1. Introduction
The speleothems, i.e., stalactites and stalagmites, representing secondary mineral deposits, could have been created by biogenic and abiogenic processes. In the abiogenic case, the mineral precipitation is due to supersaturation of the solution due to pH changes, outgassing, and evaporation. In the case of microorganisms contribution, biomineralization processes might occur ([
1] and references within). The microorganisms can biologically mediate mineral formation in several ways, either directly by creating minerals (external or internal) or passively by accelerating the deposition, by encrustation or extracellular polymeric substance (EPS), or by changing the ambient conditions such as pH [
2]. The microorganisms contributing to speleothem formation are mainly Archaea, Algae, and Bacteria, including Cyanobacteria. Multicellular organisms, such as fungi, lichens, and mosses, can also contribute. The most common cave-inhabitant microorganisms are chemolithoautotrophic or chemoheterotrophic, i.e., non-photosynthetic [
3]. Photosynthetic microorganisms usually inhabit the cave entrance, where sunlight is reaching. However, some Cyanobacteria species, even if in general they are photosynthetic species, can adjust to the darkness and become heterotrophic, such as
Fisherella,
Calothrix,
Geitleria calcarea, and
Scytonema julianum [
4,
5,
6]. The adjustment of the photosynthetic Cyanobacteria is important, as they can play a key role in speleogenetic processes since they can contribute to the dissolution or precipitation of the minerals.
Biodiversity in caves and biomineralization processes are subjects that only recently started to be studied and intercorrelated. Caves represent natural laboratories where microbe–mineral interactions under extreme conditions can be studied [
7]. In Greece, several studies concerning speleothems and speleothems-climatic changes have been conducted (e.g., [
8,
9,
10,
11]). Moreover, studies concerning only biodiversity have taken place in the last years (e.g., [
12,
13,
14,
15,
16,
17,
18,
19]).
This paper aims to study stalactites in a hot spring cave in the Aedipsos area (Euboea Island, Greece) and the involved geomicrobiological processes. The mineralogical composition and mineral chemistry, the environmental conditions, and the Cyanobacteria species diversity will be assessed to evaluate the biomineralization processes of calcium carbonate minerals.
2. Geological Setting
The study site is located at NNW edge of Aedipsos (NW Euboea Island, Greece). The geological formations of the area belong to the Pelagonian geotectonic unit of the Hellenides [
20,
21,
22], and the main geological formations are: a metamorphic crystalline basement (pre-middle to middle Carboniferous age), basic volcanoclastic complex series (Permian–Triassic age), shallow marine carbonate and clastic rocks (middle Triassic age) with volcanic rocks intercalations [
23,
24], alluvial deposits and thermogenic travertine depositions (
Figure 1).
Several hot springs occur at the NW Euboea island, mainly in the Aedipsos area, which belongs to seawater-dominated, tectonically controlled, and volcanic-related geothermal systems [
25,
26,
27]. The Lichades volcanic center, composed by trachyandesite lava, is located several kilometers away; it was dated to 0.5 Ma old (K-Ar method; [
28]).
The temperature in the hot springs at Aedipsos reaches up to 84 °C, and the hydrothermal fluids are of sodium-chloride type. Among others, pH is almost neutral, and the springs present chemical similarities [
25,
27]. They are interpreted as deep-old geothermal fluids migrating from deep basement bedrocks with volcanic origin affinities.
The Aedipsos hot springs commonly deposit thermogenic travertine [
27,
29,
30,
31,
32]. In addition, they present macro- and micro-facies [
27,
30,
31], with bio-mineralization processes resulting in the creation of hybrid travertines [
27,
29,
33,
34,
35], i.e., biotic and abiotic contributions.
3. Materials and Methods
Samples were extracted from stalactites from a small open cave with a hot spring at the base. During the sampling process, sterile metal tweezers and chisels were used. The unstable water parameters of the hot spring (i.e., temperature, salinity, and pH) were measured in situ once, during sampling, using portable apparatus.
From each sampling site, two sub-samples were collected. The first one was incubated into sterile transparent vials in the field. The second sub-sample was stored in a formaldehyde solution (2.5%). Enriched cultures were obtained in flasks and Petri dishes with BG11 and BG 11
0 culture media [
36]. Cultures were maintained in an incubator (Sanyo, Gallenkamp, Cambridge, UK) under stable conditions and a natural diurnal cycle (north-facing window) at room temperature.
The samples were studied under an optical microscope and a stereo-microscope. For species identification, the classical and recent literature was used ([
37,
38,
39,
40] and references within) at the Faculty of Biology, National and Kapodistrian University of Athens.
The mineralogical study was conducted on polished sections studied under an optical microscope and powders using X-ray diffraction (Bruker D8 Advanced Diffractometer, using Ni filtered Cu-Kα radiation, operating at 40 kV and 40 mA and employing a Bruker Lynx Eye fast detector; Bruker-AXS, Billerica, MA, USA). The XRD results were evaluated using the DIFFRACplus EVA software (Bruker-AXS, Billerica, MA, USA) and the ICDD Powder Diffraction File (2006 version) at the Department of Geology, University of Patras. Selected dehydrated samples in an alcohol series (30–100%), critical point dried, gold-coated, and were studied under SEM (Jeol JSM 5600; JEOL USA, Inc., Peabody, MA, USA) at the Faculty of Geology and Geoenvironment, National and Kapodistrian University of Athens. SEM-EDS analyses were carried out using a Jeol JSM-IT500 SEM instrument (JEOL USA, Inc., Peabody, MA, USA) equipped with an Oxford 100 Ultramax analytical device (Oxford Instruments, Abingdon, UK) at the Hellenic Survey of Geology & Mineral Exploration.
The ArcGIS software was used to modify the geological map presented by Kanellopoulos et al. [
25].
4. Results
4.1. Sampling Sites Description
In Aedipsos, several hot springs occur; very few of them are located inside small caves. The study site is a small open cave with a hot spring at the base (
Figure 2). As it was verified from the thermal photos, hot-water circulation occurs at the cave walls (including the roof). The hot spring temperature, just below the stalactites, was 49.2 °C, the pH was 6.05, and the salinity was 20‰. Samples of the stalactites were collected above the hot spring.
4.2. Mineralogy and Facies
According to XRD analyses and optical microscopy, the main mineral phase of stalactites is calcite (
Figure 3). Based on SEM-EDS microanalyses, the calcite contains up to 0.48 wt.% Na
2O, up to 0.73 wt.% MgO, up to 4.19 wt.% SO
3, up to 0.16 wt.% SrO and up to 2.21 wt.% Yb
2O
3 (
Table 1).
The studied samples display mainly lamination (
Figure 4A and
Figure 5A) and shrub (
Figure 5B) facies. The laminas could be from a few micrometers to a few millimeters thick. The laminas usually consist of micritic crystals and alternate with the next laminae, which is similar in mineralogical composition but differs in crystal size and density. Some laminas consist of shrubs (
Figure 5B) with thicknesses up to ca. 1 mm. These are stubby, dense crystalline masses of calcite crystals that expand upward by irregular branching.
In a few cases, diatoms are trapped in thin laminas consisting of non-dense micritic crystals (
Figure 5C,D). Moreover, in several cases, zones parallel to the lamination (
Figure 2C) or nest areas were identified where traces of endolithic Cyanobacteria were present, i.e., holes and grooves, occur.
4.3. Cyanobacteria Microflora
In
Figure 6, the Cyanobacteria orders are presented based on the latest classification system [
41]. As it can be seen, Synechococcales and Oscillatoriales dominate with 28% and 27%, respectively. The orders Chroococcales and Nostocales follow with 21%, and finally, Spirulinales are also present with only 3%.
By studying the fresh and cultured material, a total of twenty-nine (29) different Cyanobacteria species, plus diatoms, were identified (
Table 2;
Figure 7). Among them, typical thermophilic species were found, such as
Spirulina subtilissima (
Figure 7L).
Chroococcus lithophilus, Leptolyngbya perforans, and
Leptolyngbya ercegovicii (
Figure 7F) are also present, which are typical limestone substrate Cyanobacteria species.
5. Discussion
5.1. Mineralogical Characterization and Facies
The main mineral phase of the studied samples is calcite (
Figure 3), which is the most stable and common CaCO
3 polymorph found in speleothems [
42]. Calcite is also the most typical main mineral phase in thermogenic travertines. However, the hot spring travertine deposits of Aedipsos have as main mineral phases either calcite, calcite, and aragonite or only aragonite [
27,
29,
30]. In the case of speleothems, it was suggested that aragonite precipitates when the water has Mg/Ca ratios >1 (usually in dolomitic settings [
42,
43]). However, the Mg/Ca ratio in the study site is less than one [
25]; additionally, based on the geological setting of the area, no dolomite occurrences have been testified nearby.
Based on SEM-EDS observations and microanalyses, the calcite, except for CaCO
3, contains several trace elements, i.e., Na, Mg, S, Sr, and Yb (
Table 1). The incorporation of Mg
2+ and Sr
2+ into calcite has been well documented (e.g., [
44]). However, it is worth mentioning that high-Mg calcites are usually observed in marine organisms [
45]. The Aedipsos hot springs have high Na-Cl content and are characterized as seawater-dominated areas [
25]. The incorporation of rare earths elements (REE), including Yb
3+ in calcite, takes place by adsorption onto calcite surfaces [
46]. In the studied samples, the Yb presented equal distribution (
Figure 4D) and reached 2.21 wt.% Yb
2O
3. Although the presence of Yb-calcite in speleothems is not common, its presence in the studied samples could be explained because they are not typical speleothems but hot-spring related, and the Yb could be attributed to the hydrothermal fluid. The presence of sulfate-containing calcite has been recently proved. A characteristic example comes from LaDuke Yellowstone hot spring, USA [
47]. Okumura et al. [
47], based on XPS, XRD, and TEM analysis, verified that sulfur was the principal foreign element in synthetic and natural (from LaDuke hot spring, USA) calcite crystals, with a mean atomic ratio of S/Ca around 5%; the chemical form of sulfur was proven to be sulfate (SO
2−4). Sulfate is usually incorporated at the carbonate site of the calcite structure (structurally substitute [
48,
49]). In the studied samples of Aedipsos, the distribution of the S presented equal distribution (
Figure 4C) and reached up to 4.19 wt.% SO
3.
The studied samples were found to display mainly lamination (
Figure 4A and
Figure 5A) and shrub (
Figure 5B) facies. These two facies are the most common among the thermogenic travertine deposition of Aedipsos [
30,
33,
34]. Moreover, lamination is the most characteristic facies among the speleothems [
42].
In a few cases, diatoms are trapped in thin laminas, consisting of not-dense micritic crystals (
Figure 5C,D). Similar structures were described in previous studies in the thermogenetic travertine of Aedipsos, and they were attributed to EPS dense net resulting in the retention of calcium carbonate crystals and diatoms [
33,
34]. Moreover, in several cases, zones parallel to the lamination (
Figure 2C) or nest areas were identified, where traces of endolithic Cyanobacteria presence, i.e., holes and grooves, occur.
5.2. Cyanobacteria Diversity
The dominant orders of Cyanobacteria are Synechococcales and Oscillatoriales, while Chroococcales, Nostocales, and Spirulinales follow. Kanellopoulos et al. [
32] studied the diversity of cyanobacterial microflora of NW Euboea Island hot spring depositions. Based on their results, the summarized cyanobacterial microflora of Aedipsos hot springs is dominated by the orders Oscillatoriales (35.7%) and Synechococcales (31.4%), followed by Chroococcales (15.9%), Spirulinales (10.1%), and Nostocales (6.6%), with Chroococcidiopsidales (0.3%) barely present. Thus, in speleothems, the dominant orders are the same as in most of the hot springs of Aedipsos, i.e., Synechococcales and Oscillatoriales. In addition, the Nostocales and Chroococcales are more abound in the speleothems. The Spirulinales are significantly decreased, and Chroococcidiopsidales are totally absent.
It is very interesting that in the same study [
33], the cyanobacterial microflora of the hot spring of the cave and the drainage channel depositions were also studied. In the case of the cave hot spring (T = 49.2 °C, Sal = 20‰, pH = 6.05, only limited access to sunlight), only two orders were identified, i.e., Oscillatoriales (71%) and Synechococcales (29%). While in the samples from the drainage channel, where there is full access to sunlight (T = 43.1–37.2 °C, Sal = 27–24‰, pH = 6.5–6.27), the Oscillatoriales (50–29%) is dominant, followed by Chroococcales (25–12%), Synechococcales (21–19%), Spirulinales (19–11%), Nostocales (11%-not identified), and Chroococcidiopsidales (3%-not identified). Thus, the speleothems present several similarities, but at the same time, also distinct differences concerning the cyanobacterial microflora of the cave hot spring and the drainage channel.
Based on fresh and cultured material, a total number of twenty-nine (29) different Cyanobacteria species, plus diatoms, were identified. By comparing these results with recent extensive studies on the Cyanobacteria diversity of Aedipsos hot spring depositions and the pioneer species [
33,
34], ca. 32% of the identified Cyanobacteria species presented here, do not appear in the Aedipsos travertine deposits (i.e.,
Anabaena cf.
iyengarii, Chroococcus lithophilus, Chroococcus subnudus,
Jaaginema thermale, Oscillatoria subbrevis, Phormidium molischii, Pseudanabaena galeata, Schizothrix cf.
lardacea and
Schizothrix sp.A) indicating the peculiarity of the specific environment.
5.3. Biomineralization Processes
Cyanobacteria biomineralization processes were identified in the outer layer of the samples (
Figure 8). The presence of endolithic cyanobacteria is detrimental for the calcite crystals, i.e., they bore holes and dig channels in the crystals (
Figure 8C–E). These structures could be a result of secretion of acidic substances or EPS.
In some cases, calcified cyanobacterial sheaths were observed in micritic crystals (
Figure 8F). The occurrence of sheath structure could be related to oxygenic photosynthesis, i.e., increase in the pH in the cell vicinity leading to carbonate oversaturation and precipitation [
50,
51], or could be related to the presence of nucleating molecules [
52].
In some cases, filamentous Cyanobacteria, along with EPS, create a dense net resulting in the retention of calcium carbonate crystals (
Figure 8G,H).
The above-mentioned biomineralization processes are similar to other biomineralization processes identified and are described recently in the thermogenic travertine deposits of Aedipsos [
33,
34]. Although, the intensity of the destructive biomineralization processes of the endolithic cyanobacteria are characteristic for the speleothems.
6. Conclusions
Speleothems are secondary mineral deposits formed under extreme conditions. In the present study, samples were collected from a cave environment where a hot spring is spouting in the Aedipsos area (NW Euboea Island, Greece).
The main mineral phase of the samples is calcite, with several trace elements, i.e., up to 0.48 wt.% Na2O, up to 0.73 wt.% MgO, up to 4.19 wt.% SO3, up to 0.16 wt.% SrO and up to 2.21 wt.% Yb2O3. The main faces of the studied stalactites are lamination and shrubs, representing the most common among the faces of the thermogenic travertines of the area.
In the outer stalactite layers, thirty (30) different Cyanobacteria species were identified belonging to the orders Synechococcales (28%), Oscillatoriales (27%), Chroococcales (21%) and Nostocales (21%), and also, Spirulinales (3%). Among the identified taxa, thermophilic species (Spirulina subtilissima) and limestone substrate species (Chroococcus lithophilus, Leptolyngbya perforans and Leptolyngbya ercegovicii) occurred. The ca. 32% of the identified Cyanobacteria species presented here were not found in the Aedipsos travertine deposits.
Based mainly on SEM observations, biomineralization processes were observed in the outer layer of the sample. Similar biomineralization processes were also documented recently in the thermogenic travertine deposits of Aedipsos. The most characteristic biomineralization process of the speleothems is the high-intensity distraction of calcite crystals by endolithic Cyanobacteria. Additionally, in rare cases, calcified cyanobacterial sheaths were found, as well as the presence of filamentous Cyanobacteria and EPS, which create a dense net resulting in the retention of calcium carbonate crystals.
This study highlighted the importance of the geomicrobiological study of speleothems, especially in the extreme environments of hot springs. These sites can be considered as natural labs of unique conditions. Further research ought to be conducted in the area, including additional study sites and DNA metagenomic analysis, in order to fully outline biodiversity in these extreme environments, and the related biomineralization processes.
Author Contributions
Conceptualization C.K., V.L. and A.E.-A.; methodology, C.K., V.L. and A.E.-A.; sampling C.K. and V.L.; biological experiments and assessment A.P., V.L. and A.E.-A.; geological experiments and assessment A.P., C.K., P.V., M.K., I.I. and L.M.; SEM analysis and geobiological assessment A.P., C.K. and V.L.; GIS C.K.; visualization C.K., V.L. and A.P.; writing—original draft C.K. and V.L.; writing—review and editing A.E.-A., C.K. and V.L., P.V., I.I., M.K. and L.M.; supervision A.E.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the local population and authorities, and especially the Director of the Public Properties Company- Aedipsos branch, Ilias Siakantaris, for their cooperation during the fieldwork. The corresponding author would like to thank George Vougioukalakis from the Greek Geological Survey (IGME, present name Hellenic Survey of Geology and Mineral Exploration, HSGME) for his support and encouragement during this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reitner, J. Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia): Formation and Concept. Facies 1993, 29, 3–40. [Google Scholar] [CrossRef] [Green Version]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; p. 324. [Google Scholar]
- Konhauser, K. Introduction to Geomicrobiology; Blackwell Publishing: Oxford, UK; Malden, MA, USA, 2007; 425p. [Google Scholar]
- Friedman, I. Geitleria calcarea n. gen. et n. sp., a new atmophytic lime-encrusting blue-green alga. Bot. Not. 1955, 108, 439–445. [Google Scholar]
- Bourrelly, P.; Depuy, P. Quelques stations françaises de Geitleria calcarea, Cyanophycee cavernicole. Schweiz. Z. Hydrol. 1973, 35, 136–140. [Google Scholar]
- Whitton, B.A. The biology of Rivulariaceae. In The Cyanobacteria—A Comparative Review; Fay, P., van Baalen, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 513–534. [Google Scholar]
- Barton, H.A.; Northup, D.E. Geomicrobiology in cave environments: Past, current and future perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Psomiadis, D.; Dotsika, E.; Albanakis, K.; Ghaleb, B.; Hillaire-Marcel, C. Speleothem record of climatic changes in the northern Aegean region (Greece) from the Bronze Age to the collapse of the Roman Empire. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 489, 272–283. [Google Scholar] [CrossRef]
- Theodorakopoulou, K.; Kyriakopoulos, K.; Athanassas, C.D.; Galanopoulos, E.; Economou, G.; Maniatis, Y.; Godelitsas, A.; Dotsika, E.; Mavridis, F.; Darlas, A. First Speleothem Evidence of the Hiera Eruption (197 BC), Santorini, Greece. Environ. Archaeol. 2021, 26, 336–348. [Google Scholar] [CrossRef]
- Antonelou, A.; Tsikouras, B.; Papoulis, D.; Hatzipanagiotou, K. Investigation of the formation of speleothems in the Agios Georgios cave, Kilkis (N. Greece). Bull. Geol. Soc. Greece 2010, 43, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Ifanti, E. Petrogenetic Processes and Deposition Conditions of Speleothems at Perama Cave. Master’s Thesis, University of Patras, Patras, Greece, 2013. (In Greek). [Google Scholar]
- Anagnostidis, K.; Economou-Amili, A.; Pantazidou, A. Studies on the microflora of the cave Perama, Ioannina, Greece. Bull. Soc. Spéléol. Grece 1982, 18, 458–530. [Google Scholar]
- Lamprinou, V.; Danielidis, D.; Economou-Amili, A.; Pantazidou, A. Distribution survey of Cyanobacteria in three Greek caves of Peloponnese. Int. J. Speleol. 2012, 41, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Lamprinou, V.; Danielidis, D.B.; Pantazidou, A.; Oikonomou, A.; Economou-Amilli, A. The show cave of Diros vs. wild caves of Peloponnese, Greece—Distribution patterns of Cyanobacteria. Int. J. Speleol. 2014, 42, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Lamprinou, V.; Hernandez-Marine, M.; Canals, T.; Kormas, K.; Economou-Amilli, A.; Pantazidou, A. Morphology and molecular evaluation of Iphinoe spelaeobios gen. nov., sp. nov. and Loriellopsis cavernicola gen. nov., sp. nov., two stigonematalean Cyanobacteria from Greek and Spanish caves. Int. J. Syst. Evol. Microbiol. 2011, 61, 2907–2915. [Google Scholar] [CrossRef] [Green Version]
- Lamprinou, V.; Hernández-Mariné, M.; Pachiadaki, M.G.; Kormas, K.A.; Economou-Amilli, A.; Pantazidou, A. New findings on the true-branched monotypic genus Iphinoe (Cyanobacteria) from geographically isolated caves (Greece). Fottea 2012, 13, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Lamprinou, V.; Pantazidou, A.; Papadogiannaki, G.; Radea, C.; Economou-Amili, A. Cyanobacteria and associated invertebrates in Leontari cave. Fottea 2009, 9, 155–164. [Google Scholar] [CrossRef]
- Lamprinou, V.; Skaraki, K.; Kotoulas, G.; Anagnostidis, K.; Economou-Amilli, A.; Pantazidou, A. A new species of Phormidium (Cyanobacteria, Oscillatoriales) from Greek Caves.—Morphological and Molecular Evaluation. Fundam. Appl. Limnol. 2012, 182, 109–116. [Google Scholar] [CrossRef]
- Lamprinou, V.; Skaraki, K.; Kotoulas, G.; Economou-Amilli, A.; Pantazidou, A. Toxopsis calypsus gen. nov., sp. nov. (Cyanobacteria, Nostocales) from cave ‘Francthi’, Peloponnese, Greece—Morphological and molecular evaluation. Int. J. Syst. Evol. Microbiol. 2012, 62, 2870–2877. [Google Scholar] [CrossRef] [Green Version]
- Mountrakis, D. The Pelagonian zone in Greece: A polyphase-deformed fragment of the Cimmerian continent and its role in the geotectonic evolution of the eastern Mediterranean. J. Geol. 1986, 94, 335–347. [Google Scholar] [CrossRef]
- Vavassis, I. Geology of the Pelagonian zone in northern Evia Island (Greece): Implications for the geodynamic evolution of the Hellenides. Ph.D. Thesis, Univ. de Lausanne, Lausanne, Switzerland, 2001. [Google Scholar]
- Jolivet, L.; Faccenna, C.; Huet, B.; Labrousse, L.; Le Pourhiet, L.; Lacombe, O.; Lecomte, E.; Burov, E.; Denèle, Y.; Brun, J.-P.; et al. Aegean tectonics: Strain localization, slab tearing and trench retreat. Tectonophysics 2013, 597–598, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Katsikatsos, G.; Mettos, A.; Vidakis, M.; Bavay, P.; Panagopoulos, A.; Basilaki, A.; Papazeti, E. Geological study of Aedipos Area—Euboea; Geothemal Studies (P.E.C.); IGME: Athens, Greece, 1982. (In Greek) [Google Scholar]
- Scherreiks, R. Platform margin and oceanic sedimentation in a divergent and convergent plate setting (Jurassic, Pelagonian Zone, NE Evvoia, Greece). Int. J. Earth Sci. 2000, 89, 90–107. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Xenakis, M.; Vakalopoulos, P.; Kranis, H.; Christopoulou, M.; Vougioukalakis, G. Seawater-dominated, tectonically controlled and volcanic related geothermal systems: The case of the geothermal area in the northwest of the Island of Euboea (Evia), Greece. Int. J. Earth Sci. 2020, 109, 2081–2112. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Christopoulou, M.; Xenakis, M.; Vakalopoulos, P. Hydrochemical characteristics and geothermometry applications of hot groundwater in Edipsos area, NW Euboea (Evia), Greece. Bull. Geol. Soc. Greece 2016, 50, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Kanellopoulos, C.; Mitropoulos, P.; Valsami-Jones, E.; Voudouris, P. A new terrestrial active mineralizing hydrothermal system associated with ore-bearing travertines in Greece (northern Euboea Island and Sperchios area). J. Geochem. Explor. 2017, 179, 9–24. [Google Scholar] [CrossRef]
- Fytikas, M.; Giuliani, O.; Innocenti, F.; Marinelli, G.; Mazzuoli, R. Geochronological data on recent magmatism of the Aegean Sea. Tectonophysics 1976, 31, T29–T34. [Google Scholar] [CrossRef]
- Kanellopoulos, C. Geochemical research on the distribution of metallic and other elements in the cold and thermal groundwater, soils and plants in Fthiotida Prefecture and N. Euboea. Environmental impact. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2011. (In Greek with English abstract). [Google Scholar]
- Kanellopoulos, C. Distribution, lithotypes and mineralogical study of newly formed thermogenic travertines in Northern Euboea and Eastern Central Greece. Cent. Eur. J. Geosci. 2012, 4, 545–560. [Google Scholar] [CrossRef]
- Kanellopoulos, C. Various morphological types of thermogenic travertines in northern Euboea and Eastern Central Greece. Bull. Geol. Soc. Greece 2013, 47, 1929–1938. [Google Scholar] [CrossRef]
- Kanellopoulos, C. Morphological types, lithotypes, mineralogy and possible bio-mineralization processes in simple and iron-rich travertines from active thermogenic travertine-forming systems in Greece. The cases of Northern Euboea and Eastern Central Greece. In Proceedings of the 19th International Sedimentological Congress, Geneva, Switzerland, 18–22 August 2014; p. 341. [Google Scholar]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Economou-Amilli, A. Insights on the biomineralization processes and related diversity of cyanobacterial microflora in thermogenic travertine deposits in Greek hot springs (North-West Euboea Island). Depos. Rec. 2022, 8, 1055–1078. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Economou-Amilli, A. Pioneer species of Cyanobacteria in hot springs and their role to travertine formation: The case of Aedipsos hot springs, Euboea (Evia), Greece. Depos. Rec. 2022, 8, 1079–1092. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Iliopoulos, I.; Kokkaliari, M.; Moforis, L.; Economou-Amilli, A. Microbial Mat Stratification in Travertine Depositions of Greek Hot Springs and Biomineralization Processes. Minerals 2022, 12, 1408. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Modern approach to the classification system of Cyanophytes 4-Nostocales. Arch. Hydrobiol. Suppl. Bd. Algol. Stud. 1989, 56, 247–345. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, Part 1: Chroococcales. In Süßwasserflora von Mitteleuropa, Bd. 19 (1); Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier GmbH: München, Germany, 1999; pp. 1–548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, Part 2: Oscillatoriales. In Süßwasserflora von Mitteleuropa, Bd. 19 (2); Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier GmbH: München, Germany, 2005; pp. 1–759. [Google Scholar]
- Komárek, J.; Kastovsky, J.; Mares, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera), using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Hauer, T.; Komárek, J. CyanoDB 2.0—On-Line Database of Cyanobacterial Genera; World-Wide Electronic Publication, Univ. of South Bohemia & Inst. of Botany AS CR: České Budějovice, Czechia, 2021; Available online: https://www.cyanodb.cz (accessed on 19 December 2021).
- Fairchild, I.J.; Baker, A. Speleothem Science: From Process to Past Environments; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 432. [Google Scholar]
- Feinberg, J.M.; Johnson, K.R. Cave and Speleothem Science: From Local to Planetary Scales. Elements 2021, 17, 81–86. [Google Scholar] [CrossRef]
- Mucci, A.; Mucci, J.W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Long, X.; Ma, Y.; Qi, L. Biogenic and synthetic high magnesium calcite—A review. J. Struct. Biol. 2014, 185, 1–14. [Google Scholar] [CrossRef]
- Möller, P.; De Lucia, M. Incorporation of Rare Earths and Yttrium in Calcite: A Critical Re-evaluation. Aquat. Geochem. 2020, 26, 89–117. [Google Scholar] [CrossRef] [Green Version]
- Okumura, T.; Kim, H.-J.; Kim, J.-W.; Kogure, T. Sulfate-containing calcite: Crystallographic characterization of natural and synthetic materials. Eur. J. Mineral. 2018, 30, 929–937. [Google Scholar] [CrossRef]
- Kampschulte, A.; Strauss, H. The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Balan, E.; Aufort, J.; Pouillé, S.; Dabos, M.; Blanchard, M.; Lazzeri, M.; Rollion-Bard, C.; Blamart, D. Infrared spectroscopic study of sulfate-bearing calcite from deep-sea bamboo coral. Eur. J. Mineral. 2017, 29, 397–408. [Google Scholar] [CrossRef]
- Riding, R. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic-Cambrian changes in atmospheric composition. Geobiology 2006, 4, 299–316. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Merz-Preiss, M.; Riding, R. Cyanobacterial tufa calcification in two freshwater streams: Ambient environment, chemical thresholds and biological processes. Sediment. Geol. 1999, 126, 103–124. [Google Scholar] [CrossRef]
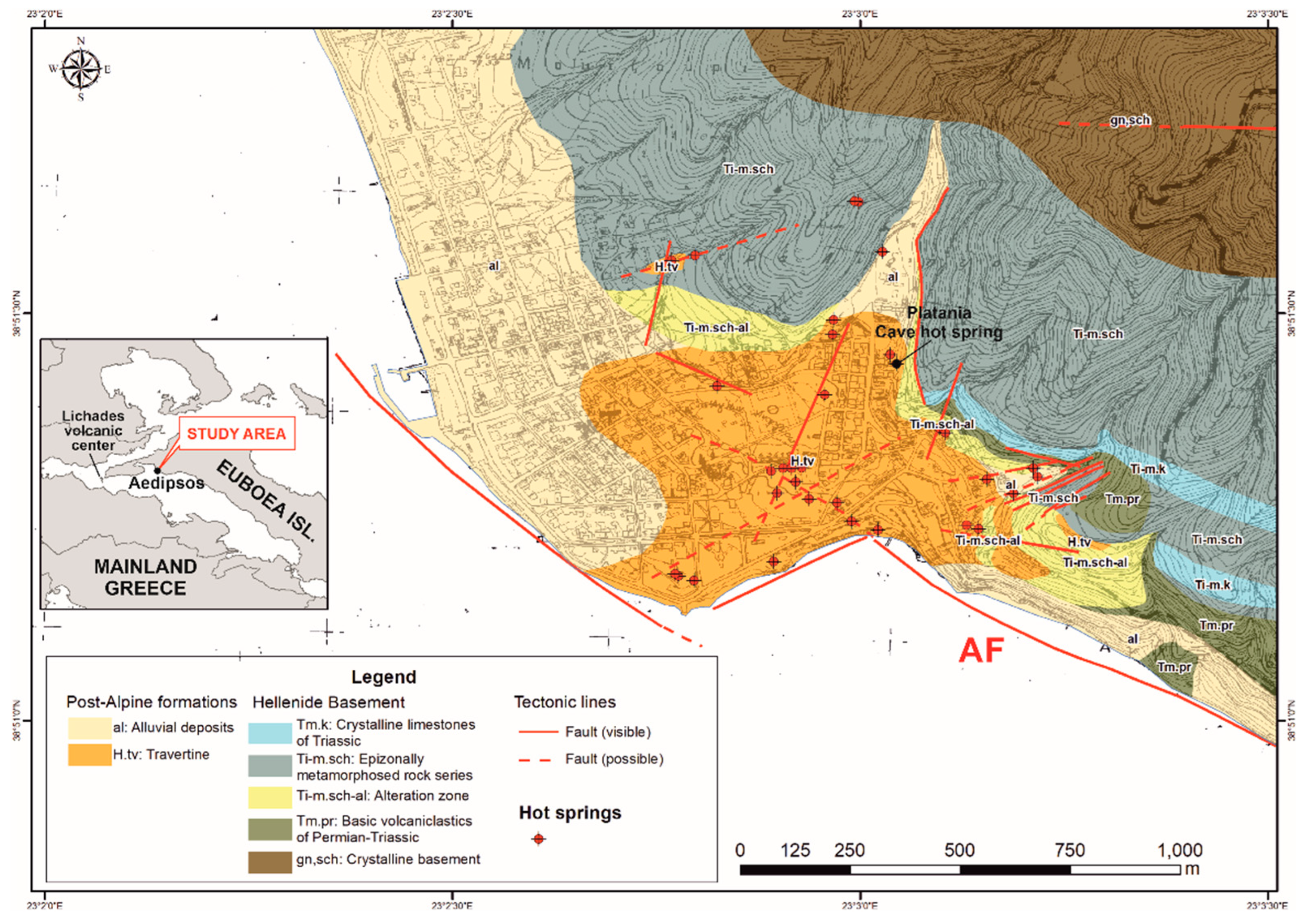
Figure 1.
Geological map of the Aedipos area, NW of Euboea (AF = Aedipsos Fault; modified after Kanellopoulos et al. [
25]). The sampling site is marked with a black dot. The geographical coordinates are in EGSA ‘87.
Figure 1.
Geological map of the Aedipos area, NW of Euboea (AF = Aedipsos Fault; modified after Kanellopoulos et al. [
25]). The sampling site is marked with a black dot. The geographical coordinates are in EGSA ‘87.
Figure 2.
(A) Overview of the study site. (B–E) Paired views of normal images (B,D) and corresponding thermal images (C,E). A column shows the temperature scale (°C) on the right side of the thermal pictures. (B,C) Photo of the stalactites where the samples were collected. (D,E) Overview of the hot spring at the bottom of the cave and some stalactites at the top.
Figure 2.
(A) Overview of the study site. (B–E) Paired views of normal images (B,D) and corresponding thermal images (C,E). A column shows the temperature scale (°C) on the right side of the thermal pictures. (B,C) Photo of the stalactites where the samples were collected. (D,E) Overview of the hot spring at the bottom of the cave and some stalactites at the top.
Figure 3.
Evaluated XRD pattern.
Figure 3.
Evaluated XRD pattern.
Figure 4.
Back-scattered electron images (BSEI) of (A) laminated stalactite from Aedipsos; (B–D) are false color BSEI results of the mapping, displaying the distribution of (B) Ca (green), (C) S (red), and (D) Yb (yellow).
Figure 4.
Back-scattered electron images (BSEI) of (A) laminated stalactite from Aedipsos; (B–D) are false color BSEI results of the mapping, displaying the distribution of (B) Ca (green), (C) S (red), and (D) Yb (yellow).
Figure 5.
Back-scattered electron images (BSEI) presenting (A) laminated facies of stalactite, (B) laminae with shrubs that expand upward by irregular branching, (C) laminae consisting of micritic crystals of calcite and into it diatoms are trapped, (D) false color BSEI, derived from the corresponding black and white BSEI (see (C)), displaying the distribution of Ca (purple), S (yellow) and Si (orange) where the diatoms are distinct, (E,F) holes and grooves in calcite crystals, suggesting the presence of endolithic Cyanobacteria.
Figure 5.
Back-scattered electron images (BSEI) presenting (A) laminated facies of stalactite, (B) laminae with shrubs that expand upward by irregular branching, (C) laminae consisting of micritic crystals of calcite and into it diatoms are trapped, (D) false color BSEI, derived from the corresponding black and white BSEI (see (C)), displaying the distribution of Ca (purple), S (yellow) and Si (orange) where the diatoms are distinct, (E,F) holes and grooves in calcite crystals, suggesting the presence of endolithic Cyanobacteria.
Figure 6.
Pie diagrams presenting the percentage of each Cyanobacteria order.
Figure 6.
Pie diagrams presenting the percentage of each Cyanobacteria order.
Figure 7.
Cyanobacterial microflora under an optical microscope. (A) 9- Brasilonema cf. angustatum (Scale bar: 50 μm), (B) Chroococcus cf. mediocris (Scale bar: 50 μm), (C) Chroococcus subnudus (Scale bar: 20 μm), (D) Jaaginema thermale (Scale bar: 20 μm), (E) Kamptonema formosum (Scale bar: 20 μm), (F) Leptolyngbya ercegovicii (Scale bar: 10 μm), (G) Nostoc punctiforme (Scale bar: 20 μm), (H) Oscillatoria crassa (Scale bar: 10 μm), (I) Oscillatoria subbrevis (Scale bar: 40 μm), (J) Oxynema acuminatum (Scale bar: 50 μm), (K) Phormidium cf. abronema (Scale bar: 20 μm), (L) Spirulina subtilissima (Scale bar: 10 μm).
Figure 7.
Cyanobacterial microflora under an optical microscope. (A) 9- Brasilonema cf. angustatum (Scale bar: 50 μm), (B) Chroococcus cf. mediocris (Scale bar: 50 μm), (C) Chroococcus subnudus (Scale bar: 20 μm), (D) Jaaginema thermale (Scale bar: 20 μm), (E) Kamptonema formosum (Scale bar: 20 μm), (F) Leptolyngbya ercegovicii (Scale bar: 10 μm), (G) Nostoc punctiforme (Scale bar: 20 μm), (H) Oscillatoria crassa (Scale bar: 10 μm), (I) Oscillatoria subbrevis (Scale bar: 40 μm), (J) Oxynema acuminatum (Scale bar: 50 μm), (K) Phormidium cf. abronema (Scale bar: 20 μm), (L) Spirulina subtilissima (Scale bar: 10 μm).
Figure 8.
Biomineralizing processes by Cyanobacteria under SEM. (A) Vertical cut section surface of stalactite. (B) Detailed view of the outer periphery of stalactite, presenting fluvial crust with characteristic zoning and filaments of the endolithic Leptolyngbya perforans and L. ercegoviccii destroying the stalactite; whereas the upper zone is covered by granular epilithic species such as Chroococcus lithophilus. (C,D) Calcite crystals with distinct holes and dig channels are occupied by filamentous Cyanobacteria. (E) Filamentous Cyanobacteria of the endolithic Leptolyngbya perforans are coming out of calcite crystal (blue arrow) and calcified sheaths of Cyanobacteria filaments (red arrow). (F) Calcified sheaths of Cyanobacteria filaments by micritic calcium carbonate crystals. (G) Retention of calcium carbonate crystals by filamentous Cyanobacteria. (H) EPS along with filaments.
Figure 8.
Biomineralizing processes by Cyanobacteria under SEM. (A) Vertical cut section surface of stalactite. (B) Detailed view of the outer periphery of stalactite, presenting fluvial crust with characteristic zoning and filaments of the endolithic Leptolyngbya perforans and L. ercegoviccii destroying the stalactite; whereas the upper zone is covered by granular epilithic species such as Chroococcus lithophilus. (C,D) Calcite crystals with distinct holes and dig channels are occupied by filamentous Cyanobacteria. (E) Filamentous Cyanobacteria of the endolithic Leptolyngbya perforans are coming out of calcite crystal (blue arrow) and calcified sheaths of Cyanobacteria filaments (red arrow). (F) Calcified sheaths of Cyanobacteria filaments by micritic calcium carbonate crystals. (G) Retention of calcium carbonate crystals by filamentous Cyanobacteria. (H) EPS along with filaments.
Table 1.
Representative microanalyses of calcite.
Table 1.
Representative microanalyses of calcite.
| No. | I | II | III | IV | V | VI | VII | VII | IX | X |
|---|
| Na2O | 0.34 | 0.32 | 0.31 | 0.24 | - | 0.19 | 0.22 | 0.28 | 0.34 | 0.48 |
| MgO | 0.59 | 0.59 | 0.71 | 0.31 | 0.5 | 0.71 | 0.51 | 0.6 | 0.61 | 0.53 |
| SO3 | 3.54 | 2.79 | 2.33 | 3.13 | 0.73 | 1.95 | 2.61 | 2.3 | 4.19 | 2.71 |
| CaO | 51.5 | 52.41 | 53.66 | 52.38 | 53.54 | 52.59 | 52.77 | 53.11 | 52.72 | 51.92 |
| SrO | - | - | - | 0.16 | - | - | 0.16 | - | - | - |
| Yb2O3 | 1.79 | 2.04 | 2.08 | 1.87 | 2.21 | 1.92 | 2.05 | 1.97 | 2 | 1.9 |
| Total | 57.76 | 58.16 | 59.09 | 58.09 | 56.98 | 57.36 | 58.31 | 58.27 | 59.86 | 57.54 |
Table 2.
Identified Cyanobacteria species.
Table 2.
Identified Cyanobacteria species.
| Anabaena cf. iyengarii Bharadwaja 1935 |
| Brasilonema cf. angustatum M.A.Vaccarino & J.R.Johansen 2012 |
| Chroococcus cf. mediocris N.L.Gardner 1927 |
| Chroococcus lithophilus Ercegovic 1925 |
| Chroococcus occidentalis (N.L.Gardner) Komárek & Komárková-Legnerová 2007 |
| Chroococcus subnudus (Hansgirg) G.Cronberg & J.Komárek 1994 |
| Cyanocohniella calida J.Kastovský, E.Berrendero, J.Hladil & J.R.Johansen 2014 |
| Gloeocapsa gelatinosa Kützing 1843 |
| Jaaginema thermale Anagnostidis 2001 |
| Kamptonema formosum (Bory ex Gomont) Strunecký, Komárek & J.Smarda 2014 |
| Leptolyngbya ercegovicii (Cado) Anagnostidis & Komárek 1988 |
| Leptolyngbya foveolara (Gomont) Anagnostidis & Komárek 1988 |
| Leptolyngbya perforans (Geitler) Anagnostidis & Komárek 1988 |
| Leptolyngbya sp.C |
| Nostoc punctiforme Hariot 1891 |
| Nostoc sp.B |
| Nostoc sp.C |
| Nostocaceae |
| Oscillatoria crassa (C.B.Rao) Anagnostidis 2001 |
| Oscillatoria sp.B |
| Oscillatoria subbrevis Schmidle 1901 |
| Oxynema acuminatum (Gomont) Chatchawan, Komárek, Strunecky, Smarda & Peerapornpisal 2012 |
| Phormidium acidophilum J.J.Copeland 1936 |
| Phormidium cf. abronema Skuja, 1901 |
| Phormidium molischii (Vouk) Anagnostidis & Komárek 1988 |
| Pseudanabaena galeata Böcher 1949 |
| Schizothrix cf. lardacea Gomont 1892 |
| Schizothrix sp.A |
| Diatoms |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).