Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records
Abstract
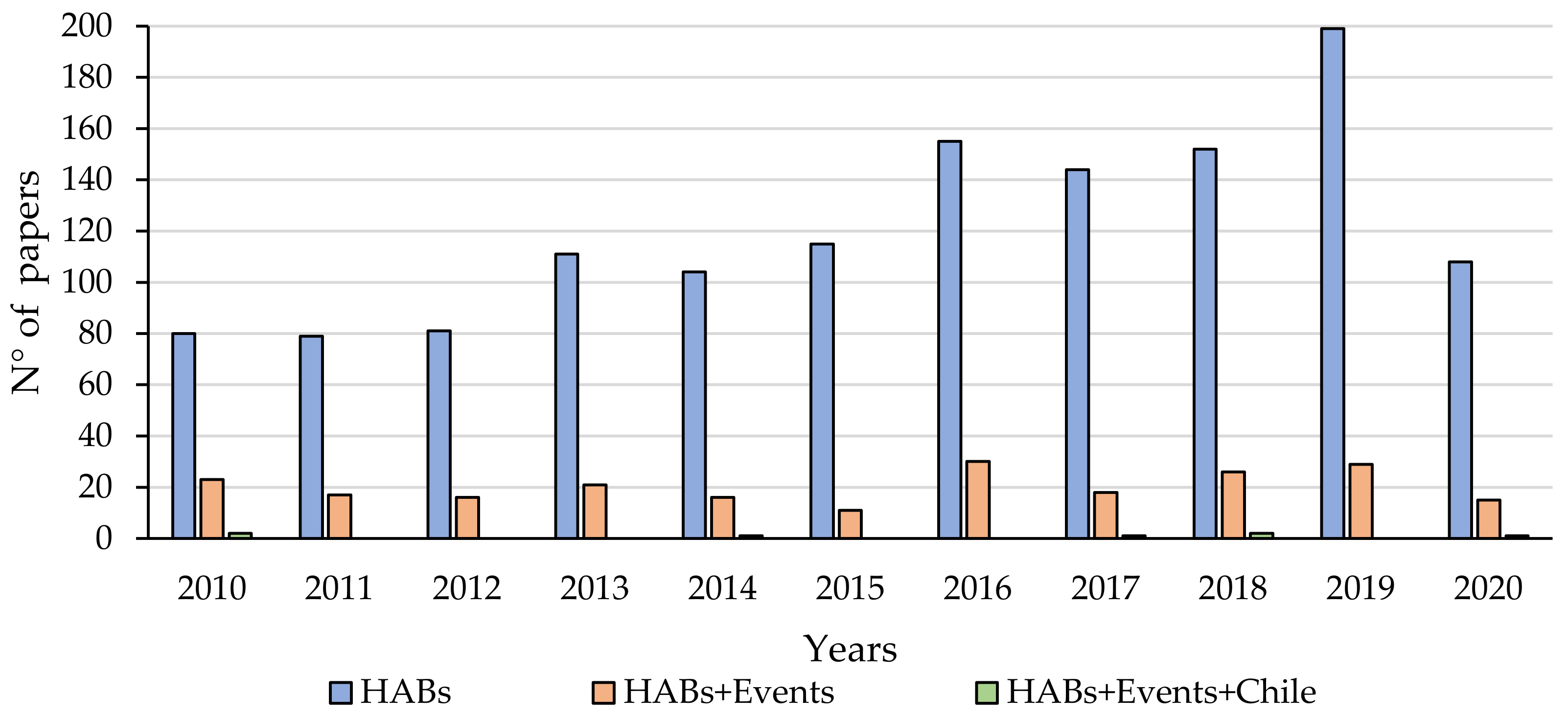
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Occurrences and Events of HABs in Chile
3.2. Toxic HAB in Chile
3.2.1. Paralytic Shellfish Poison in Chile (PSP)
3.2.2. Lipophilic Marine Toxins
3.2.3. Amnesic Shellfish Poison in Chile (ASP)
3.3. HABs: Ichthyotoxic or Capable of Causing Damage to Fish
3.3.1. Harmful Diatom Species
3.3.2. Dinoflagellates
3.3.3. Silicoflagellate, Raphidophyceae and Haptophyte
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belin, C.; Soudant, D.; Amzil, Z. Three Decades of Data on Phytoplankton and Phycotoxins on the French Coast: Lessons from Rephy and Rephytox. Harmful Algae 2020, 102, 101733. [Google Scholar] [CrossRef] [PubMed]
- Calvo Vargas, E.; Berrocal Artavia, K.; Boza Abarca, J. Floraciones Algales Nocivas Durante El Periodo 2008-2010 En El Golfo de Nicoya, Costa Rica Harmful Algal Blooms during 2008-2010 in the Gulf of Nicoya, Costa Rica. Rev. Mar. Cost 2016, 8, 129–149. [Google Scholar] [CrossRef] [Green Version]
- Coutteau, P. Micro-Algae. In Manual on the Production and Use of Live Food for Aquaculture; FAO Fisheries Technical Paper 361; Lavens, P., Sorgeloos, P., Eds.; FAO: Rome, Italy, 1996; pp. 7–43. ISBN 9251039348. [Google Scholar]
- Hallegraeff, G. Harmful Algal Bloom: A Global Overview. In Manual on Harmful Marine Microalgae. Monographs on Oceanographic Methodology; Hallegraeff, G., Anderson, D., Cembella, A., Eds.; UNESCO Publishing: Paris, France, 2003; pp. 25–50. ISBN 9231039482. [Google Scholar]
- Al-Ghelani, H.; AlKindi, A.; Amer, S.; Al-Akhzami, Y. Harmful Algal Blooms: Physiology, Behavior, Population Dynamics and Global Impacts- A Review. Sultan Qaboos Univ. J. Sci. [SQUJS] 2005, 10, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Lassus, P.; Chomérat, N.; Hess, P.; Nézan, E. Toxic and Harmful Microalgae of the World Ocean. In IOC Manuals and Guides No. 68; UNSECO: Paris, France, 2016; ISBN 9788799082766. [Google Scholar]
- Anderson, D.; Cembella, A.; Hallegraeff, G. Progress in Understanding Harmful Algal Blooms (HABs): Paradigm Shifts and New Technologies for Research, Monitoring and Management. Ann Rev Mar Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mello, D.F.; Antonio, L.; Proença, D.O.; Barracco, M.A. Comparative Study of Various Immune Parameters in Three Bivalve Species during a Natural Bloom of Dinophysis acuminata in Santa Catarina Island, Brazil. Toxins 2010, 2, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Seguel, M.; Sfeir, A. Distribución de las toxinas marinas y quistes de dinoflagelados tóxicos en los canales occidentales de la región de Aysén. Cienc. Technol. Mar. 2010, 33, 43–55. [Google Scholar]
- Burkholder, J.M. Harmful Algal Blooms. Encycl. Inl. Waters 2009, 264–285. [Google Scholar] [CrossRef]
- Coquereau, L.; Jolivet, A.; Hégaret, H.; Chauvaud, L. Short-Term Behavioural Responses of the Great Scallop Pecten maximus Exposed to the Toxic Alga Alexandrium minutum Measured by Accelerometry and Passive Acoustics. PLoS ONE 2016, 11, e0160935. [Google Scholar] [CrossRef] [Green Version]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of Harmful Algal Blooms on the Aquaculture Industry: Chile as a Case Study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Shumway, S.E.; Burkholder, J.M.; Morton, S.L. Harmful Algal Blooms. In A Compendium Desk Reference.; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 667. ISBN 9781118994696. [Google Scholar]
- Assunção, J.; Malcata, F. Dinoflagellates and Toxin Production. In Marine Macro- and Microalgae: An Overview.; Malcata, F., Pinto, I., Guedes, A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 216–234. ISBN 9781498705332. [Google Scholar]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global Harmful Algal Bloom Status Reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Fuenzalida, G.; Zenteno, K.; Alves-de-Souza, C.; Astuya, A.; Dorantes-Aranda, J.J. Salinity-Growth Response and Ichthyotoxic Potency of the Chilean Pseudochattonella verruculosa. Front. Mar. Sci. 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Sunesen, I.; Méndez, S.M.; Mancera-Pineda, J.E.; Bottein, M.-Y.D.; Enevoldsen, H. The Latin America and Caribbean HAB status report based on OBIS and HAEDAT maps and databases. Harmful Algae 2021, 102, 101920. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallegraeff, G.M. A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef] [Green Version]
- García-Lagunas, N.; Romero-Geraldo, R.D.J.; Hernández-Saavedra, N.Y. Changes in Gene Expression and Histological Injuries as a Result Ofexposure of Crassostrea gigas to the Toxic Dinoflagellate Gymnodinium catenatum. J. Molluscan Stud. 2016, 82, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Wang, D.; Wei, Q.; Wang, Q.; Yang, D.; Liu, H.; Dong, Z.; Zhang, X.; Zhang, Q.; Zhao, J. Integrative Biomarker Assessment of the Influence of Saxitoxin on Marine Bivalves: A Comparative Study of the Two Bivalve Species Oysters, Crassostrea gigas, and Scallops, Chlamys farreri. Front. Physiol. 2018, 9, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembeye, G. 6.3 Harmful Algal Blooms in the Austral Chilean Channels and Fjords; Comité Oceanográfico Nacional: Valparaíso, Chile, 2006; pp. 99–103. [Google Scholar]
- Velásquez, C.; Navarro, J.M. Feeding and Intoxication-Detoxification Dynamics in Two Populations of the Mussel Mytilus chilensis (Hupé, 1854) with Different Histories of Exposure to Paralytic Shellfish Poisoning (PSP). Mar. Freshw. Behav. Physiol. 2014, 47, 185–195. [Google Scholar] [CrossRef]
- Lembeye, G. Distribución de quistes de Alexandrium catenella y otros dinoflagelados en sedimentos de la zona SurAustral de Chile. Cienc. Tecnol. Mar. 2004, 27, 21–31. [Google Scholar]
- Borbor-Córdova, M.J.; Pozo-Cajas, M.; Cedeno-Montesdeoca, A.; Saltos, G.M.; Kislik, C.; Espinoza-Celi, M.E.; Lira, R.; Ruiz-Barzola, O.; Torres, G. Risk Perception of Coastal Communities and Authorities on Harmful Algal Blooms in Ecuador. Front. Mar. Sci. 2018, 5, 365. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- De Romero-Geraldo, R.J.; Hernández-Saavedra, N.Y. Stress Gene Expression in Crassostrea Gigas (Thunberg, 1793) in Response to Experimental Exposure to the Toxic Dinoflagellate Prorocentrum lima (Ehrenberg) Dodge, 1975. Aquac. Res. 2014, 45, 1512–1522. [Google Scholar] [CrossRef]
- Bazzoni, A.M.; Cangini, M.; Mudadu, A.G.; Lorenzoni, G.; Arras, I.; Sanna, G.; Pino, F.; Milandri, A.; Virgilio, S. Recent Findings of Paralytic Shellfish Toxins Linked to the Genus Alexandrium Halim in Mediterranean Mollusc Production Areas. Toxicon 2020, 174, 48–56. [Google Scholar] [CrossRef]
- Avaria, S.; Cáceres, M.; Muñoz, P.; Palma, S.; Vera, P. Plan Nacional Sobre Floraciones Algales Nocivas En Chile; Comité Oceanográfico Nacional: Valparaíso, Chile, 1999; pp. 1–31. [Google Scholar]
- SUBPESCA Res. Ex. 177-2009. Declara Área de Florecimiento Algal Nocivo (FAN) Sector que Indica. Subsecretaria de Pesca y y Acuicultura, Valparaíso, Chile. 2009, pp. 1–3. Available online: https://www.subpesca.cl/portal/615/w3-article-9253.html (accessed on 9 November 2022).
- Buschmann, A.H.; Riquelme, V.A.; Hernández-González, M.C.; Varela, D.; Jiménez, J.E.; Henríquez, L.A.; Vergara, P.A.; Guíñez, R.; Filún, L.A. Review of the Impacts of Salmonid Farming on Marine Coastal Ecosystems in the Southeast Pacific. ICES J. Mar. Sci. 2006, 63, 1338–1345. [Google Scholar] [CrossRef]
- Cuellar-Martinez, T.; Ruiz-Fernández, A.C.; Alonso-Hernández, C.; Amaya-Monterrosa, O.; Quintanilla, R.; Carrillo-Ovalle, H.L.; Arbeláez, M.N.; Díaz-Ascencio, L.; Méndez, S.M.; Vargas, M.; et al. Addressing the Problem of Harmful Algal Blooms in Latin America and the Caribbean- A Regional Network for Early Warning and Response. Front. Mar. Sci. 2018, 5, 409. [Google Scholar] [CrossRef]
- Farabegoli, F.; Blanco, L.; Rodríguez, L.P.; Vieites, J.M.; Cabado, A.G. Phycotoxins in Marine Shellfish: Origin, Occurrence and Effects on Humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glibert, P.M. Harmful Algae at the Complex Nexus of Eutrophication and Climate Change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Müller, M.N.; Mardones, J.I.; Dorantes-Aranda, J.J. Editorial: Harmful Algal Blooms (HABs) in Latin America. Front. Mar. Sci. 2020, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.B.; Whitton, B.A.; Higgins, S.N.; Paerl, H.W.; Brooks, B.W.; Wehr, J.D. Harmful Algal Blooms. In Freshwater Algae of North America. Ecology and Classification; Wehr, J.D., Sheath, R.G., Kociolek, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 873–920. ISBN 9780123858764. [Google Scholar]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful Algal Blooms and Climate Change: Learning from the Past and Present to Forecast the Future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [Green Version]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Cultivo de Bivalvos en Criadero. Un Manual Práctico. FAO Doc. Técnico Pesca 2006, 471, 1–184. [Google Scholar]
- León-Munõz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic Conditions Trigger Record Harmful Algal Bloom in Western Patagonia (Summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello, F.C.; Godfrey, H.P. Harmful Algal Blooms (HABs), Marine Ecosystems and Human Health in the Chilean Patagonia. Rev. Chil. Infectología 2016, 33, 559–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SERNAPESCA Anuario Estadístico de Pesca y Acuicultura 2019. Servicio Nacional de Pesca y Acuicultura, Valparaíso, Chile. Available online: http://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura (accessed on 9 November 2022).
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental Issues in Chilean Salmon Farming: A Review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the Environmental Processes Responsible for the World’s Largest Farmed Fish-Killing Harmful Algal Bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef] [PubMed]
- SERNAPESCA. RES. EX. N1: 6073. Declara Pre-Alerta Acuícola por Floración de Alga Nociva, Conforme lo Dispuesto en el D.S No 320 DE 2001. 2018, Servicio Nacional de Pesca y Acuicultura, Valparaíso, Chile. pp. 1–5. Available online: http://www.sernapesca.cl/normativa-relacionada/resex-ndeg-6073-24122018-declara-pre-alerta-acuicola-por-floracion-de-alga (accessed on 9 November 2022).
- SERNAPESCA. Manual de Inocuidad y Certificación. Parte II: Sección I Control en Origen. Ministerio de Economía Fomento y Turismo, Santiago, Chile. 2018, pp. 1–56. Available online: https://www.sernapesca.cl/manuales-publicaciones/manual-de-inocuidad-y-certificacion (accessed on 9 November 2022).
- Suárez-Isla, B.; Guzmán-Méndez, L. Floraciones de Algas Nocivas. Mareas Rojas y Toxinas Marinas; Orientaciones en Ciencias; Tecnología y Cultura, Editorial Universitaria: Santiago, Chile, 1998; 98p. [Google Scholar]
- SERNAPESCA. Res. Ex. 529-2009. Programa de Vigilancia, Detección y Control de la plaga Alexandrium catenella, 2009, Servicio Nacional de Pesca y Acuicultura, Valparaíso, Chile. pp. 1–11. Available online: http://www.sernapesca.cl/sites/default/files/res.ex_.6004-2019.pdf (accessed on 9 November 2022).
- Cruzat, F.A.; Muñoz, C.; González-saldía, R.R.; Inostroza, A.; Andree, K.B. High Genetic Variability of Alexandrium catenella Directly Detected in Environmental Samples from the Southern Austral Ecosystem of Chile. Mar. Pollut. Bull. 2018, 127, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, J.M.; Contreras, A.M. An Integrative Response by Mytilus Chilensis to the Toxic Dinoflagellate Alexandrium catenella. Mar. Biol. 2010, 157, 1967–1974. [Google Scholar] [CrossRef]
- Llewellyn, L.E. Saxitoxin, a Toxic Marine Natural Product That Targets a Multitude of Receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef]
- Guzmán, L.; Campodonico, G.Í. Marea Roja En La Region de Magallanes. Publ. Inst. Pat. Ser. Mon 1975, 9, 44. [Google Scholar]
- Crawford, D.W.; Montero, P.; Daneri, G. Blooms of Alexandrium catenella in Coastal Waters of Chilean Patagonia: Is Subantarctic Surface Water Involved? Front. Mar. Sci. 2021, 8, 612628. [Google Scholar] [CrossRef]
- Rodríguez, L. Observaciones sobre fitoplancton y temperatura superficial en la Bahía San Jorge, Antofagasta, Chile. Rev. Biol. Mar. Valparaíso 1987, 23, 1–29. [Google Scholar]
- Uribe, J.C. Antecedentes Sobre Un tercer Brote de Veneno Paralizante de Moluscos (VPM) en La Region de Magallanes. Ans. Inst. Pat. Ser. Cs. Nts. Punta Arenas 1988, 18, 97–101. [Google Scholar]
- Guzmán, L.; Pacheco, H.; Pizarro, G.; Alarcón, C. Alexandrium catenella y Veneno Paralizante de Los Mariscos En Chile. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Monografías del Instituto Español de Oceanografía: Vigo, Spain, 2002; pp. 235–255. [Google Scholar]
- Molinet, C.; Lafon, A.; Lembeye, G.; Moreno, C.A. Spatial and Temporal Distribution Patterns of Blooms of Alexandrium catenella (Whedon & Kofoid) Balech 1985, on Inland Seas of Northwest Patagonia, Chile. Rev. Chil. Hist. Nat. 2003, 76, 681–698. [Google Scholar] [CrossRef] [Green Version]
- Cassis, D.; Muñoz, P.; Avaria, S. Variación Temporal del Fitoplancton entre 1993 y 1998 en una Estación Fija del Seno Aysén, Chile (45o26′ S 73o00′ W). Rev. Biol. Mar. Oceanogr. 2002, 37, 43–65. [Google Scholar] [CrossRef] [Green Version]
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL—ISP-Servicios de Salud Año 2002. Ministerio de Salud, Santiago, Chile. 2003, pp. 2–6. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2003doc.pdf (accessed on 9 November 2022).
- Navarro, J.M.; Contreras, A.M.; Chaparro, Ó.R. Short-Term Feeding Response of the Mussel Mytilus chilensis Exposed to Diets Containing the Toxic Dinoflagellate Alexandrium Catenella. Rev. Chil. Hist. Nat. 2008, 81, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL–ISP—Servicios de Salud Año 2003. Ministerio de Salud, Santiago, Chile. 2004, pp. 1–6. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2004doc.pdf (accessed on 9 November 2022).
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL—ISP-Servicios de Salud Año 2004. Ministerio de Salud, Santiago, Chile. 2005, pp. 1–9. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2004doc.pdf (accessed on 9 November 2022).
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL—ISP-Servicios de Salud Año 2005. Ministerio de Salud, Santiago, Chile. 2006, pp. 1–9. Available online: www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2004doc.pdf (accessed on 9 November 2022).
- Fuentes, C.; Clément, A.; Aguilera, A. Summer Alexandrium catenella Bloom and the Impact on Fish Farming, in the XI Aysén Region, Chile. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 August 2006; pp. 183–186. [Google Scholar]
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL—ISP-SEREMIS Año 2006. Ministerio de Salud, Santiago, Chile. 2007, pp. 1–8. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2007doc.pdf (accessed on 9 November 2022).
- Instituto de Salud Pública. Informe Programa de Vigilancia de la Marea Roja en Chile. MINSAL—ISP-SEREMIS Año 2007. Ministerio de Salud, Santiago, Chile. 2008, pp. 1–8. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/INFORME.Programa%20de%20Marea%20Roja2008doc.pdf (accessed on 9 November 2022).
- Álvarez, G.; Uribe, E.; Vidal, A.; Ávalos, P.; González, F.; Mariño, C.; Blanco, J. Paralytic Shellfish Toxins in Argopecten purpuratus and Semimytilus algosus from Northern Chile. Aquat. Living Resour. 2009, 22, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Mardones, J.; Clément, A.; Rojas, X.; Aparicio, C. Alexandrium catenella during 2009 in Chilean Waters, and Recent Expansion to Coastal Ocean. Harmful Algae News 2010, 41, 8–9. [Google Scholar]
- Instituto de Salud Pública. Informe Programa de Vigilancia de Fenómenos Algales Nocivos (FAN) en Chile MINSAL—ISP-SEREMIS Año 2009. Ministerio de Salud, Santiago, Chile. 2010, pp. 1–15. Available online: https://www.ispch.cl/sites/default/files/documento/2013/05/Informe%20Programa%20de%20Marea%20Roja%202010%20VFF%20MINSAL.pdf (accessed on 9 November 2022).
- Guzmán-Méndez, L.; Vidal-Santana, G.; Vivanco-Tapia, X.; Arenas-Sepúlveda, V.; Iriarte-Bustamante, L.; Mercado-Leal, S.; Alarcón-Zapata, C.; Pacheco-Valles, H.; Palma-Alarcón, M.; Espinoza-Alvarado, C.; et al. Informe Final Corregido Tomo I (Texto Informe) Manejo y Monitoreo de Las Mareas Rojas, En Las Regiones de Los Lagos, Aysén y Magallanes 2008–2009; IFOP: Paris, France, 2010; 210p. [Google Scholar]
- Buschmann, A.; Farías, L.; Tapia, F.; Varela, D.; Vásquez, M. Comisión Marea Roja. Universidad de Los Lagos, Puerto Montt, Chile. 2016; 60p. Available online: https://www.economia.gob.cl/wp-content/uploads/2016/11/InfoFinal_ComisionMareaRoja_24Nov2016-1.compressed.pdf (accessed on 9 November 2022).
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef]
- SERNAPESCA Boletín Estado de Floraciones de Algas Nocivas (FAN). Ministerio de Economía Fomento y Turismo, Valparaíso, Chile. 2017, pp. 1–4. Available online: http://www.sernapesca.cl/sites/default/files/boletin_ndeg2_fan_2017.pdf (accessed on 9 November 2022).
- SERNAPESCA Boletín Informativo Floraciones de Algas Nocivas (FAN). Ministerio de Economía, Fomento y Turismo, Valparaíso, Chile. 2018, pp. 1–4. Available online: http://www.sernapesca.cl/sites/default/files/boletin_2_diciembre_2018.pdf (accessed on 9 November 2022).
- Guzmán-Méndez, L.; Espinoza-González, O.; Carbonell-Arias, P.; Martínez-Gonzáles, R.; Mardones-Sánchez, J.; Pizarro-Nova, G.; Salgado-Garrido, P.; Fuenzalida del Río, G.; Pinilla-Matamala, E.; Besoaín-Meneses, V.; et al. Informe Final Corregido. Convenio Desempeño 2018: Programa de Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, Etapa XII 2018-19. IFOP 2019, 12, 107. [Google Scholar]
- SERNAPESCA Boletín Estado de Floraciones de Algas Nocivas (FAN). Ministerio de Economía, Fomento y Turismo, Valparaíso, Chile. 2019, pp. 1–4. Available online: http://www.sernapesca.cl/sites/default/files/boletin_5_febrero_2019.pdf (accessed on 9 November 2022).
- AQUA Marea Roja En Magallanes: Cierran Sector de Isla Morton Para Extracción de Almejas. Available online: https://www.aqua.cl/2019/10/07/marea-roja-en-magallanes-cierran-sector-de-isla-morton-para-extraccion-de-almejas/# (accessed on 9 November 2022).
- Seremi de Salud Región de Magallanes y Antártica. Autoridad sanitaria prohibe extracción de mariscos en sector Isla Carlos por presencia de marea roja. Available online: https://seremi12.redsalud.gob.cl/autoridad-sanitaria-prohibe-extraccion-de-mariscos-en-sector-isla-carlos-por-presencia-de-marea-roja/ (accessed on 9 November 2022).
- Salgado-Garrido, P.; Díaz-Galindo, L.; Pesse-Lastra, N.; Vivanco-Tapia, X.; Guzmán-Méndez, L. Monitoreo de Alexandrium catenella En Zona No Declarada de La Región de Atacama y Coquimbo. In Informe Final Convenio Asesoría Integral Para La Toma de Decisiones En Pesca y Acuicultura; IFOP: Paris, France, 2012; 41p. [Google Scholar]
- Salgado, P.; Riobó, P.; Rodríguez, F.; Franco, J.M.; Bravo, I. Differences in the Toxin Profiles of Alexandrium ostenfeldii (Dinophyceae) Strains Isolated from Different Geographic Origins: Evidence of Paralytic Toxin, Spirolide, and Gymnodimine. Toxicon 2015, 103, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, C.; Díaz, P.A.; Molinet, C.; Seguel, M. Exceptional Climate Anomalies and Northwards Expansion of Paralytic Shellfish Poisoning Outbreaks in Southern Chile. Harmful Algae News 2016, 54, 1–2. [Google Scholar]
- Tubaro, A.; Dell’Ovo, V.; Sosa, S.; Florio, C. Yessotoxins: A Toxicological Overview. Toxicon 2010, 56, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, G.; Alarcón, C.; Franco, J.; Palma, M.; Escalera, L.; Reguera, B.; Vidal, G.; Guzmán, L. Distribución espacial de Dinophysis spp. y detección de toxinas DSP en el agua mediante resinas Diaion (Verano 2006, Región de Los Lagos, Chile). Rev. Cienc. Y Tecnol. Del Mar. 2011, 34, 31–48. [Google Scholar]
- Álvarez, G.; Uribe, E.; Regueiro, J.; Blanco, J.; Fraga, S. Gonyaulax taylorii, a New Yessotoxins-Producer Dinoflagellate Species from Chilean Waters. Harmful Algae 2016, 58, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Trefault, N.; Krock, B.; Delherbe, N.; Cembella, A.; Vásquez, M. Latitudinal Transects in the Southeastern Pacific Ocean Reveal a Diverse but Patchy Distribution of Phycotoxins. Toxicon 2011, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Avaria, S.; Sievers, H.; Prado, R. Presencia de Dinoflagelados Tóxicos del Género Dinophysis en El Seno Aysén, Chile. Rev. Biol. Mar. 1992, 27, 187–212. [Google Scholar]
- Campodonico, G.Í.; Guzmán, M.L. Marea roja producida por Amphidoma sp. en el Estrecho de Magallanes. Ans. Inst. Pat. Ser. Cs. Nts. Punta Arenas 1974, 5, 209–213. [Google Scholar]
- Villarroel, G.O. Detección de toxinas paralizante, diarreica y amnésica en mariscos de la XI Región por cromatografía líquida de alta resolución (HPLC) y bioensayo en ratones. Cienc. Tecnol. Mar. 2004, 27, 33–42. [Google Scholar]
- Reguera, B. Establecimiento de Un Programa de Seguimiento de Microalgas Toxicas. In Floraciones Algales Nocivas En El Cono Sur Americano; Sar, E., Ferrario, M., Reguera, B., Eds.; Monografía; Monografías del Instituto Español de Oceanografía: Vigo, Spain, 2002; pp. 19–54. [Google Scholar]
- Diaz, P.; Molinet, C.; Cáceres, M.A.; Valle-Levinson, A. Seasonal and Intratidal Distribution of Dinophysis spp. in a Chilean Fjord. Harmful Algae 2011, 10, 155–164. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic Toxins in Chile: History, Producers and Impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef]
- Uribe, J.C.; García, C.; Rivas, M.; Lagos, N. First Report of Diarrhetic Shellfish Toxins in Magellanic Fjords, Southern Chile. J. Shellfish Res. 2001, 20, 69–74. [Google Scholar]
- García, C.; Mardones, P.; Sfeir, A.; Lagos, N. Simultaneous Presence of Paralytic and Diarrheic Shellfish Poisoning Toxins in Mytilus chilensis Samples Collected in the Chiloe Island, Austral Chilean Fjords. Biol. Res. 2004, 37, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Álvarez, G.; Uribe, E. Identification of Pectenotoxins in Plankton, Filter Feeders, and Isolated Cells of a Dinophysis acuminata with an Atypical Toxin Profile, from Chile. Toxicon 2007, 49, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Méndez, L.; Vidal-Santana, G.; Vivanco-Tapia, X.; Palma-Alarcón, M.; Espinoza-Alvarado, C.; Mejías-Wagner, P.; Ulloa-Herrera, R.; Iriarte-Bustamante, L.; Arenas-Sepúlveda, V.; Mercado-Leal, S.; et al. Informe Final SUBPESCA. Manejo y monitoreo de las mareas rojas en las regiones de Los Lagos, Aysén y Magallanes. IFOP 2007, 1, 141. [Google Scholar]
- Guzmán-Méndez, L.; Vidal-Santana, G.; Vivanco-Tapia, X.; Arenas-Sepúlveda, V.; Iriarte-Bustamante, L.; Mercado-Leal, S.; Alarcón-Zapata, C.; PAcheco-Valle, H.; Palma-Alarcón, M.; Espinoza-Alvarado, C.; et al. Manejo y monitoreo de las mareas rojas en las regiones de Los Lagos, Aysén y Magallanes (período 2007–2008). IFOP 2009, 2, 187. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Seguel, M.; Marín, A.; Krock, B. First Detection of Pectenotoxin-2 in Shellfish Associated with an Intense Spring Bloom of Dinophysis acuminata on the Central Chilean Coast. Mar. Pollut. Bull. 2020, 158, 111414. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Seguel, C.G.; Valderrama, K.; Tillmann, U. Pectenotoxins and Yessotoxin from Arica Bay, North Chile as Determined by Tandem Mass Spectrometry. Toxicon 2009, 54, 364–367. [Google Scholar] [CrossRef]
- Alves-de-Souza, C.; Varela, D.; Contreras, C.; de La Iglesia, P.; Fernández, P.; Hipp, B.; Hernández, C.; Riobó, P.; Reguera, B.; Franco, J.M.; et al. Seasonal Variability of Dinophysis spp. and Protoceratium reticulatum Associated to Lipophilic Shellfish Toxins in a Strongly Stratified Chilean Fjord. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 101, 152–162. [Google Scholar] [CrossRef]
- Díaz, P.A.; Peréz-Santos, I.; Álvarez, G.; Garreaud, R.; Pinilla, E.; Díaz, M.; Sandoval, A.; Araya, M.; Álvarez, F.; Rengel, J.; et al. Multiscale Physical Background to an Exceptional Harmful Algal Bloom of Dinophysis acuta in a Fjord System. Sci. Total Environ. 2021, 773, 145621. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Méndez, L.; Vidal-Santana, G.; Pizarro-Nova, G.; Vivanco-Tapia, X.; Iriarte-Bustamante, L.; Alarcón-Zapata, C.; Arenas-Sepúlveda, V.; Mercado-Leal, S.; PAcheco-Valles, H.; Mejías-Wagner, P.; et al. Informe Final Manejo y Monitoreo de Las Mareas Rojas, en las Regiones de Los Lagos, Aysén y Magallanes. IV Etapa, 2010. IFOP 2011, 4, 205. [Google Scholar]
- Guzmán-Méndez, L.; Vidal, G.; Pizarro, G.; Vivanco, X.; Iriarte, L.; Alarcón, C.; Arenas, V.; Mercado, S.; Pacheco, H.; Mejías, P.; et al. Informe Final Manejo y Monitoreo de Las Mareas Rojas en las Regiones de Los Lagos, Aysén y Magallanes (V. Etapa). IFOP 2012, 5, 61. [Google Scholar]
- Guzmán-Méndez, L.; Vidal-Santana, G.; Pizarro-Nova, G.; Vivanco-Tapia, X.; Iriarte-Bustamante, L.; Alarcón-Zapata, C.; Arenas-Sepúlveda, V.; Mercado-Leal, S.; PAcheco-Valle, H.; Mejías-Wagner, P.; et al. Informe Final Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, VI Etapa 2012-13. IFOP 2013, 6, 21. [Google Scholar]
- Guzmán-Méndez, L.; Vidal-Santana, G.; Pizarro-Nova, G.; Salgado-Garrido, P.; Vivanco-Tapia, X.; Iriarte-Bustamante, L.; Mercado-Leal, S.; PAcheco-Valle, H.; Pesse-Lastra, N.; Alarcón-Zapata, C.; et al. Informe Final Convenio III: Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos. Aysén y Magallanes (VII Etapa) Programa de Manejo y Monitoreo de las Mareas Rojas en Las Regiones de Los Lagos, Aysén y Magallanes, Etapa VII. 2013-14. IFOP 2014, 7, 18. [Google Scholar]
- Pizarro, G.; Arévalo, F.; Moroño, A.; Riobó, P.; Franco, J.; Zamora, C.; Guzmán, L. Emergent Lipophilic Shellfish Toxins in the Magellan Region (47–55 oS), Chile. In Proceedings of the 16th International Conference on Harmful Algae, Michael Fowler Centre, Wellington, New Zealand, 27–31 October 2014; p. 124. [Google Scholar]
- IFOP Convenio de Desempeño 2015: Programa de Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, Etapa IX 2015-16. IFOP 2016, 9, 1–141.
- Guzmán-Méndez, L.; Espinoza-González, O.; Carbonell-Arias, P.; Martínez-Gonzáles, R.; Besoaín-Meneses, V.; Mardones-Sánchez, J.; Pizarro-Nova, G.; Pinilla-Matamala, E.; Vivanco-Tapia, X.; Calderón-Nash, M.; et al. Informe Final Textos Convenio Desempeño 2016: Programa de Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, Etapa X 2016-17. IFOP 2017, 10, 83. [Google Scholar]
- IFOP Floraciones Nocivas Durante Enero y Febrero de 2017 En Las Regiones de Los Lagos y Aysén Artículos Relacionados. Available online: https://www.ifop.cl/floraciones-nocivas-durante-enero-y-febrero-de-2017-en-las-regiones-de-los-Lagos-y-Aysen/ (accessed on 9 November 2022).
- Guzmán-Méndez, L.; Espinoza-González, O.; Carbonell-Arias, P.; Martínez-Gonzáles, R.; Besoaín-Meneses, V.; Mardones-Sánchez, J.; Pizarro-Nova, G.; Salgado-Garrido, P.; Fuenzalida del Río, G.; Pinilla-Matamala, E.; et al. INFORME FINAL Programa de Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, Etapa XI 2017-18. IFOP 2018, 9, 77. [Google Scholar]
- Guzmán-Méndez, L.; Espinoza-González, O.; Carbonell-Arias, P.; Martínez-Gonzáles, R.; Pizarro-Nova, G.; Salgado-Garrido, P.; Mardones-Sánchez, J.; Fuenzalida del Río, G.; Besoaín-Meneses, V.; Cascales-Hellman, E.; et al. Informe Final Corregido Tomo I F C T I Programa de Manejo y Monitoreo de las Mareas Rojas en el Sistema de Fiordos y Canales de Chile, XIII Etapa Año 2019-2020. IFOP 2020, 13, 149. [Google Scholar]
- Álvarez, G.; Uribe, E.; Díaz, R.; Braun, M.; Mariño, C.; Blanco, J. Bloom of the Yessotoxin Producing dinoflagellate Protoceratium reticulatum (Dinophyceae) in Northern Chile. J. Sea Res. 2011, 65, 427–434. [Google Scholar] [CrossRef]
- Suárez-Isla, B..; Barrera, F.; Carrasco, D.; Cigarra, L.; López-Rivera, A..; Rubilar, I.; Alcayaga, C.; Contreras, V.; Seguel, M. Comprehensive Study of the Occurrence and Distribution of Lipophilic Marine Toxins in Shellfish from Production Areas in Chile. In Harmful Algae 2018—From Ecosystems to Socioecosystems. Proceedings of the 18th International Conference on Harmful Algae (ICHA), Nantes, France, 21–26 October 2018; Hess, P., Ed.; International Society for the Study of Harmful Algae (ISSHA): Copenhagen, Denmark, 2020; pp. 163–166. ISBN 9788799082773. [Google Scholar]
- Fux, E.; Smith, J.L.; Tong, M.; Guzmán, L.; Anderson, D.M. Toxin Profiles of Five Geographical Isolates of Dinophysis spp. from North and South America. Toxicon 2011, 57, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, G.; Uribe, E.; Ávalos, P.; Mariño, C.; Blanco, J. First Identification of Azaspiracid and Spirolides in Mesodesma donacium and Mulinia edulis from Northern Chile. Toxicon 2010, 55, 638–641. [Google Scholar] [CrossRef]
- Yasumoto, T.; Takizawa, A. Fluorometric Measurement of Yessotoxins in Shellfish by High-Pressure Liquid Chroraatography. Biosci. Biotechnol. Biochem. 1997, 61, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, G.; Paz, B.; Alarcón, C.; Toro, C.; Frangópulos, M.; Salgado, P.; Olave, C.; Zamora, C.; Pacheco, H.; Guzmán, L. Winter Distribution of Toxic, Potentially Toxic Phytoplankton, and Shellfish Toxins in Fjords and Channels of the Aysén Region, Chile. Lat. Am. J. Aquat. Res. 2018, 46, 120–139. [Google Scholar] [CrossRef]
- Álvarez, G.; Rengel, J.; Álvarez, F.; Ganuza, I.; Pino, R.; Muñoz, P.; Rosales, S.A.; Hevia, V.; Araya, M.; Díaz, P.A.; et al. Mass Mortality of Marine Invertebrates Associated with the Presence of Yessotoxins in Northern Chile. Harmful Algal News 2020, 64, 6–7. [Google Scholar]
- King, T.L.; Nguyen, N.; Doucette, G.J.; Wang, Z.; Bill, B.D.; Peacock, M.B.; Madera, S.L.; Elston, R.A.; Trainer, V.L. Hiding in Plain Sight: Shellfish-Killing Phytoplankton in Washington State. Harmful Algae 2021, 105, 102032. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Norambuena, L.; Paredes, J.; Fuenzalida, G.; Dorantes-Aranda, J.J.; Chang, K.J.L.; Guzmán, L.; Krock, B.; Hallegraeff, G. Unraveling the Karenia selliformis Complex with the Description of a Non-Gymnodimine Producing Patagonian Phylotype. Harmful Algae 2020, 98, 101892. [Google Scholar] [CrossRef] [PubMed]
- López-Rivera, A.; O’Callaghan, K.; Moriarty, M.; O’Driscoll, D.; Hamilton, B.; Lehane, M.; James, K.J.; Furey, A. First Evidence of Azaspiracids (AZAs): A Family of Lipophilic Polyether Marine Toxins in Scallops (Argopecten purpuratus) and Mussels (Mytilus chilensis) Collected in Two Regions of Chile. Toxicon 2010, 55, 692–701. [Google Scholar] [CrossRef]
- Tillmann, U.; Trefault, N.; Krock, B.; Parada-Pozo, G.; De La Iglesia, R.; Vásquez, M. Identification of Azadinium poporum (Dinophyceae) in the Southeast Pacific: Morphology, Molecular Phylogeny, and Azaspiracid Profile Characterization. J. Plankton Res. 2017, 39, 350–367. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Isla, B..; López, A.; Hernández, C.; Clément, A.; Guzmán, L. Impacto Económico de Las Floraciones de Microalgas Nocivas En Chile y Datos Recientes Sobre La Ocurrencia de Veneno Amnésico de Los Mariscos. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E., Ferrario, M., Reguera, B., Eds.; Monografías del Instituto Español de Oceanografía: Vigo, Spain, 2002; pp. 259–268. ISBN 8495877015. [Google Scholar]
- Teitelbaum, J..; Zatorre, R.J.; Carpenter, S.; Gendron, D.; Evans, A..; Gjedde, A.; Cashman, N. Neurologic Sequelae of Domoic Acid Intoxication Due to the Ingestion of Contaminated Mussels. N. Engl. J. Med. 1990, 322, 1781–1787. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic Acid Production by Pseudo-Nitzschia australis and Pseudo-Nitzschia calliantha Isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- López-Rivera, A.; Pinto, M.; Insinilla, A.; Suárez-Isla, B.; Uribe, E.; Alvarez, G.; Lehane, M.; Furey, A.; James, K. The Occurrence of Domoic Acid Linked to a Toxic Diatom Bloom in a New Potential Vector: The Tunicate Pyura chilensis (Piure). Toxicon 2009, 54, 754–762. [Google Scholar] [CrossRef]
- Guzmán-Méndez, L. Tomo I Informe Final: Capítulos 1 Al 5: Textos Convenio Desempeño 2014: Programa de Manejo y Monitoreo de Las Mareas Rojas En Las Regiones de Los Lagos, Aysén y Magallanes, Etapa VIII 2014-15. IFOP 2015, 8, 182. [Google Scholar]
- Visión-Acuícola Detectan Veneno Amnésico de Los Moluscos En Sector Costero Entre Pue Calbuco. Available online: https://www.soychile.cl/Puerto-Montt/Vision-Acuicola/2020/12/19/686466/Detectan-veneno-amnesico-de-los-moluscos-en-sector-costero-de-Puerto-Montt.aspx (accessed on 9 November 2022).
- Álvarez, G.; Rengel, J.; Araya, M.; Álvarez, F.; Pino, R.; Uribe, E.; Díaz, P.A.; Rossignoli, A.E.; López-Rivera, A.; Blanco, J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins 2020, 12, 698. [Google Scholar] [CrossRef]
- Espinoza-González, O.; Guzmán-Méndez, L.; Norambuena-Subiabre, L.; Cascales-Hellman, E.; Cáceres-Chamizo, J.; López-Rivera, L.; Labra-Holzapfel, G.; Olivares-Olivares, B.; Soto-Castillo, C.; Palma-Alarcón, M. Distribución Abundancia de Pseudo-Nitzschia Toxina Amnésica de los Mariscos (TAM) Fiordo e Reloncavi. Cent. Estud. Algas Nocivas/IFOP 2020, 1, 1–14. [Google Scholar]
- Uribe, J.C.; Ruiz, M. Gymnodinium Brown Tide in the Magellanic Fjords, Southern Chile. Rev. Biol. Mar. Oceanogr. 2001, 36, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Montes, R.M.; Rojas, X.; Artacho, P.; Tello, A.; Quiñones, R.A. Quantifying Harmful Algal Bloom Thresholds for Farmed Salmon in Southern Chile. Harmful Algae 2018, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Hurtado, C.; Paredes Herbach, E.; Mejia, M.; Aguilera, A.; Uribe, C. Floraciones Algales Nocivas y Su Impacto en Peces. Puerto Montt, Chile. 2017, pp. 12–14. Available online: https://fdocuments.ec/document/primer-semestre-21-primer-semestre-microalgas-microalgas-primer-semestre-21.html?page=1 (accessed on 9 November 2022).
- Iriarte, J.L.; Pantoja, S.; González, H.E.; Silva, G.; Paves, H.; Labbé, P.; Rebolledo, L.; Van Ardelan, M.; Häussermann, V. Assessing the Micro-Phytoplankton Response to Nitrate in Comau Fjord (42 S) in Patagonia (Chile), Using a Microcosms Approach. Environ. Monit. Assess. 2013, 185, 5055–5070. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, J.L. Natural and Human Influences on Marine Processes in Patagonian Subantarctic Coastal Waters. Front. Mar. Sci. 2018, 5, 360. [Google Scholar] [CrossRef]
- Müller, M.N.; Mardones, J.I.; Dorantes-Aranda, J.J. Harmful Algal Blooms (HABS) in Latin America; Frontiers Media SA: Lausanne, Switzerland, 2020; pp. 6–9. [Google Scholar] [CrossRef]
- Sandoval, A. Efecto combinado de la temperatura y salinidad sobre parámetros fisiológicos de las especies ictiotóxicas Heterosigma akashiwo y Pseudochattonella verruculosa. Univ. Concepción Fac. Ciencias Nat. Ocean. 2020, 64, 53. [Google Scholar]
- Mardones, J.I. Screening of Chilean Fish-Killing Microalgae Using a Gill Cell-Based Assay. Lat. Am. J. Aquat. Res. 2020, 48, 329–335. [Google Scholar] [CrossRef]
- Fuica, N.; Rojas, X.; Clément, A.; Bittner, V.; Silva, M.; Uribe, C. Ocurrencia e Impacto de las FANs en la Salmonicultura en el Sur de Chile: Análisis del Programa de Monitoreo de INTESAL de SalmonChile. Salmo Cienc. 2007, 2, 61–71. [Google Scholar]
- Sepúlveda, R. Supervisan Retiro de Peces Muertos por Florac151ión Algal Estero Córdova en Magallanes. Biobio Chile. Available online: https://www.biobiochile.cl/noticias/nacional/region-de-magallanes/2017/11/23/supervisan-retiro-de-peces-muertos-por-floracion-algal-en-estero-cordova-en-magallanes.shtml (accessed on 9 November 2022).
- SERNAPESCA Boletín Estado de Floraciones de Algas Nocivas (FAN). Ministerio de Economía Fomento y Turismo, Valparaíso, Chile. 2020, pp. 1–5. Available online: http://www.sernapesca.cl/sites/default/files/boletin_fan_20200526.pdf (accessed on 9 November 2022).
- Mardones, J.I.; Dorantes-Aranda, J.J.; Seger, A.; Nichols, P.; Hallegraeff, G.M. Avances en el Estudio de Ictiotoxinas Asociadas con Floraciones Algales Nocivas (FANs); Salmonexpert: Bergen, Norway, 2015; pp. 38–44. [Google Scholar]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish Gill Damage by the Dinoflagellate Alexandrium catenella from Chilean Fjords: Synergistic Action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in Understanding Algal Bloom-mediated Fish Kills: The Role of Superoxide Radicals, Phycotoxins and Fatty Acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardones, J.I.; Müller, M.N.; Hallegraeff, G.M. Toxic Dinoflagellate Blooms of Alexandrium catenella in Chilean Fjords: A Resilient Winner from Climate Change. ICES J. Mar. Sci. 2016, 74, 988–995. [Google Scholar] [CrossRef]
- Clément, A.; Seguel, M.; Arzul, G.; Guzmán, L.; Alarcón, A. Widespread Outbreak of a Haemolytic, Ichthyotoxic Gymnodinium sp. in Southern Chile. In Harmful Algal Blooms 2000, Proceedings of the Ninth International Conference on Harmful Algal Blooms, Hobart, Australia, 7–11 February 2000; Hallegraeff, G., Blackburn, S., Lewis, R., Eds.; Japan Oceanographic Data Center: Nago, Japan, 2001; pp. 66–69. [Google Scholar]
- Uribe, E.; Álvarez, G.; Rengel, J.; Blanco, J. Prorocentrum lima, a New Diarrhetic Shellfish Toxins Producer in Northern Chile. In Proceedings of the 18th International Conference on Harmful Algae—From Ecosystems to Socioecosystems, Nantes, France, 21–26 October 2018. [Google Scholar] [CrossRef]
- Chang, F.H. Cytotoxic Effects of Vicicitus globosus (Class Dictyochophyceae) and Chattonella marina (Class Raphidophyceae) on Rotifers and Other Microalgae. J. Mar. Sci. Eng. 2015, 3, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Treasurer, J.W.; Hannah, F.; Cox, D. Impact of a Phytoplankton Bloom on Mortalities and Feeding Response of Farmed Atlantic Salmon, Salmo salar, in West Scotland. Aquaculture 2003, 218, 103–113. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, B.S.; Wang, P.; Kim, J.H.; Youn, S.H.; Han, M.S. Cyst Morphology and Germination in Heterosigma skashiwo (Raphidophyceae). Phycologia 2015, 54, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.; Clément, A.; Rojas, X. Monitoring Potentially Ichthyotoxic Phytoflagellates in Southern Fjords of Chile. Harmful Algae News 2012, 45, 6–7. [Google Scholar]















| Toxin Syndrome | Toxin | Thresholds Limits |
|---|---|---|
| PSP | Saxitoxin (equivalent) | ≥80 µg eq. STX/100 g |
| ASP | Domoic acid | ≥20 mg/Kg |
| DSP (Lipophilic toxins) | Okadaic acid (OA) and Dinophysistoxins (DTX1 and DTX2) | ≥160 µg/kg |
| Pectenotoxins (PTX1 and PTX2) | ||
| Azaspiracids (AZA1, AZA2 and AZA3) | ≥160 µg eq. AZA/kg | |
| Yesotoxins (YTX, 45-OH-YTX, homo-YTX, 45-OH-homo-YTX) | ≥3.75 mg eq. YTX/kg |
| Microalgae | Threshold Limits (Cell × mL−1) | Mechanism of Action |
|---|---|---|
| Alexandrium catenella | >300 | Ichthyotoxic |
| Azadinium spp. | Unknown | n.i |
| Chaetoceros convolutus | >5 | Mechanic |
| Chaetoceros cryophilus | >5 | Mechanic |
| Octactis speculum | >75 | Mechanic |
| Eucampia zodiacus | >400 | Mechanic |
| Gymnodinium spp. | Unknown | n.i. |
| Heterosigma akashiwo | >20 | Ichthyotoxic |
| Karenia mikimotoi | >40 | Ichthyotoxic |
| Karenia spp. | >40 | Ichthyotoxic |
| Leptocylindrus danicus | >2500 | Mechanic |
| Leptocylindrus minimus | >2000 | Mechanic |
| Pseudochattonella cf. verruculosa | >50 | Ichthyotoxic |
| Rhizosolenia aff. setigera | >500 | Mechanic |
| Thalassiosira pseudonana | >3000 | Rheotoxicity |
| Haptophyte | Unknown | n.i. |
| Magallanes | Aysén | Los Lagos | Biobío | Coquimbo | Atacama | Antofagasta | Tarapacá | Arica- Parinacota | |
|---|---|---|---|---|---|---|---|---|---|
| Dinophyceae (Dinoflagellate) | |||||||||
| Alexandrium catenella | x | x | x | x * | |||||
| Alexandrium spp. | x | x | |||||||
| Amphidoma sp. | x * | ||||||||
| Azadinium sp. | x | ||||||||
| Dinophysis acuminata | x | x | x | x | x | x | x | ||
| Dinophysis acuta | x | x | |||||||
| Dinophysis spp. | x | x | |||||||
| Gonyaulax spinifera | x | ||||||||
| Gonyaulax taylorii | x | ||||||||
| Gymnodinium spp. | x | x | x | ||||||
| Karenia mikimotoi | x | x | |||||||
| Karenia selliformes | x | x | |||||||
| Karenia spp. | x | ||||||||
| Karlodinium australe | x | ||||||||
| Kryptoperidinium triquetrum | x | x | x | ||||||
| Lepidodinium chlorophorum | x | ||||||||
| Lepidodinium spp. | x | x | |||||||
| Margalefidinium polykrikoides | x | ||||||||
| Prorocentrum lima | x | ||||||||
| Prorocentrum micans | x | x | x | ||||||
| Protoceratium reticulatum | x | x | x | x | |||||
| Bacillariophyceae (Diatoms) | |||||||||
| Chaetoceros convolutus | x | x | x | ||||||
| Chaetoceros criophilus | x | x | |||||||
| Eucampia zodiacus | x | x | x | ||||||
| Leptocylindrus danicus | x | x | |||||||
| Leptocylindrus minimus | x | x | x | ||||||
| Pseudo-nitzschia australis | x | x | x | x | x | x | |||
| Pseudo-nitzschia calliantha | x | x | |||||||
| Pseudo-nitzschia delicatissima | x | ||||||||
| Pseudo-nitzschia pseudodelicatissima | x | x | x | x | x | ||||
| Pseudo-nitzschia seriata | x | ||||||||
| Pseudo-nitzschia spp | x | x | x | x | |||||
| Pseudo-nitzschia subfraudulenta | x | x | |||||||
| Rhrizosolenia setigera | x | x | x | ||||||
| Thalassiosira pseudonana | x | x | |||||||
| Dictyophyceae (Silicoflagellate) | |||||||||
| Octactis speculum | x | x | |||||||
| Pseudochatonella spp. | x | ||||||||
| Pseudochatonella verruculosa | x | x | x | ||||||
| Vicicitus globosus | x | ||||||||
| Raphidophyceae | |||||||||
| Heterosigma akashiwo | x | ||||||||
| Haptophyta | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barría, C.; Vásquez-Calderón, P.; Lizama, C.; Herrera, P.; Canto, A.; Conejeros, P.; Beltrami, O.; Suárez-Isla, B.A.; Carrasco, D.; Rubilar, I.; et al. Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records. J. Mar. Sci. Eng. 2022, 10, 1868. https://doi.org/10.3390/jmse10121868
Barría C, Vásquez-Calderón P, Lizama C, Herrera P, Canto A, Conejeros P, Beltrami O, Suárez-Isla BA, Carrasco D, Rubilar I, et al. Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records. Journal of Marine Science and Engineering. 2022; 10(12):1868. https://doi.org/10.3390/jmse10121868
Chicago/Turabian StyleBarría, Camila, Piera Vásquez-Calderón, Catalina Lizama, Pablo Herrera, Anahi Canto, Pablo Conejeros, Orietta Beltrami, Benjamín A. Suárez-Isla, Daniel Carrasco, Ignacio Rubilar, and et al. 2022. "Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records" Journal of Marine Science and Engineering 10, no. 12: 1868. https://doi.org/10.3390/jmse10121868
APA StyleBarría, C., Vásquez-Calderón, P., Lizama, C., Herrera, P., Canto, A., Conejeros, P., Beltrami, O., Suárez-Isla, B. A., Carrasco, D., Rubilar, I., Guzmán, L., Durán, L. R., & Oliva, D. (2022). Spatial Temporal Expansion of Harmful Algal Blooms in Chile: A Review of 65 Years Records. Journal of Marine Science and Engineering, 10(12), 1868. https://doi.org/10.3390/jmse10121868






