Abstract
To evaluate the effects of antifouling paints and biocides on larval settlement and metamorphosis, newly hatched swimming larvae of the compound ascidian Botryllus schlosseri, a dominant species of soft-fouling in coastal communities, were exposed to (i) substrata coated with seven antifouling paints on the market containing different biocidal mixtures and types of matrices and (ii) sea water containing various concentrations of eight biocidal constituents. All antifouling paints showed high performance, causing 100% mortality and metamorphic inhibition, with ≥75% not-settled dead larvae. All antifouling biocides prevented the settlement of larvae. The most severe larval malformations, i.e., (i) the formation of a bubble encasing the cephalenteron and (ii) the inhibition of tail resorption, were observed after exposure to metal and organometal compounds, including tributyltin (TBT) at 1 μM (325.5 µg L−1), zinc pyrithione (ZnP) at 1 μM (317.7 µg L−1), and CuCl at 0.1 μM (98.99 µg L−1), and to antimicrobials and fungicides, including Sea-Nine 211 at 1 μM (282.2 µg L−1) and Chlorothalonil at 1 μM (265.9 µg L−1). The herbicides seemed to be less active. Irgarol 1051 was not lethal at any of the concentrations tested. Diuron at 250 μM (58.2 mg L−1) and 2,3,5,6-tetrachloro-4-(methylsulphonyl)pyridine (TCMS pyridine) at 50 μM (14.8 mg L−1) completely inhibited larval metamorphosis. These results may have important implications for the practical use of different antifouling components, highlighting the importance of their testing for negative impacts on native benthic species.
1. Introduction
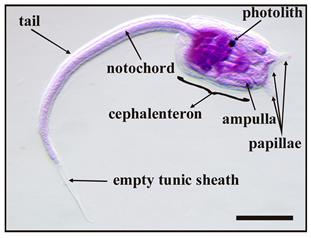
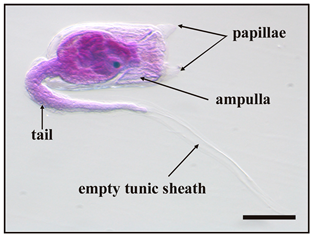
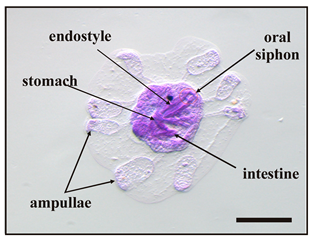
Ascidians are the most common members of the urochordate subphylum. They are the closest relatives to vertebrates, and are the only chordates able to reproduce both sexually and asexually [1]. During the larval stage, known as the “tadpole” stage, they share with vertebrates the same body plan in the tail formed of a dorsal notochord and a tubular nervous system, both flanked by striated muscle. They are sessile occupants of hard substrata in coastal environments, where they often represent the dominant component of soft-fouling. As worldwide filter-feeding organisms living at the water–sediment interface, many solitary and compound species are considered useful bioindicators of various environmental pollutants. The developmental biology of ascidians with particular attention to both the larval stage, which is the dispersal and colonisation stage of the life cycle, and its metamorphosis have been studied extensively [2,3,4,5]. After a short dispersal period, during which the ascidian larvae actively swim, moving the tail according to a positive phototropism and a negative geotropism, the larvae select a substratum and attach to it following a photo- and geotropism reversal. They explore and contact the substratum by means of a series of rapid touches with the anterior area of the cephalenteron, which contains special sensory structures, namely “papillae”. These structures, protruding from the anterior epidermis and from which an adhesive substance is secreted, act as a control centre for the initiation of metamorphosis and are fundamental for larval settlement. Recently, many inducers of metamorphosis have been identified in papillae as specific gene expressions, growth factors, several neurotransmitters, and transient Ca2+ signals [6,7,8]. The temporary settlement with papillae is rapidly substituted by a stable settlement due to the protrusion of anterior blind-sac vessels, namely “ampullae”, which expand onto the substratum and secrete a glue substance from an apical glandular epithelial. At the same time, tail resorption occurs with complete dismantling of the axial complex. Successively, metamorphosis continues inside the cephalenteron with the visceral rotation and the dismantling of other larval structures, such as ocellus and statocyst. Finally, the adult organ primordia complete the development, and the opening of both siphons occurs and the juvenile begins to filter-feed.
Generally, ascidians are pre-disposed to rapid and competitive colonisation of hard substrata. However, geographic invasion and the impact of fouling by ascidians on shipping, aquaculture, pleasure boating, oil and gas installations, and other industries are significant, with numerous species responsible for infesting anthropogenic structures [9]. Although the larval phase is short (24–48 h), the global spread is favoured by translocation via shipping since the hull fouling can be considered the most important vector. The massive ascidian fouling is potentially responsible for physical, economical, and ecological damage since it concerns the coverage of clean surfaces, loss of efficiency of submerged pipelines and harbour/industrial structures, infestation of shellfish and finfish aquacultures, increased fuel consumption during boat navigation, and decreased biodiversity in benthic communities.
For these reasons, beginning from the second half of the 1960s, antifouling compounds have been massively introduced in the formulation of paints to prevent the settlement of the most problematic foulers on submerged structures, such as ship’s hulls and propellers, buoys, wharves, and platforms. The most successful antifouling paints at the time contained organotin compounds as biocides, mainly represented by tributyltin (TBT), triphenyltin (TPT), and their derivatives. These compounds proved to be harmful to the benthic marine communities, as they caused severe impacts on the oyster aquaculture and were persistent in the environment in the long term [10,11,12,13]. After the total ban on organotin compounds by the International Marine Organisation—Marine Environment Protection Committee (IMO-MEPC) in 1998, and subsequently by the Ordinance No. 782/2003, 14 April 2003, of the European Commission, the paint industry developed substitutive tin-free formulations. The new paint formulations mainly contained biocidal combinations of specific synthesis, e.g., Sea-Nine 211, or pesticides coming from the pharmacology industry (antimicrobials) or agriculture (herbicides, fungicides, insecticides). The aim of these formulations was not only to prevent the settlement of algal propagules and invertebrate larvae of macrofoulers, but also the formation of bacterial and microalgal microfilm, or “biofilm”, from which the ecological succession of the hard-substratum community begins.
Because of this choice, a number of substances (Table 1) are at present in various commercial formulations of new-generation antifouling paints. The biocidal compounds play various roles, i.e., as alternatives to organotin compounds or as boosters, with the latter serving to increase the toxic performance of the antifouling paints towards a wider spectrum of fouling organisms. Before the introduction of these compounds in paint formulations, tests of acute and chronic toxicity were performed only on laboratory mammals and freshwater model fish. As a consequence, many new contaminants with potential accumulation and deleterious effects on coastal communities have been introduced worldwide from both direct and indirect pollution sources, which have followed the increase in productivity of agro-industrial, tourism, and commercial shipping sectors.

Table 1.
Common biocidal substances used in formulations of antifouling paints in EU countries.
Recently, toxic effects on aquatic organisms have arisen, with mechanisms of action involving various cell targets [14]. The risk assessment of these emerging contaminants in marine ecosystems is now a priority due to the continuous uncontrolled leaching from antifouling paints and the synergistic interactions, which could affect the primary production and the survival and reproduction of marine fish and invertebrates [15,16,17,18].
Organotin compounds and a few alternative biocides are known to provoke embryotoxicity in solitary ascidians such as Styela plicata and Ciona intestinalis [19,20]. These species are oviparous and both the spawned gametes and embryos might be exposed to biocides in the water column, with important effects on fertilisation and offspring. At present, no study has been performed on compound ascidians, which are ovoviviparous [21,22,23], and therefore, as a difference from solitary ascidians, only hatched larvae might be exposed. They can be considered a better model than the solitary ascidians for the evaluation of the effects on post-embryonic stages, settlement, and metamorphosis.
Botryllus schlosseri, commonly called the star ascidian, earns its name from the stellate colony formed by a group of asexually reproducing individuals. It is a cosmopolitan compound species, is easy to collect, and breed in aquaria. In peculiar ecosystems with transitional waters such as the Lagoon of Venice, during autumn, more than 90% of the community comprises solitary and colonial ascidians, predominantly botryllids (Botryllus schlosseri and Botrylloides leachii), forming a stable biocoenosis described as a “Botryllus community” [24]. The organism has recently emerged as a simple and important model species for morphogenesis, regeneration, allorecognition, and apoptosis and for studying a variety of biological problems, such as comparative immunobiology, sexual and asexual reproduction, stem cell differentiation, and regeneration [25,26,27].
In the present study, the compound ascidian B. schlosseri was used as an experimental model for the evaluation of the effects of (i) TBT-based and tin-free (alternative to TBT) antifouling paints and (ii) antifouling biocides on larval viability, settlement, and metamorphosis. The biocides considered have been distinguished into three groups: (i) metals and organometals, i.e., CuCl, TBT, and zinc pyrithione (ZnP); (ii) antimicrobials and fungicides, i.e., Sea-Nine 211 and Chlorothalonil; and (iii) herbicides, i.e., Irgarol 1051, Diuron, 2,3,5,6-tetrachloro-4-(methylsulphonyl)pyridine (TCMS pyridine). Experiments were performed in two steps by exposing larvae to substrata coated with seven trade antifouling paints containing mixtures of the above-reported biocides and two different types of matrices (contact-leaching or self-polishing), and to various concentrations of each type of biocide. Different antifouling performances and metamorphic abnormalities have also been considered, and the mechanisms of actions of larval toxicity have been proposed.
2. Materials and Methods
2.1. Larval Collection
B. schlosseri reproductive colonies were collected in spring (April–May) from the southern basin of the Lagoon of Venice and transferred to large aquaria, in which they were reared on glass slides in aerated filtered sea water (FSW) (salinity of 35 ± 1 psu, temperature of 19 ± 0.5 °C, pH 8.1). A semi-static system was used for animal maintenance, with seawater renewal every other day, and colonies were fed with Microbe-Lift®/Phyto-Plus B (Ecological Laboratories, Inc., Cape Coral, FL, USA) and microalgae (Isochrysis galbana). Newly hatched swimming larvae were easily identified under a dissection binocular stereomicroscope Wild Heerbrugg with 50× maximum magnification. They were immediately collected with a glass micropipette, counted, and temporarily transferred to a 30 mL glass-evaporating dish.
Five main stages were identified during metamorphosis, from the beginning of settlement to fully metamorphosed oozoids, as listed in Table 2.

Table 2.
Main stages of the metamorphosis of B. schlosseri larva 1.
2.2. Antifouling Paints
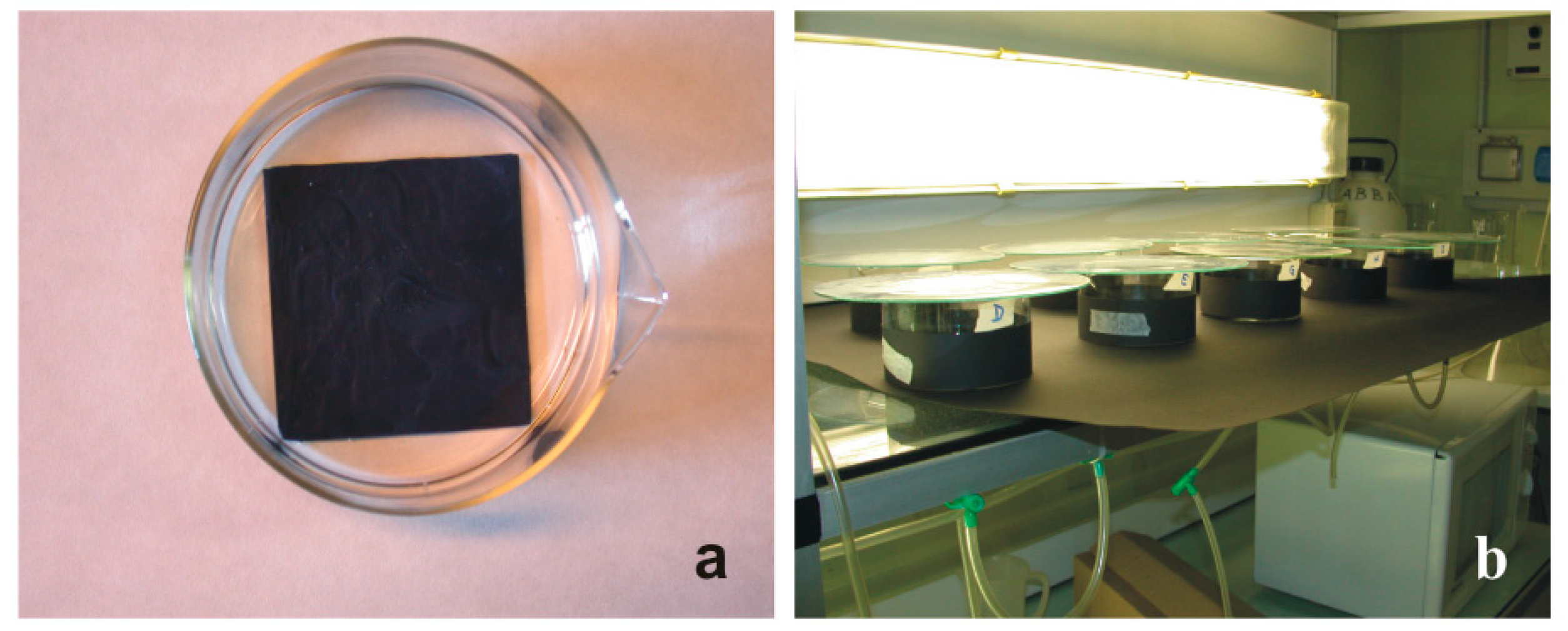
In the first series of experiments, seven (A–G) antifouling paints were tested (Table 3) with two replicates for a total exposure of 700 larvae. In each replicate, 50 swimming larvae were put into a glass-crystallising dish filled with 200 mL of FSW and with a 7.5 × 7.5 × 0.15 cm glass plate on the bottom that was previously coated with an antifouling paint. During exposure, the glass containers were covered outside and on the bottom with a black paper simulating a dark substratum, which favoured the settlement of larvae with positive geotropism and negative phototropism (Figure 1). The exposure occurred at 22 °C under artificial light until all larvae (100%) metamorphosed (48 h) in the reference glass-crystallising dishes, the latter without a painted plate on the bottom.

Table 3.
Antifouling paints used to coat the glass plates for the assays of larval settlement and metamorphosis.
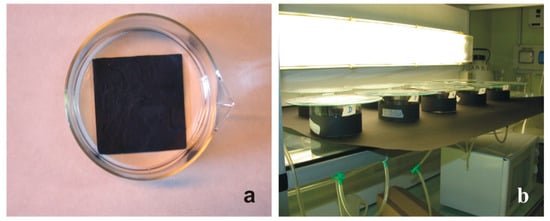
Figure 1.
Experimental set-up for the evaluation of the settlement and metamorphosis ability of B. schlosseri larvae. (a) Glass-crystallising dish with a plate coated with an antifouling paint on the bottom. (b) Arrangement of the various glass-crystallising dishes in the thermostatic room at 22 °C.
2.3. Antifouling Biocides
In the second series of experiments, eight biocidal compounds were separately tested with three different concentrations and two replicates for a total exposure of 2400 larvae. For every replicate of biocidal concentration, 50 larvae were incubated in a glass-crystallising dish filled with 200 mL of biocidal solution in FSW. Concentration ranges of biocidal solutions were chosen based on previous studies of immunotoxicity in vitro on the haemocytes of this species [28,29,30,31,32]. The biocides considered were tributyltin (TBT) as monochloride (Sigma-Aldrich, Burlington, MA, USA), zinc pyrithione (ZnP, Sigma-Aldrich), copper(I) chloride (CuCl, purified, >99%, Sigma-Aldrich), Sea Nine 211 (Rohm & Haas, Philadelphia, PA, USA), Chlorothalonil (Fluka), Irgarol 1051 (Riedel-de Haën GmbH, Seelze, Germany), Diuron (Sigma-Aldrich), and TCMS pyridine (Avecia, Manchester, UK).
Stock solutions were prepared at the nominal concentrations of 1 mM in FSW for CuCl; 10 mM in 95% ethanol for TBT, Sea Nine 211, Diuron, and TCMS pyridine; and 10 mM in dimethylsulfoxide (DMSO purum; >99%, Fluka Chemie Gmbh, Buchs, Switzerland) for ZnP, Chlorothalonil, and Irgarol 1051. They were freshly diluted to working concentrations in FSW as follows: 0.1, 1, 10 μM for TBT (corresponding to 32.5, 325.5, 3255 µg L−1); 0.1, 0.5, 1 µM for ZnP (corresponding to 31.7, 158.5, 317.7 µg L−1 and 6.5 × 10−7, 3.2 × 10−6, 6.5 × 10−6 Zn wt%); 0.01, 0.1, 1 μM for CuCl (corresponding to 9.8, 98.99, 989.9 µg L−1 and 1.8 × 10−6, 1.8 × 10−5, 1.8 × 10−4 Cu wt%); 0.1, 1, 10 µM for Sea-Nine 211 (corresponding to 28.2, 282.2, 2822 µg L−1); 0.1, 1, 10 μM for Chlorothalonil (corresponding to 26.5, 265.9, 2659 µg L−1); 50, 100, 200 μM for Irgarol 1051 (corresponding to 12.6, 25.3, 50.6 mg L−1); 100, 250, 500 μM for Diuron (corresponding to 23.3, 58.2, 116.5 mg L−1); and 25, 50, 75 μM for TCMS pyridine (corresponding to 7.4, 14.8, 22.2 mg L−1). In controls, larvae were incubated with FSW containing the maximum solvent concentration employed in the experiments with biocides, i.e., 0.02% DMSO or 0.01% 95%-ethanol. These concentrations had no effect on survival or metamorphosis. After all larvae (100%) unexposed to biocides (48 h at 22 °C) reached the oozoid stage, the number of living (motile) and dead (immotile) larvae were recorded, as well as the metamorphosis stage reached (developmental delay) and the presence of abnormalities under a Leica MZ16F stereomicroscope.
2.4. Statistical Analysis
Data are reported as the mean percentage ± standard deviation (SD). The statistical analysis was performed with IBM SPSS Statistics v. 25 software. The probit method was used to calculate the median lethal concentration (LC50) and the median effective concentration (EC50), the latter defined here as the toxicant concentration that reduced normal larvae by 50%, considering both the larval settlement and metamorphosis as endpoints and their 95% confidence intervals. Significant differences (p < 0.05) (i) between the control group and test concentrations, (ii) between the control group and test paints, and (iii) among the test concentrations or the paint groups were evaluated with one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test and Dunnett’s multiple comparison test. In the case of values expressed as percentages, the raw data were analysed after arcsine transformation to achieve normality.
In the experiments of exposure to antifouling paints and biocides, a clustering analysis with average linkage between groups was used to obtain hierarchy dendrograms after a χ2 test with Yates’ p-value correction applied on the contingency table. Regarding the experiments of exposure to antifouling paints, the basic criterion of this test is to verify if the seven paints are significantly associated with different outcomes. Since it leads to rejecting the null hypothesis of independence between paints and outcomes, the clustering analysis can be applied to determine which paints can be considered similar and then grouped into clusters. In the case of the χ2 test for the evaluation of the different outcomes related to the concentrations of the eight biocidal compounds, only Irgarol 1051 did not give significantly (p = 0.999) different outcomes at different concentrations. For all the other biocides, the different concentrations were significantly (p < 0.001) associated with different outcomes. For the combinations of biocides and concentrations, clustering analysis groups similar objects in clusters that are most homogeneous within them and most heterogeneous among them.
3. Results and Discussion
3.1. Effects of Antifouling Paints
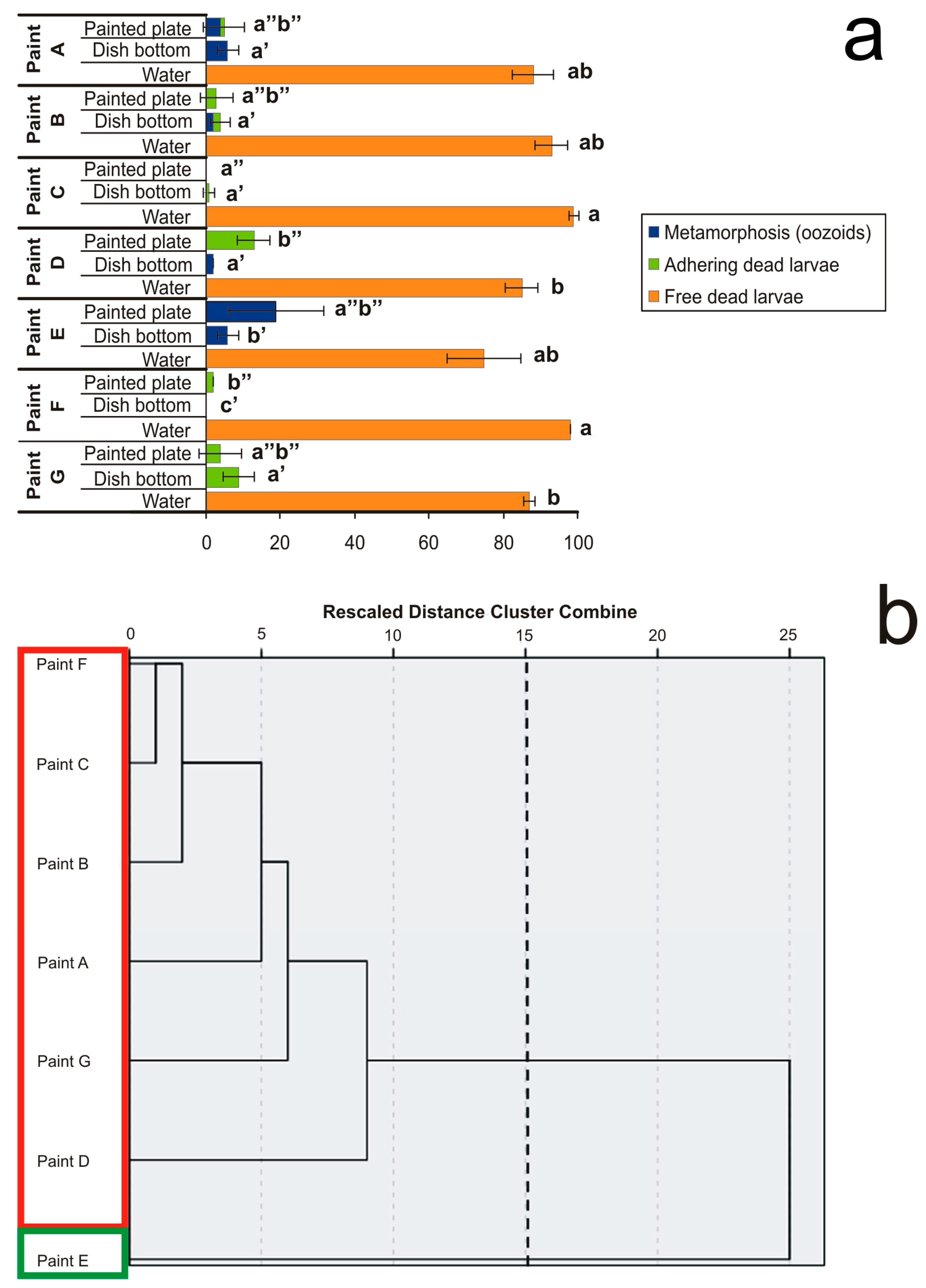
In the experiments with plates coated with seven antifouling paints, larvae were placed inside glass-crystallising dishes filled with sea water and with a coated plate on the bottom. At the end of exposure, considered when all control larvae in filtered sea water metamorphosed forming completed filter-feeding oozoids (100% survival rate), three effects were evaluated (Figure 2a): (i) complete metamorphosis with the oozoid formation, (ii) settled but dead larvae, and (iii) not-settled but free-floating dead larvae. These effects were also evaluated in three different conditions: (i) individuals floating in the sea water, (ii) individuals settled on the dish’s glass bottom surrounding the coated plate, and (iii) individuals settled on the coated paint.
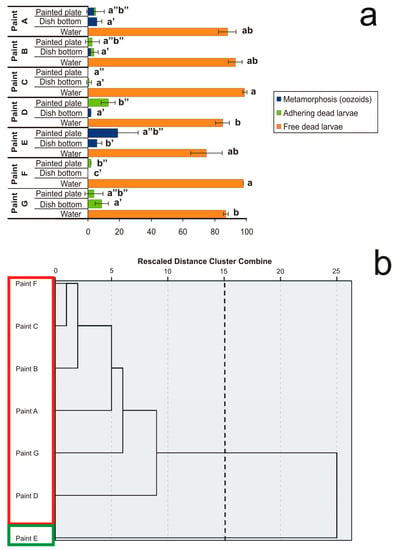
Figure 2.
Effects of plates coated with seven antifouling paints on larval settlement and metamorphosis observed after exposure until all control larvae metamorphosed in the reference glass-crystallising dishes forming filter-feeding oozoids. (a) Percentages of three effects on metamorphosis, i.e., (i) complete metamorphosis with oozoid formation (blue), (ii) settled but dead larvae (green), and (iii) not-settled but free-floating dead larvae (orange). Larval mortality and abnormality were considered in three conditions: (i) individuals floating in the sea water (“water”), (ii) individuals adhering to the dish glass bottom peripheral to the painted plate (“dish bottom”), and (iii) individuals settled on the antifouling-coated plate (“painted plate”). Significant (p < 0.05) differences inside each of the three conditions obtained with Dunnett’s test are expressed with different letters. (b) Dendrogram obtained from cluster analysis with average linkage between groups. The cutting point at 15 corresponds to the maximum margin showing two clusters bordered by different colours.
All antifouling paints demonstrated a remarkable performance because they caused both high mortality and inhibition of metamorphosis. Paints containing CuSCN + ZnP + Diuron + Sea-Nine 211 (Paint C) and Cu2O + Chlorothalonil + Irgarol 1051 (Paint F) prevented larval adhesion. Paints containing ZnP + Zineb + Endosulfan (Paint B), CuSCN + Diuron + Sea-Nine 211 (Paint D), and TCMS pyridine + Diuron (Paint G) allowed minor larval adhesion, but prevented metamorphosis. Paints containing TBT (Paint A) or Cu2O alone (Paint E) occasionally allowed adhesion and metamorphosis, but killed the oozoids. It is noteworthy that the antifouling components generally do not act only through direct contact—the presence of dead and partially metamorphosed larvae, both free-floating and adhering to the glass surrounding the painted plates, suggests a certain degree of leaching of the biocides from the antifouling paints. A large number of free-floating dead larvae were observed in all cases corresponding to ≥75% with small numbers of settled larvae, which died without completing metamorphosis (Figure 2a). Nevertheless, the analysis of variance did not permit us to clearly distinguish the different effects of the various antifouling paints.
From the hierarchy dendrogram obtained from the clustering analysis with average linkage between groups (Figure 2b), only two clusters emerged. These clusters represent the mean percentages of five variables, i.e., (i) free-floating dead larvae in sea water, (ii) oozoids on the dish glass bottom, (iii) dead larvae settled on the dish glass bottom, (iv) oozoids on the coated plate, and (v) dead larvae settled on the coated plate.
The first cluster only includes Paint E with 75% of free-floating larvae, 19% of oozoids on the coated plate, and 6% of oozoids on the dish glass bottom. The second cluster is represented by all the other paints with 91.7% of free-floating larvae, 3.8% and 2% of dead larvae settled on the coated plate and on the dish-glass bottom, respectively, and 1.7% and 0.7% of oozoids adhering to the dish glass bottom and to the coated plate, respectively. However, these results are difficult to interpret because the toxic effects are similar. The only relevant consideration is that Paint E, which showed a lower toxic effect than the other paints, is a unique antifouling paint without booster biocides and contains the highest amount of CuCl (41%). This concentration is similar to that of Paint F, which also contained Chlorothalonil and Irgarol 1051 as booster biocides and is based on self-polishing technology rather than on an insoluble matrix. In conclusion, the interactions of a variety of principal and booster biocides on one hand and different matrix technologies on the other hand are complex in commercial paints. The leaching rate of biocides from the paints, which is fundamental for the risk assessment, is often not reported in manufacturers’ datasheets. It varies with the mixture of concentrations of biocides in the paint formulation and depends on the interaction with boosters and other additives; environmental abiotic parameters such as temperature, salinity, and pH; and the type of matrix [33,34].
Generally, the most commonly used approach for understanding the action of antifouling compounds is the direct exposure of embryos or larvae of marine invertebrates to various concentrations of a single biocide in sea water with controlled parameters, totally excluding the interaction of matrix, pigments, solvents, and other additives [35,36,37,38,39,40,41]. In this way, larval toxicity and the effects on metamorphosis can be clearly observed, elucidating the possible mechanisms of action at both cellular and subcellular levels.
3.2. Effects of Antifouling Biocides
At the end of the experiments, all control larvae in filtered sea water metamorphosed, forming complete filter-feeding oozoids (100% survival rate). The order of toxicity of the antifouling biocides assayed with the evaluation of the median lethal and median effective concentrations (LC50 and EC50) able to cause mortality and inhibition of larval settlement/metamorphosis, respectively, in B. schlosseri (Table 4) is CuCl > ZnP > TBT > Chlorothalonil > Sea Nine 211 > TCMS pyridine > Irgarol 1051 > Diuron.

Table 4.
Median lethal concentrations (LC50) of antifouling biocides on Botryllus schlosseri larvae and median effective concentrations (EC50) on larval settlement and metamorphosis as endpoints after 48 h exposure at 20–22 °C to antifouling biocides compared with data reported for the solitary ascidian Ciona intestinalis.
Both the LC50 and EC50 found for B. schlosseri are higher than the environmental concentrations reported in the literature, with the exception of ZnP and CuCl. The concentration ranges known in the sea water column are <0.02–31.7 μg L−1 for ZnP [45], 0.15–26 μg L−1 for CuCl [46,47], <0.001–3.3 μg L−1 for Sea Nine 211, <0.01–1.4 μg L−1 for Chlorothalonil, <0.001–1.7 μg L−1 for Irgarol 1051, and <0.001–6.7 μg L−1 for Diuron [18]. B. schlosseri appears to be more sensitive to both ZnP and CuCl than the solitary ascidian Ciona intestinalis [43,44]. On the contrary, C. intestinalis is about 4× and 1.5× more sensitive than B. schlosseri in terms of the reduction in larval settlement by 50% in the presence of TBT [42] and Sea-Nine 211 or Chlorothalonil [37], respectively, whereas the toxicity concerning Irgarol 1051 is similar [37]. Generally, CuCl and ZnP showed the highest toxicity, causing 50% larval mortality at 0.35 and 0.46 μM (34.6 and 146 μg L−1), respectively, and reducing the larval settlement and metamorphosis by 50% at 0.04 and 0.06 μM (3.96 and 19 μg L−1), respectively. This fact supports the hypothesis that the CuCl and ZnP concentrations usually employed in antifouling paints are too high for their goal and the continuous leaching of these compounds is of great concern considering their impact on coastal ecosystems, particularly the possible long-term negative effects on non-target benthic organisms. Therefore, restrictions and bans regarding the use of copper- and zinc-based antifouling paints are essential for the development of “eco-friendly” antifouling paints [48].
The clustering analysis with average linkage between groups was performed on 25 objects representing the linkages of biocides and test concentrations (Figure 3).
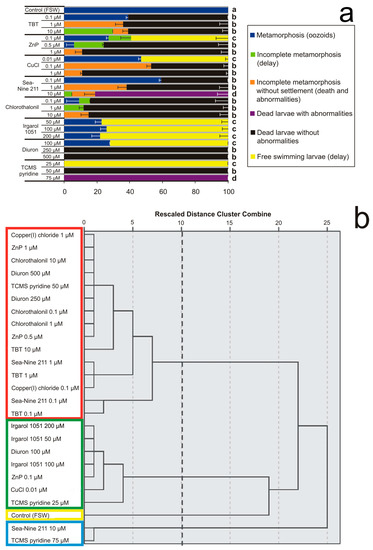
Figure 3.
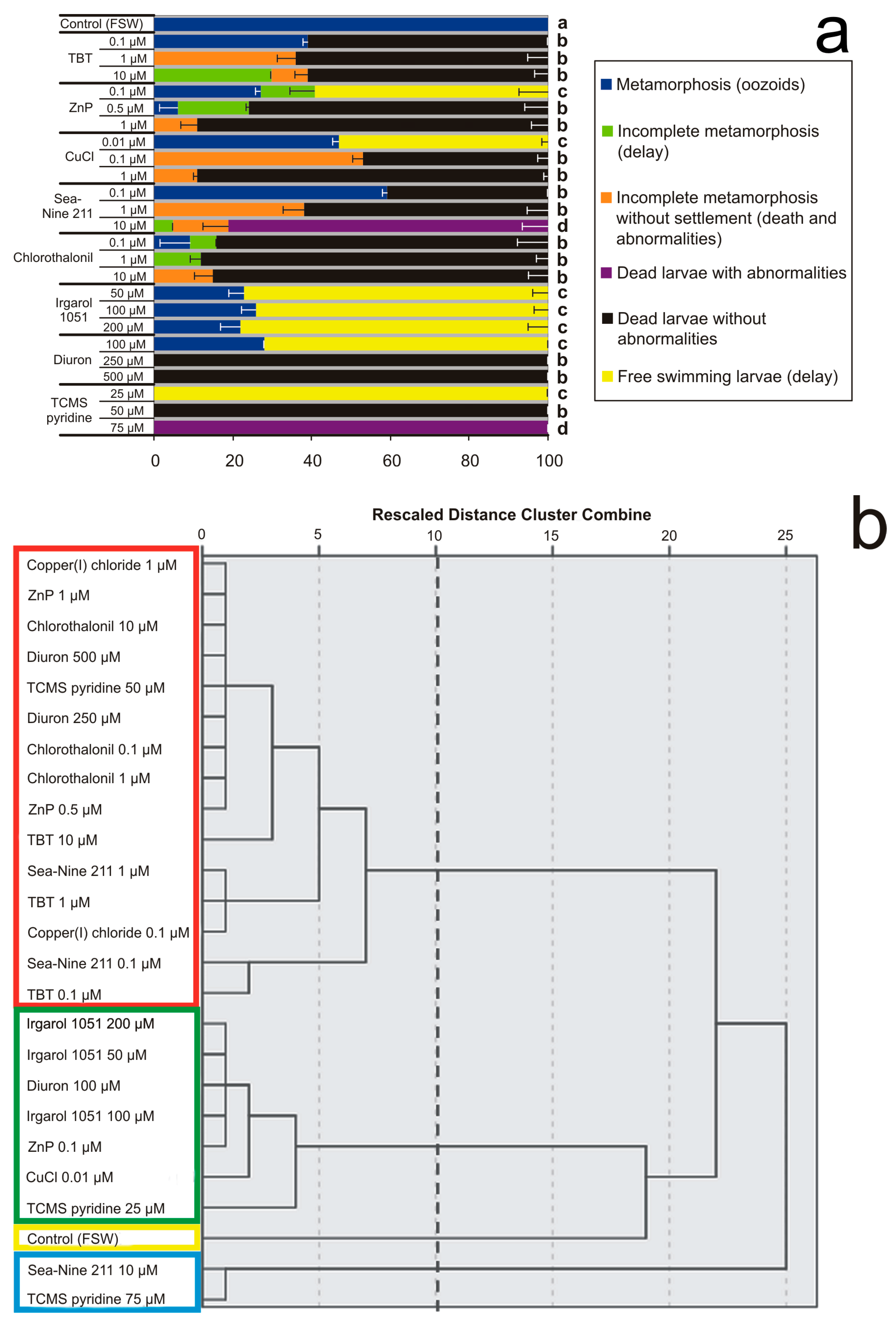
Effects of various concentrations of antifouling biocides in sea water on larval settlement and metamorphosis. (a) Percentages of larval mortality and abnormality observed after exposure until all control larvae in FSW completed the metamorphosis forming filter-feeding oozoids. Significant (p < 0.05) differences obtained with Dunnett’s test are expressed with different letters. (b) Dendrogram obtained from cluster analysis with average linkage between groups. The cutting point at 10 corresponds to the maximum margin showing four clusters bordered by different colours.
The hierarchy dendrogram obtained shows four clusters with mean percentages of six effects. The effects considered were (i) complete metamorphosis with the oozoid formation (blue, in the legend of Figure 3a), (ii) settled larvae with incomplete metamorphosis or metamorphic delay without abnormalities (green), (iii) incomplete metamorphosis without settlement of dead larvae with abnormalities (orange), (iv) not-settled dead larvae with abnormalities (violet), (v) not-settled dead larvae without recognisable abnormalities (black), and (vi) free-swimming larvae as the result of inhibition or severe delay of metamorphosis (yellow).
The first cluster (“a”, in Figure 3a; yellow frame, in Figure 3b) is represented by the unique effect observed in the control, i.e., 100% complete metamorphosis and survival. The second cluster (“b”, red frame) has 15 objects, represented by all concentrations of TBT and Chlorothalonil, and the middle concentrations of ZnP, CuCl, Sea Nine 211, Diuron, and TCMS pyridine. In this cluster, 76.5% of dead larvae without abnormalities prevail, followed by 11.5% incomplete metamorphosis without settlement, 7.5% complete metamorphosis, and 4.5% incomplete metamorphosis with settlement. The third cluster (“c”, green frame) includes seven objects represented by all Irgarol 1051 concentrations, and the lowest concentrations of ZnP, CuCl, Diuron, and TCMS pyridine, with 73.3% free-swimming larvae, 24.7% completed metamorphosis, and 2% incomplete metamorphosis of settled larvae without abnormalities. The fourth cluster (“d”, cyan frame) contains two objects, represented by the highest concentrations of Sea Nine 211 and TCMS pyridine, with 90.5% dead larvae with abnormalities, 7% incomplete metamorphosis without settlement, and 2.5% incomplete metamorphosis with settlement.
These results show that although all biocides are able to prevent larval settlement on the substratum and provoke severe metamorphic malformations, the herbicides Diuron and TCMS pyridine at their lowest concentrations and Irgarol 1051 at all concentrations are not lethal.
3.3. Larval Abnormalities
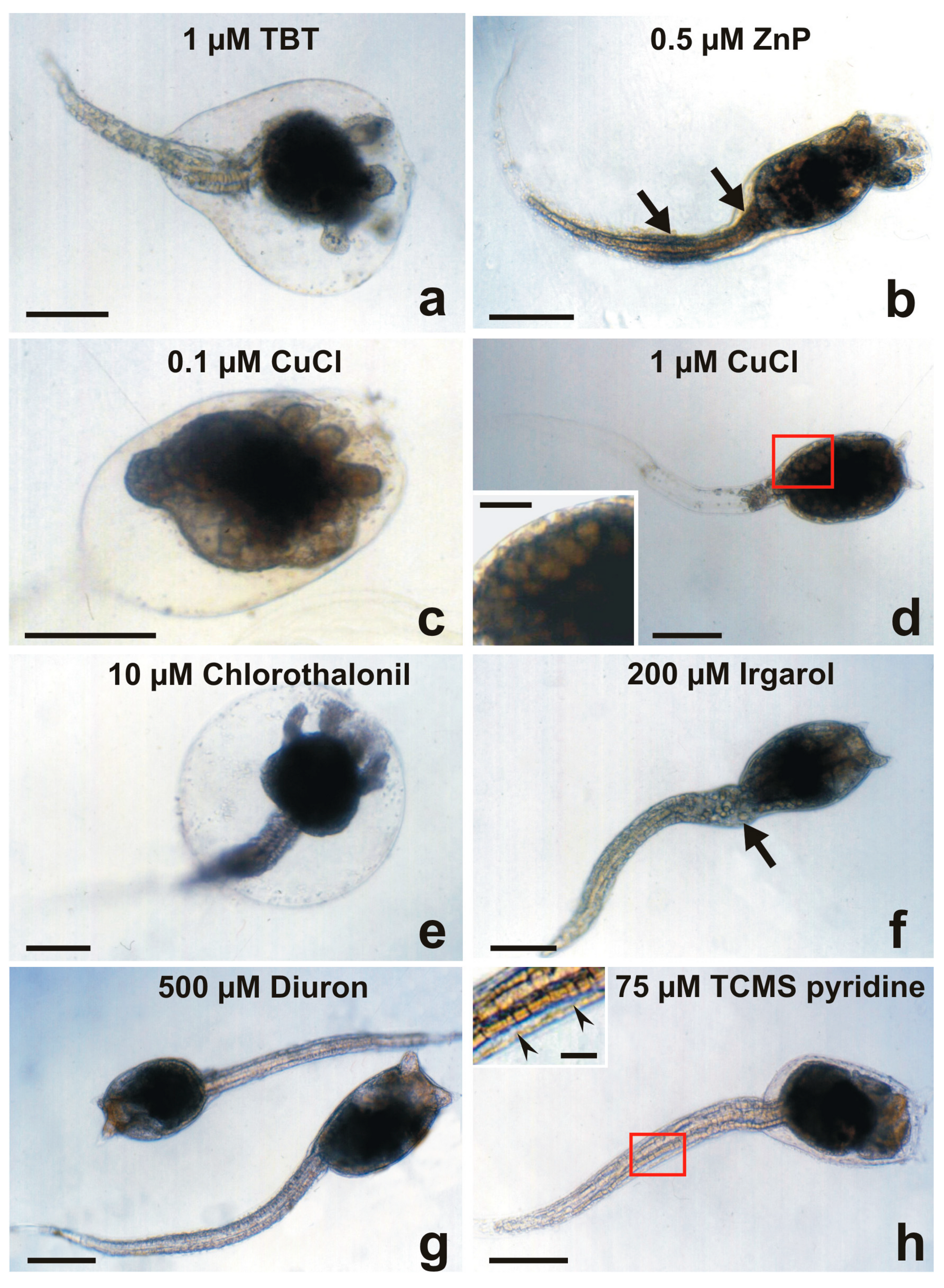
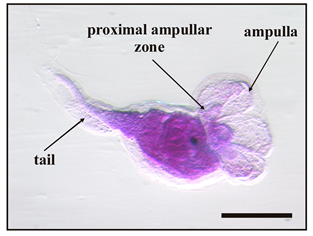
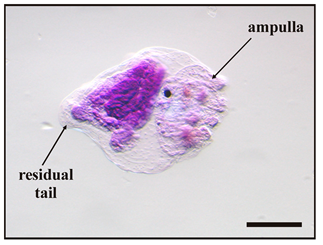
The larval abnormalities of the experiments reported in Section 3.2 were analysed under a light microscope (Figure 4). The most severe morphological effects in metamorphic development have been observed after exposure to 1 and 10 μM TBT (Figure 4a), Sea-Nine 211 and Chlorothalonil, 1 μM ZnP, and 0.1 μM CuCl (Figure 4c). In these cases, the initial metamorphosis was evident with the protrusion of ampullae and tail resorption at various degrees, but cephalenteron always appeared to be encased by an anomalous large bubble filled with a colourless fluid with small, scattered cells. Organotin compounds, zinc, copper, and Chlorothalonil are known to cause significant changes in hydromineral fluxes and membrane permeability, mechanisms that maintain osmotic homeostasis [49,50,51,52]. The bubble formation in B. schlosseri larvae could be the result of the disruption of osmotic control systems due to the direct action on lipid composition and/or ion transport across the plasma membranes. Regarding the tail resorption, TBT, Sea-Nine 211, and Chlorothalonil caused an abnormal extensive detaching of spherical-shaped cells throughout the entire tail length. These compounds are known to directly and indirectly inhibit a series of enzymes. In particular, Ca2+-ATPase is inhibited via biocide interaction with calmodulin [53]. This pump inhibition increases intracellular Ca2+ concentrations, which in turn provoke the depolymerisation of cytoskeletal components [54] and induce cell apoptosis [55]. The incomplete tail resorption with the persistence of apoptotic cells dissociated from the axial complex indicates the absence of phagocytosis, which is usually carried out by motile phagocytes able to engulf all dead cells and tissue debris [56], confirming the immunosuppressive activity of these biocides [28,29].
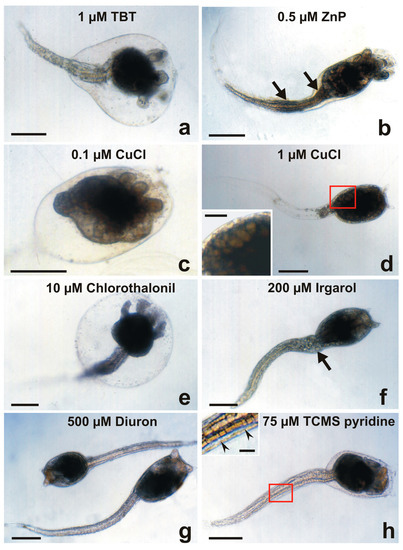
Figure 4.
Larval abnormalities in Botryllus schlosseri after exposure to antifouling biocides. (a) Protruded ampullae inside an anomalous bubble encasing the cephalenteron, and extensive spherical-shaped cells detaching from the tail tissues. (b) Protruded ampullae and tail with proximal and median bulges (arrows). (c) Advanced metamorphosis (protruded ampullae and tail resorption) inhibited by the formation of a bubble encasing the whole body. (d) Tail resorption and inhibition of cephalenteron metamorphosis due to extensive organ regression forming vacuolated cells (inset in (d)). (e) Protruded ampullae inside a bubble encasing the cephalenteron, and normal tail resorption. (f) Detaching of cells from the proximal tail zone (arrow). (g) Absence of metamorphosis. (h) Initial protrusion of ampullae, and separation of the lateral tail muscles from the epidermis by large intercellular spaces (arrowheads in inset in (h)). Bar length: 200 µm in (a–h); 30 µm inset in (d); 40 µm inset in (h).
At 0.5 μM ZnP (Figure 4b) and 1 μM CuCl (Figure 4d), the cephalenteron was not encased in a bubble. In the case of ZnP, protrusion of ampullae occurred, but not the tail resorption. The formation of proximal and median bulges of detached cells was recognisable along the tail, probably due to the inhibition of phagocytosis [32]. In the case of CuCl, tail resorption appeared to be nearly completed, but no protrusion of ampullae occurred and the cephalenteron organs underwent a massive regression, recognisable by the appearance of large vacuolated cells instead of the organ primordia.
Many heavy metals are essential to the growth of marine organisms. Low concentrations of copper ions stimulate metamorphosis in ascidians [57], but at high concentrations, metal ions become toxic. Metals can significantly decrease the synthesis of ATP and negatively alter the metabolic activity causing cell death because they act as uncouplers of oxidative phosphorylation or via opening pores in the mitochondrial membrane [58].
Regarding the herbicides Irgarol 1051 (Figure 4f), Diuron (Figure 4g), and TCMS pyridine (Figure 4h), the metamorphosis was completely inhibited. The larvae did not swim or settle because they were dead only in the cases of 250 μM Diuron and 50 μM TCMS pyridine. As a minor difference from Diuron, an initial protrusion of ampullar rudiments was observed after exposure to TCMS pyridine. These compounds are known to cause disturbances in the mitochondrial respiratory chains and induce cell apoptosis due to severe oxidative stress [59,60]. After the exposure to 500 μM Diuron (Figure 4g), larvae were without evident malformations but immotile. The absence of signs of tail resorption indicates an alteration of communication with the cephalenteron. It could be triggered through the abnormal activation by xenobiotics of stress-related signal transduction pathways, such as the stress-inducible protein HSP90, which in turn activates the enzyme nitric oxide synthase (NOS). The consequent NO production is responsible for the metamorphosis repression in ascidians, as observed in Boltenia villosa larvae, which failed in tail resorption and protrusion of the ampullar rudiments [61]. Cell dissociation occurred in the proximal region of the tail of a small number of larvae after exposure to the highest concentration (200 μM) of Irgarol 1051 (Figure 4f). An extensive separation of the lateral tail muscles from the caudal epidermis by intercellular spaces was recognisable after exposure to 75 μM TCMS pyridine (inset of Figure 4h). These events could be related indirectly to HSP90/NO activation and directly to sudden increases in intracellular Ca2+ concentrations. The latter induce both apoptosis through the activation of endonucleases, triggering DNA fragmentation [30,31], and the loss of Ca2+-dependent cell adhesion molecules such as cadherins, causing extensive tissue detachment [62,63].
Finally, it must be emphasised that in all cases of absence of tail resorption, the increase in intracellular calcium ions might cause extensive depolymerisation of the microfilaments of the cells of the caudal epidermis. In such a way, the F-actin responsible for contractions that provide the driving force in tail resorption [2] failed.
4. Conclusions
The experiments carried out on the free-swimming larvae of the compound ascidian B. schlosseri provide new evidence for better understanding the effects of trade antifouling compounds on settlement ability, metamorphic development, and viability, arranging them in an order of decreasing toxicity: metals and organometals > antimicrobials and fungicides > herbicides. These results confirm the high toxicity of TBT, ZnP, and copper(I)-based antifouling compounds, but also reveal that Sea-Nine 211 and Chlorothalonil, recently introduced by “green chemistry” in new antifouling paint formulations as TBT alternatives, are effective in causing larval death and abnormalities. Moreover, the herbicides Irgarol 1051, Diuron, and TCMS pyridine, although introduced in paint formulations only to prevent the settlement and growth of algae, showed toxicity towards animals. Since these compounds have been in commerce for decades, and, consequently, widespread in the marine environment, a real risk for the coastal biocoenoses appears to be undeniable. In many cases, their partitioning between water and sediment, modality and half-time of biotic and abiotic degradation, speciation, binding to various ligands, formation of complexes, environmental fate, and bioaccumulation in the trophic chains are not yet well known.
The effects of exposure to antifouling paints are much more difficult to interpret than those of exposure to antifouling biocides directly introduced in the sea water. The latter approach is preferable because it can be better controlled and not affected by the modality of leaching of a biocidal mixture from paints, which could vary with various environmental parameters and also determine a network of synergistic effects with booster biocides, other paint additives, and types of matrices. Paints with a contact-leaching matrix are more resistant to abrasion and rubbing than those with a self-polishing matrix, but they tend to oxidise with time, decreasing the biocidal release. By comparing the biocidal release rates from contact-leaching and self-polishing paint technologies, although the initial value of the latter is low, a constant release soon occurs throughout the lifetime of the paint.
Therefore, according to the Biocides Directive (98/8/EC) [64], more assays of acute and chronic toxicity on various target and non-target marine organisms should be performed before new potential pollutants enter the market, in order to prevent the same errors that already occurred with TBT in both the ecological and economical fields due to the deterioration of environments and habitats.
Author Contributions
Conceptualisation, resources, validation, supervision, F.C.; investigation, methodology, data curation, formal analysis, R.V.; writing—original draft preparation and editing, F.C. and R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Italian MIUR (DOR 2020) to F.C. and by a funding agreement between P. Zarantonello’s “Resimix s.r.l.” (Brendola, Vicenza, Italy, http://www.resimix.com/it/) and the University of Padova, Italy, for a Ph.D. fellowship in Biosciences to R.V. (rep. #1488, prot. #186788, 6 May 2019).
Institutional Review Board Statement
The authors followed all applicable international, national, and/or institutional guidelines for the care and use of animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
The authors thank Fabrizio Longo for photos of the stages of B. schlosseri metamorphosis of Table 2, and Andrea Sambo, in-charge technician of the staff at the Umberto D’Ancona Hydrobiological Station of Chioggia (Venice, Italy), for assistance in ascidian collection and boat driving.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Delsuc, F.; Tsagkogeorga, G.; Lartillot, N.; Philippe, H. Additional molecular support for the new chordate phylogeny. Genesis 2008, 46, 592–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloney, R.A. Ascidian larvae and the events of metamorphosis. Am. Zool. 1982, 22, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Karaiskou, A.; Swalla, B.J.; Sasakura, Y.; Chambon, J.-P. Metamorphosis in solitary ascidians. Genesis 2014, 53, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Dauga, D.; Manni, L. The ontology of the anatomy and development of the solitary ascidian Ciona: The swimming larva and its metamorphosis. Sci. Rep. 2020, 10, 17916. [Google Scholar] [CrossRef]
- Sasakura, Y.; Hozumi, A. Formation of adult organs through metamorphosis in ascidians. WIREs Dev. Biol. 2018, 7, e304. [Google Scholar] [CrossRef]
- Caicci, F.; Zaniolo, G.; Burighel, P.; Degasperi, V.; Gasparini, F.; Manni, L. Differentiation of papillae and rostral sensory neurons in the larva of the ascidian Botryllus schlosseri (Tunicata). J. Comp. Neurol. 2010, 518, 547–566. [Google Scholar] [CrossRef]
- Pennati, R.; Rothbächer, U. Bioadhesion in ascidians: A developmental and functional genomics perspective. Interface Focus 2015, 5, 20140061. [Google Scholar] [CrossRef]
- Wakai, M.K.; Nakamura, M.J.; Sawai, S.; Hotta, K.; Oka, K. Two-round Ca2+ transient in papillae by mechanical stimulation induces metamorphosis in the ascidian Ciona intestinalis type A. Proc. R. Soc. B 2021, 288, 20203207. [Google Scholar] [CrossRef]
- Aldred, N.; Clare, A.S. Mini-review: Impact and dynamics of surface fouling by solitary and compound ascidians. Biofouling 2014, 30, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bryan, G.W.; Gibbs, P.E.; Burt, G.R.; Hummerstone, L.G. The decline of the gastropod Nucella lapillus around southwest England: Evidence for the effects of tributyltin from antifouling paints. J. Mar. Biol. Assoc. UK 1986, 66, 611–640. [Google Scholar] [CrossRef]
- Henderson, A.S.; Salazar, S.M. Flowthrough bioassay studies on the effects of antifouling TBT leachates. In Organotin: Environmental Fate and Effects; Champ, M.A., Seligman, P.F., Eds.; Chapman & Hall: London, UK, 1996; pp. 281–303. [Google Scholar]
- Hoch, M. Organotin compounds in the environment: An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Cima, F.; Craig, P.J.; Harrington, C. Organotin compounds in the environment. In Organometallic Compounds in the Environment; Craig, P.J., Ed.; Wiley & Sons Ltd.: Chichester, UK, 2003; pp. 101–149. [Google Scholar]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of using antifouling biocides in aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef]
- Voulvoulis, N.; Scrimshaw, M.D.; Lester, J.N. Alternative antifouling biocides. Appl. Organomet. Chem. 1999, 13, 135–143. [Google Scholar] [CrossRef]
- Evans, S.M.; Birchenough, A.C.; Brancato, M.S. The TBT ban: Out of the frying pan into the fire? Mar. Pollut. Bull. 2000, 40, 204–211. [Google Scholar] [CrossRef]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Cima, F.; Ballarin, L.; Bressa, G.; Martinucci, G.B.; Burighel, P. Toxicity of organotin compounds on embryos of a marine invertebrate (Styela plicata; Tunicata). Ecotoxicol. Environ. Saf. 1996, 35, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zega, G.; Pennati, R.; Candiani, S.; Pestarino, M.; De Bernardi, F. Solitary ascidians embryos (Chordata, Tunicata) as model organisms for testing coastal pollutant toxicity. Invertebr. Surv. J. 2009, 6, S29–S34. [Google Scholar]
- Zaniolo, G.; Burighel, P.; Martinucci, G. Ovulation and placentation in Botryllus schlosseri (Ascidiacea): An ultrastructural study. Can. J. Zool. 1987, 65, 1181–1190. [Google Scholar] [CrossRef]
- Manni, L.; Zaniolo, G.; Burighel, P. Ultrastructural study of oogenesis in the compound ascidian Botryllus schlosseri (Tunicata). Acta Zool. 1994, 75, 101–113. [Google Scholar] [CrossRef]
- Gasparini, F.; Manni, L.; Cima, F.; Zaniolo, G.; Burighel, P.; Caicci, F.; Franchi, N.; Schiavon, F.; Rigon, F.; Campagna, D.; et al. Sexual and asexual reproduction in the colonial ascidian Botryllus schlosseri. Genesis 2014, 53, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Cima, F.; Ballarin, L. A proposed integrated bioindex for the macrofouling biocoenosis of hard substrata in the lagoon of Venice. Estuar. Coast. Shelf Sci. 2013, 130, 190–201. [Google Scholar] [CrossRef]
- Manni, L.; Zaniolo, G.; Cima, F.; Burighel, P.; Ballarin, L. Botryllus schlosseri: A model ascidian for the study of asexual reproduction. Dev. Dyn. 2007, 236, 335–352. [Google Scholar]
- Manni, L.; Anselmi, C.; Cima, F.; Gasparini, F.; Voskoboynik, A.; Martini, M.; Peronato, A.; Burighel, P.; Zaniolo, G.; Ballarin, L. Sixty years of experimental studies on the blastogenesis of the colonial tunicate Botryllus schlosseri. Dev. Biol. 2019, 448, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hamo, O.; Rinkevich, B. Botryllus schlosseri—A model colonial species in basic and applied studies. In Handbook of Marine Model Organisms in Experimental Biology; Established and Emerging; Boutet, A., Schierwater, B., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 385–402. [Google Scholar]
- Cima, F.; Ballarin, L.; Bressa, G.; Sabbadin, A. Immunotoxicity of butyltins in tunicates. Appl. Organomet. Chem. 1995, 9, 567–572. [Google Scholar] [CrossRef]
- Cima, F.; Bragadin, M.; Ballarin, L. Toxic effects of new antifouling compounds on tunicate haemocytes—I. Sea-Nine 211™ and chlorothalonil. Aquat. Toxicol. 2008, 86, 299–312. [Google Scholar] [CrossRef]
- Menin, A.; Ballarin, L.; Bragadin, M.; Cima, F. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins—II. The case of diuron and TCMS pyridine. J. Environ. Sci. Health 2008, 43B, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Ballarin, L. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins: III—The case of copper(I) and Irgarol 1051. Chemosphere 2012, 89, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Ballarin, L. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins—IV. The case of zinc pyrithione. Comp. Biochem. Physiol. 2015, 169, 16–24. [Google Scholar] [CrossRef]
- Kiil, S.; Dam-Johansen, K.; Weinell, C.E.; Pedersen, M.S.; Codolar, S.A. Estimation of polishing and leaching behaviour of antifouling paints using mathematical modelling: A literature review. Biofouling 2003, 19, 37–43. [Google Scholar] [CrossRef]
- Takahashi, K. Release rate of biocides from antifouling paints. In Ecotoxicology of Antifouling Biocides; Arai, T., Harino, H., Ohji, M., Langston, W.J., Eds.; Springer: Tokyo, Japan, 2009; pp. 3–22. [Google Scholar]
- Marin, M.G.; Moschino, V.; Cima, F.; Celli, C. Embryotoxicity of butyltin compounds to the sea urchin Paracentrotus lividus. Mar. Environ. Res. 2000, 50, 231–235. [Google Scholar] [CrossRef]
- Khandeparker, L.; Desai, D.; Shirayama, Y. Larval development and post-settlement metamorphosis of the barnacle Balanus albicostatus Pilsbry and the serpulid polychaete Pomatoleios kraussii Baird: Impact of a commonly used antifouling biocide, Irgarol 1051. Biofouling 2005, 21, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J. Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates. Sci. Total Environ. 2006, 367, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.; Buono, S.; Cremisini, C. Toxic effects of irgarol and diuron on sea urchin Paracentrotus lividus early development, fertilization, and offspring quality. Arch. Environ. Contam. Toxicol. 2006, 51, 61–68. [Google Scholar] [CrossRef]
- Mai, H.; Morin, B.; Pardon, P.; Gonzalez, P.; Budzinski, H.; Cachot, J. Environmental concentrations of irgarol, diuron and s-metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Gallo, A.; Tosti, E. Reprotoxicity of the antifoulant chlorothalonil in ascidians: An ecological risk assessment. PLoS ONE 2015, 10, e0123074. [Google Scholar] [CrossRef]
- Dumollard, R.; Gazo, I.; Gomes, I.D.L.; Besnardeau, L.; McDougall, A. Ascidians: An emerging marine model for drug discovery and screening. Curr. Top. Med. Chem. 2017, 17, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef]
- Bellas, J. Toxicity assessment of the antifouling compound zinc pyrithione using early developmental stages of the ascidian Ciona intestinalis. Biofouling 2005, 21, 289–296. [Google Scholar] [CrossRef]
- Bellas, J.; Beiras, R.; Vazquez, E. Sublethal effects of trace metals (Cd, Cr, Cu, Hg) on embryogenesis and larval settlement of the ascidian Ciona intestinalis. Arch. Environ. Contam. Toxicol. 2004, 46, 61–66. [Google Scholar] [CrossRef]
- Mackie, D.S.; van den Berg, C.M.G.; Readman, J.W. Determination of pyrithione in natural waters by cathodic stripping voltametry. Anal. Chim. Acta 2004, 511, 47–53. [Google Scholar] [CrossRef]
- Ranke, J.; Jastorff, B. Multidimensional risk analysis of antifouling biocides. Environ. Sci. Pollut. Res. 2000, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 2011, 213, 1–26. [Google Scholar] [PubMed]
- Lagerström, M.; Ytreberg, E. Quantification of Cu and Zn in antifouling paint films by XRF. Talanta 2021, 223, 121820. [Google Scholar] [CrossRef]
- Hartl, M.G.J.; Hutchinson, S.; Hawkins, L.E. Organotin and osmoregulation: Quantifying the effects of environmental concentrations of sediment-associated TBT and TPhT on the freshwater-adapted European flounder, Platichthys flesus (L.). J. Exp. Mar. Biol. Ecol. 2001, 256, 267–278. [Google Scholar] [CrossRef]
- Bregante, M.; Carpaneto, A.; Piazza, V.; Sbrana, F.; Vassalli, M.; Faimali, M.; Gambale, F. Osmoregulated chloride currents in hemocytes from Mytilus galloprovincialis. PLoS ONE 2016, 11, e0167972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Polo, A.; Scrimshaw, M.D. Challenges for the development of a biotic ligand model predicting copper toxicity in estuaries and seas. Environ. Toxicol. Chem. 2012, 31, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.N.; Eom, H.-J.; Nam, S.-E.; Shin, Y.K.; Rhee, J.-S. Chlorothalonil induces oxidative stress and reduces enzymatic activities of Na+/K+-ATPase and acetylcholinesterase in gill tissues of marine bivalves. PLoS ONE 2019, 14, e0214236. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Dominici, D.; Mammi, S.; Ballarin, L. Butyltins and calmodulin: Which interaction? Appl. Organomet. Chem. 2002, 16, 182–186. [Google Scholar] [CrossRef]
- Cima, F.; Ballarin, L. Tributyltin induces cytoskeletal alterations in the colonial ascidian Botryllus schlosseri phagocytes via interaction with calmodulin. Aquat. Toxicol. 2000, 48, 419–429. [Google Scholar] [CrossRef]
- Cima, F.; Ballarin, L. TBT-induced apoptosis in tunicate haemocytes. Appl. Organomet. Chem. 1999, 13, 697–703. [Google Scholar] [CrossRef]
- Schiaffino, S.; Burighel, P.; Nunzi, M.G. Involution of the caudal musculature during metamorphosis in the ascidian, Botryllus schlosseri. Cell Tissue Res. 1974, 153, 293–305. [Google Scholar] [CrossRef]
- Whittaker, J.R. Copper as a factor in the onset of ascidian metamorphosis. Nature 1964, 202, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Bragadin, M.; Manente, S.; Marton, D.; Cima, F.; Rigobello, M.P.; Bindoli, A. The interaction of zinc pyrithione with mitochondria from rat liver and a study of the mechanism of inhibition of ATP synthesis. Appl. Organomet. Chem. 2003, 17, 869–874. [Google Scholar] [CrossRef]
- Bragadin, M.; Cima, F.; Ballarin, L.; Manente, S. Irgarol inhibits the synthesis of ATP in mitochondria from rat liver. Chemosphere 2006, 65, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Bragadin, M.; Iero, A.; Cima, F.; Ballarin, L.; Manente, S. TCMS inhibits ATP synthesis in mitochondria: A systematic analysis of the inhibitory mechanism. Toxicol. In Vitro 2007, 21, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D.; Bates, W.R.; Brandhorst, B.P. Regulation of metamorphosis in ascidians involves NO/cGMP signaling and HSP90. J. Exp. Zool. 2001, 289, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Ferruzza, S.; Scarino, M.L.; Rotilio, G.; Ciriolo, M.R.; Santaroni, P.; Muda, A.O.; Sambuy, Y. Copper treatment alters the permeability of tight junctions in cultured human intestinal Caco-2 cells. Am. J. Physiol. 1999, 277, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Chung-Hsun, L.; I-Hui, C.; Chia-Rong, L.; Chih-Hsien, C.; Ming-Che, T.; Jin-Lian, T.; Hsiu-Fen, L. Inhibition of gap junctional intercellular communication in WB-F344 rat liver epithelial cells by triphenyltin chloride through MAPK and PI3-kinase pathways. J. Occup. Med. Toxicol. 2010, 5, 17. [Google Scholar]
- European Commission. Directive 98/8/EC of the European Parliament and of the Council of 16 February Concerning the Placing of Biocidal Products on the Market. Off. J. Eur. Comm. 1998, 41, 123. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).