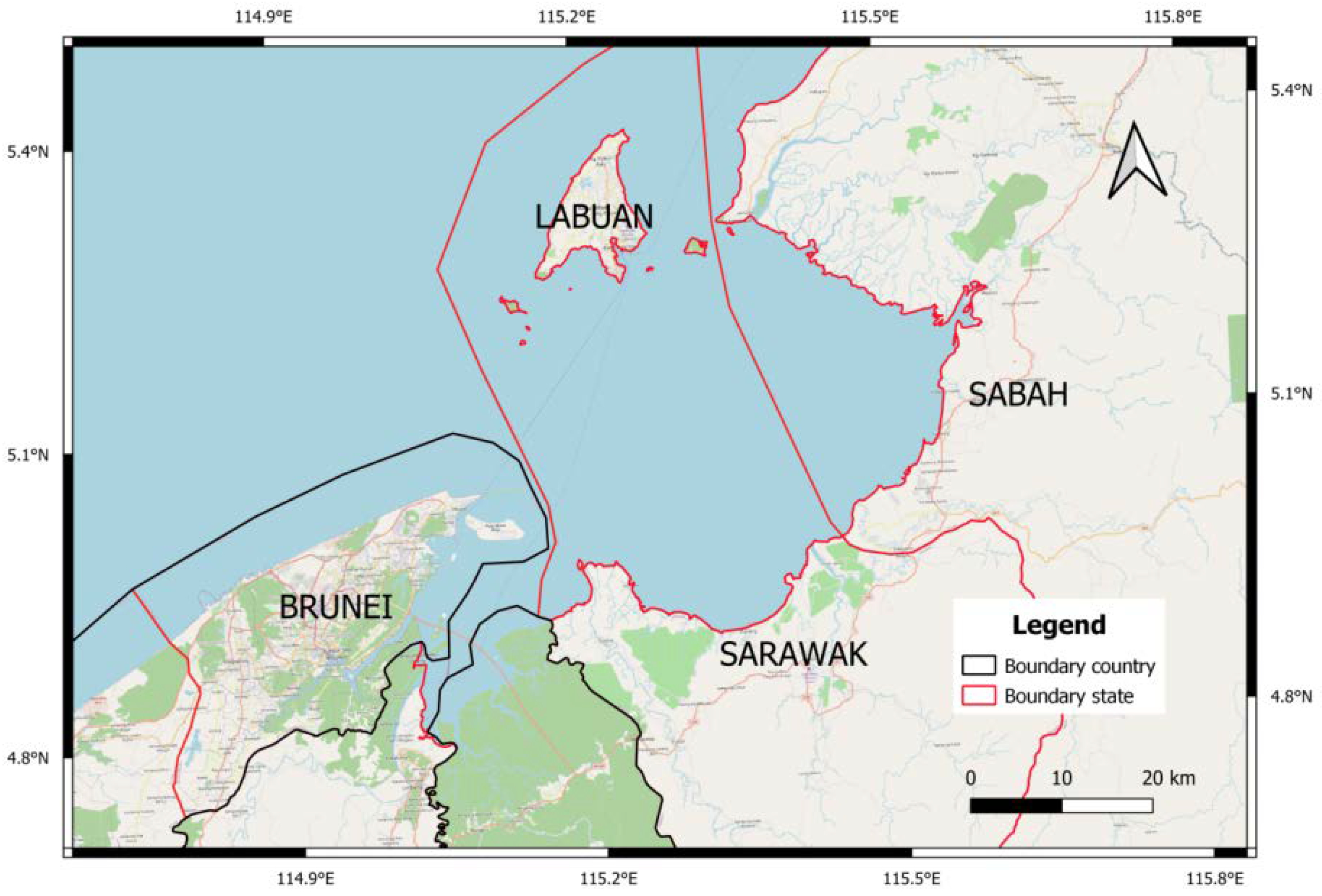
1.1. Study Site
1.2. Irrawaddy Dolphin (Orcaella brevirostris)
1.3. Water Surface Behaviour and Influencing Factors
1.4. Objectives of the Study
- (a)
- To determine the behavioural categories (feeding, socializing and travelling) that will be displayed by Irrawaddy dolphins.
- (b)
- To determine how the behavioural categories displayed by Irrawaddy dolphin are affected by the abiotic factors (depth, turbidity, sea surface temperature and salinity).
- (c)
- To determine whether the number of individuals in a group of Irrawaddy dolphins is affected by the measured abiotic factors (depth, turbidity, sea surface temperature and salinity).
2. Materials and Methods
2.1. Boat-Based Survey
2.2. Behavioural Events
- (a)
- Feeding: surfacing many times, raising flukes and long diving for fish capturing, sometimes pausing (snorkelling) for a while. Fish capturing and tossing fish and prey are often seen in dolphins’ mouths. Following trawlers and preying fish near trawling net. Water spitting.
- (b)
- Socializing: prolonged body contact with other individuals. Leaping or surfacing out of the water. Fighting and chasing between individuals. Bow riding in front of boats or survey vessels. Spy-hopping to observe around. Overlapping swim between individuals.
- (c)
- Travelling: swimming, diving and surfacing synchronously. Grouping and moving in the same direction simultaneously.
3. Results
3.1. Survey Effort and Sightings
3.2. Behavioural Activities of Irrawaddy Dolphin
3.3. Behavioural Activities in Relation to the Number of Individuals in a Group
3.4. Behavioural Categories in Relation to Abiotic Factors
4. Discussion
4.1. Survey Effort and Sightings
4.2. Behavioural Activities
4.3. Behavioural Activities in Relation to the Number of Individuals in a Group
4.4. Behavioural Categories and Abiotic Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, C.; Poh, A.N.Z.; Ngeian, J.; Tuen, A.A.; Minton, G. Identifying Habitat Characteristics and Critical Areas for Irrawaddy Dolphin, Orcaella brevirostris: Implications for Conservation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 225–238. [Google Scholar]
- Mahmud, A.I.; Jaaman, S.A.; Muda, A.M.; Muhamad, H.M.; Zhang, X.; Scapini, F. Factors influencing the behaviour of Irrawaddy dolphins Orcaella brevirostris (Owen in Gray, 1866) in Brunei Bay, Malaysia. J. Ethol. 2018, 36, 169–180. [Google Scholar] [CrossRef]
- Nor Balqis, M.Z. Seawater Properties in Brunei Bay, Sabah. Master’s Thesis, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia, 2007. [Google Scholar]
- Joseph, B.; Adiana, G.; Shazili, N.A.M.; Ong, M.C.; Juahir, H.; Shaari, H.; Yii, M.W.; Kamaruddin, M.K.A.; Gasim, M.B.; Maulud, K.N.A.; et al. The Evaluation of Brunei Bay Sediment Cores Sedimentation Rate Using 210Pb Radiometric Dating Technique. Int. J. Eng. Technol. 2018, 7, 107–114. [Google Scholar]
- Jiang, Y.; Liu, Z.; Yang, C.; Lü, L.G.; Yu, X.; Huang, L.; Zhang, X.; Yang, Z.; Yang, G.; Sun, L.; et al. High-frequency whistles of Irrawaddy dolphins (Orcaella brevirostris) recorded in Brunei Bay. Mar. Mamm. Sci. 2020, 36, 846–857. [Google Scholar] [CrossRef]
- Swee, Y.P.; Joo, H.T.; Suratman, S.; Simoneit, B.R.T.; Tahir, N.M. Input of organic matter in Brunei Bay, East Malaysia, as indicated by sedimentary steroids and multivariate statistics. Mar. Pollut.Bull. 2020, 156, 111269. [Google Scholar]
- Jaafar, M.H.; Subramaniam, S. Occurrences of red tide in Brunei Darussalam and methods of monitoring and surveillance. Toxic Red Tides and Shellfish Toxicity in Southeast Asia. In Proceedings of the Consultative Meeting, Singapore, 11–14 September 1984; White, A.W., Anraku, M., Hooi, K.-K., Eds.; pp. 17–24. [Google Scholar]
- Shams, S.; Juani, R.H.M. Flow Assessment of Brunei River due to the Impact of Climate Change. In Proceedings of the 4th International Conference on Environmental, Energy and Biotechnology, Madrid, Spain, 15–16 June 2015; Volume 85, pp. 2–8. [Google Scholar]
- Sulaiman, Z.H.; Rahman, K.H.A.; Ying, T.Y.; Taha, H.H.; Taha, N.Q.H.M. Genetic population structure of red snapper (Lutjanus malabaricus) and orange-spotted grouper (Epinephelus coiodes) in Brunei and Sabah. Integr. Zool. 2018, 3, 208–215. [Google Scholar] [CrossRef]
- Lamit, N.; Tanaka, Y.; Majid, H.M.A. Seagrass diversity in Brunei Darussalam: First records of three species. Sci. Bruneiana 2017, 16, 48–52. [Google Scholar] [CrossRef]
- Linden, O.; Ganning, B.; Lindestrom, L. Studies on Plankton, Primary Production and Fish in The Inner Brunei Bay. Mar. Res. Indones. 1992, 28, 55–80. [Google Scholar] [CrossRef]
- Stacey, P.J.; Arnold, P.W. Orcaella brevirostris. Mammalian Species. Am. Soc. Mammologists 1999, 616, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Parra, G.J.; Azuma, C.; Preen, A.R.; Corkeron, P.J.; Marsh, H. Distribution of Irrawaddy dolphins, Orcaella brevirostris, in Australian waters. Raffles Bull. Zool. 2002, 141–154. [Google Scholar]
- Smith, B.D. Irrawaddy dolphin, Orcaella brevirostris. In Encyclopedia of Marine Mammals; Perrin, W., Wursig, B., Thewissen, J.G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 638–642. [Google Scholar]
- Cziko, G. The Things We Do: Using the Lessons of Bernard and Darwin to Understand the What, How, and Why of Our Behavior; The MIT Press: Cambridge, MA, USA, 2000; 290p. [Google Scholar]
- Sutaria, D. Species Conservation in a Complex Socio-Ecological System: Irrawaddy Dolphins, Orcaella brevirostris in Chilika Lagoon, India. Ph.D. Thesis, James Cook University, Douglas, Australia, 2009. [Google Scholar]
- Baumgartner, M.F.; Mate, B.R. Summer and fall habitat of North Atlantic right whales (Eubalaena glacialis) inferred from satellite telemetry. Can. J. Fish Aquat. Sci. 2005, 62, 527–543. [Google Scholar] [CrossRef]
- Gaskin, D.E. Distribution of Delphinidae (Cetacea) in relation to sea surface temperatures off Eastern and Southern New Zealand. N. Z. J. Mar. Freshw. Res. 1968, 2, 527–537. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Leatherwood, S.; Webber, M.A. Marine Mammals of the World, FAO Species Identification Guide; FAO: Rome, Italy, 1993; p. 320. [Google Scholar]
- Jaaman, S.A.; Lah-Anyi, Y.U. Dugongs (Dugong dugon Muller, 1776) in East Malaysian Waters. ASEAN Rev. Biodivers. Environ. Conserv. (ARBEC) Online J. 2003. Available online: http://www.arbec.com.my/dugongs (accessed on 8 September 2022).
- Rajamani, L. The Conservation Biology of the Dugong (Dugong dugon) and Its Seagrass Habitat in Sabah, Malaysia: A Basis for Conservation Planning. Ph.D. Thesis, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia, 2009. [Google Scholar]
- Rajamani, L.; Marsh, H. Using parallel regional- and local-scale initiatives to inform conservation management of rare wildlife: A case study of the dugong Dugong dugon in Sabah, Malaysia. Endanger Species Res. 2010, 13, 17–23. [Google Scholar] [CrossRef]
- Jaaman, S.A. Marine Mammals in East Malaysia: Distribution and Interactions with Fisheries. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2010. [Google Scholar]
- Jaaman, S.A.; Ahmad-Kamil, E.I.; Bali, J.; Redzwan, K.; Rajamani, L.; Ponnampalam, L.S.; Syed Abdullah, S.A.K.; Mohd Lazim, M.S.; Azlina, A. UNEP CMS Dugong Project 2011. In Proceedings of the Southeast Asia Sub-Regional Meeting on Dugongs and Workshop on Developing Standard Analysis Protocols for Dugong Questionnaire Survey Project Data for Southeast Asia Region, Sarawak, Malaysia, 27–29 July 2011; pp. 1–21. [Google Scholar]
- Lim, H.C.; Kamaruzzan, A.S. EMMCE Workshop (Sabah Chapter) and Marine Mammal Survey-Narrative Report, 2nd ed.; Report prepared for Talisman Malaysia Limited (TML); Malaysian Nature Society: Kuala Lumpur, Malaysia, 2012; Unpublished. [Google Scholar]
- Muhamad, H.M.; Xu, X.; Zhang, X.; Jaaman, S.A.; Muda, A.M. Whistle description of Irrawaddy dolphins (Orcaella brevirostris) in Bay of Brunei, Sarawak, Malaysia. J. Acoust. Soc. Am. 2018, 143, 2708. [Google Scholar] [CrossRef]
- Parra, G.J. Behavioural Ecology of Irrawaddy Dolphins, Orcaella brevirostris (Owen in Gray, 1866), and Indo-Pacific Humpback Dolphins, Sousa chinensis (Osbeck, 1765), in Northeast Queensland, Australia: A Comparative Study’. Ph.D. Thesis, James Cook University, Townsville, Australia, 2005. [Google Scholar]
- Minton, G.; Peter, C.; Zulkifli Poh, A.N.; Ngeian, J.; Braulik, G.; Hammond, P.S.; Tuen, A.A. Population estimates and distribution patterns of Irrawaddy dolphins (Orcaella brevirostris) and Indo-Pacific finless porpoises (Neophocaena phocaenoides) in the Kuching Bay, Sarawak. Raffles Bull. Zool. 2013, 61, 877–888. [Google Scholar]
- Khalifa, M.A.; Kamal, M.M.; Adiwilaga, E.M.; Sunuddin, A. Preliminary Study on the Distribution of Irrawaddy Dolphin, Orcaella brevirostris, in Banten Bay. Open J. Mar. Sci. 2014, 4, 338–343. [Google Scholar] [CrossRef]
- Yet, Y.H.; Suratman, S. Physico-Chemical Parameters Profile During Dry and Wet Seasons in Southern South China Sea: Brunei Bay. Asian J. Chem. 2016, 28, 2146–2152. [Google Scholar]
- Ariff, M.R.M.; Bakeri, A. Hubungan Perdagangan Sumber Perikanan Sarawak-Negara Brunei Darussalam: Kajian Kes Daerah Perikanan Miri. Jati 2001, 6, 1–28. [Google Scholar]
- Jaaman, S.A.; Muda, A.M.; Abdul-Raman, A.; Bali, J.; Munsang, T.K.; Muhamad, H.M. Surveys of marine mammals in the Bay of Brunei, Malaysia. In Scientific Expedition to Brunei Bay; Suratman, S., Ed.; Universiti Malaysia Terengganu: Terengganu, Malaysia, 2016; pp. 151–174. [Google Scholar]
- Tubbs, S.E.; Keen, E.; Jones, A.L.; Thap, R. On the distribution, behaviour and seasonal variation of Irrawaddy dolphins (Orcaella brevirostris) in the Kep Archipelago, Cambodia. Raffles Bull. Zool. 2020, 68, 137–149. [Google Scholar]
- Smith, B.D.; Beasley, I.; Buccat, M.; Calderon, V.; Evina, R.; Valle, L.D.; Cadigal, A.; Tura, E.; Visitasion, Z. Status, ecology and conservation of Irrawaddy dolphins Orcaella brevirostris in Malampaya Sound, Palawan, Philippines. J. Cetacean Res. Manag. 2004, 6, 41–52. [Google Scholar]
- Kamaruzzan, A.S. Occurrence and Behaviour of Indo-Pacific Humpback (Sousa chinensis) And Irrawaddy (Orcaella brevirostris) Dolphins in Cowie Bay, Sabah and Their Relationships with Water Parameters. MSc Thesis, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia, 2011; pp. 1–162. [Google Scholar]
- Mahmud, A.I. Population Estimation and Behavioural Patterns of Irrawaddy Dolphins, Orcaella brevirostris (Owen in Gray 1866) in the Brunei Bay. Erasmus Mundus. Master Course in Tropical Biodiversity and Ecosystem. Master’s Thesis, Universiti Malaysia Terengganu, Terengganu, Malaysia, 2016. [Google Scholar]
- Wang, X.; Wu, F.; Turvey, S.T.; Rosso, M.; Zhu, Q. Seasonal group characteristics and occurrence patterns of Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, Fujian Province, China. J. Mammal. 2016, 97, 1026–1032. [Google Scholar] [CrossRef]
- Ismail, F.D.H.; Azizul, N.; Jaaman, S.A.; Muda, A.M.; Momin, M.V.; Abdullah, B.; Zhang, X.; Muhamad, H.M. Seasonal Occurrence, Distribution and Group Size of Irrawaddy Dolphins (Orcaella brevirostris) in the Bay of Brunei, Brunei Darussalam. Sains Malays. 2021, 50, 3159–3169. [Google Scholar] [CrossRef]
- Matsumoto, B.M.M. Fish and Fisheries Resources. In Coastal Environmental Profile of Brunei Bay; Saleem, S.M., Ejria, S., Eds.; Universiti Malaysia Sabah: Kota Kinabalu, Malaysia, 2007; pp. 95–133. [Google Scholar]
- Vargas, L.H.R.; Rajamani, L.; Dolar, L.; Porter, L. Population size, distribution and daylight behaviour of Irrawaddy dolphins (Orcaella brevirostris) in Penang Island, Malaysia. Raffles Bull. Zool. 2019, 67, 671–683. [Google Scholar]
- Karczmarski, L.; Cockcroft, V.G.; McLachLan, A. Group size and seasonal pattern of occurrence of Humpback dolphins Sousa chinensis in Algoa Bay, South Africa. S. Afr. J. Mar. Sci. 1999, 21, 89–97. [Google Scholar] [CrossRef]
- Burgess, E.A. Foraging Ecology of Common Dolphins (Delphinus sp.) in THE Hauraki Gulf, New Zealand. Master’s Thesis, Massey University Albany, Auckland, New Zealand, 2006. [Google Scholar]
- Baird, I.G.; Beasley, I.L. Irrawaddy dolphin Orcaella brevirostris in the Cambodian Mekong River: An initial survey. Oryx 2005, 39, 301–310. [Google Scholar] [CrossRef]
- Hoekstra, P.; Lindeboom, H.; Bak, R.; Van Den Bergh, G.; Tiwi, D.A.; Douven, W.; Heun, J.; Hobma, T.; Hoitink, T.; Kiswara, W.; et al. Teluk Banten Research Programme: An Integrated Coastal Zone Management Study (1995–2001). In Proceedings of the Scientific Programme Indonesia-Netherlands, Bandung, Indonesia, 12 February 2002. [Google Scholar]
- Kreb, D.; Budiono. Cetacean diversity and habitat preferences in tropical waters of East Kalimantan, Indonesia. Raffles Bull. Zool. 2005, 53, 149–155. [Google Scholar]
- Pardo, P.C. Environmental factors governing the distribution of the bottlenose (Tursiops truncatus) and the spotted dolphin (Stenella attenuata) in Golfo Dulce, South Pacific, off Costa Rica. Investig. Mar. 2007, 35, 15–23. [Google Scholar]











| Year | Month | Days Surveyed | No of Sighting | Distance (km) | Time (Hour) | Survey Effort (km·h) | Sighting Rate (per 100 km·h) |
|---|---|---|---|---|---|---|---|
| 2016 | January (NEM) | 2 | 1 | 133.8 | 6.57 | 486.083 | 0.21 |
| April (IM) | 2 | 2 | 105.5 | 4.45 | 236.155 | 0.85 | |
| July (SWM) | 3 | 4 | 223.6 | 16.91 | 1270.17 | 0.32 | |
| October (IM) | 5 | 6 | 323.5 | 30.78 | 2009.58 | 0.30 | |
| 2017 | January (NEM) | 4 | 3 | 281.1 | 22.65 | 1591.66 | 0.19 |
| April (IM) | 4 | 5 | 277.1 | 20.42 | 1414.70 | 0.35 | |
| July (SWM) | 4 | 7 | 280.8 | 24.15 | 1720.41 | 0.41 | |
| October (IM) | 4 | 5 | 263.2 | 21.06 | 1435.15 | 0.35 | |
| 2018 | January (NEM) | 4 | 5 | 277 | 21.91 | 1532.64 | 0.33 |
| April (IM) | 4 | 11 | 273.6 | 20.23 | 1385.39 | 0.79 | |
| July (SWM) | 4 | 8 | 274.3 | 18.94 | 1303.95 | 0.61 | |
| October (IM) | 4 | 7 | 285.3 | 20.39 | 1458.98 | 0.48 | |
| TOTAL | 44 | 64 | 2998.8 | 228.46 | 15,844.9 | 0.40 | |
| Months | Number of Sightingse (% of Total) | Sighting Rate (Per 100 km·h) | Number of Groups with Calf | Group Size | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | ||||
| January (NEM) | 9 (14%) | 0.25 | 6 | 6.9 | 4.2 | 7.0 | 1–15 |
| April (IM) | 18 (28%) | 0.59 | 3 | 4.9 | 14.7 | 4.0 | 1–15 |
| July (SWM) | 19 (30%) | 0.44 | 4 | 3.4 | 2.7 | 2.0 | 1–10 |
| October (IM) | 18 (28%) | 0.37 | 1 | 2.7 | 1.5 | 2.0 | 1–6 |
| TOTAL | 64 | 0.40 | 14 | 4.1 | 3.3 | 3.0 | 1–15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizul, N.; Jaaman, S.A.; Ismail, F.D.H.; Muda, A.M.; Zhang, X.; Muhamad, H.M.; Momin, M.V.; Abdullah, B. Water Surface Behaviour of Irrawaddy Dolphin Orcaella brevirostris (Owen in Gray, 1866) and Influencing Factors in the Bay of Brunei, Brunei Darussalam. J. Mar. Sci. Eng. 2022, 10, 1711. https://doi.org/10.3390/jmse10111711
Azizul N, Jaaman SA, Ismail FDH, Muda AM, Zhang X, Muhamad HM, Momin MV, Abdullah B. Water Surface Behaviour of Irrawaddy Dolphin Orcaella brevirostris (Owen in Gray, 1866) and Influencing Factors in the Bay of Brunei, Brunei Darussalam. Journal of Marine Science and Engineering. 2022; 10(11):1711. https://doi.org/10.3390/jmse10111711
Chicago/Turabian StyleAzizul, Nurlisa, Saifullah Arifin Jaaman, Farah Dayana Haji Ismail, Azmi Marzuki Muda, Xuelei Zhang, Hairul Masrini Muhamad, Mohammad Vol Momin, and Bohari Abdullah. 2022. "Water Surface Behaviour of Irrawaddy Dolphin Orcaella brevirostris (Owen in Gray, 1866) and Influencing Factors in the Bay of Brunei, Brunei Darussalam" Journal of Marine Science and Engineering 10, no. 11: 1711. https://doi.org/10.3390/jmse10111711
APA StyleAzizul, N., Jaaman, S. A., Ismail, F. D. H., Muda, A. M., Zhang, X., Muhamad, H. M., Momin, M. V., & Abdullah, B. (2022). Water Surface Behaviour of Irrawaddy Dolphin Orcaella brevirostris (Owen in Gray, 1866) and Influencing Factors in the Bay of Brunei, Brunei Darussalam. Journal of Marine Science and Engineering, 10(11), 1711. https://doi.org/10.3390/jmse10111711








