Functional Histology and Ultrastructure of the Digestive Tract in Two Species of Chitons (Mollusca, Polyplacophora)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection Site, Digestive Tract Content and Measurements
2.2. Tissue Processing for Light and Transmission Electron Microscopy
2.3. Histochemistry
2.4. Ultrastructural Detection of Arylsulphatase Activity
3. Results
3.1. General Morphology and Content of the Digestive Tract
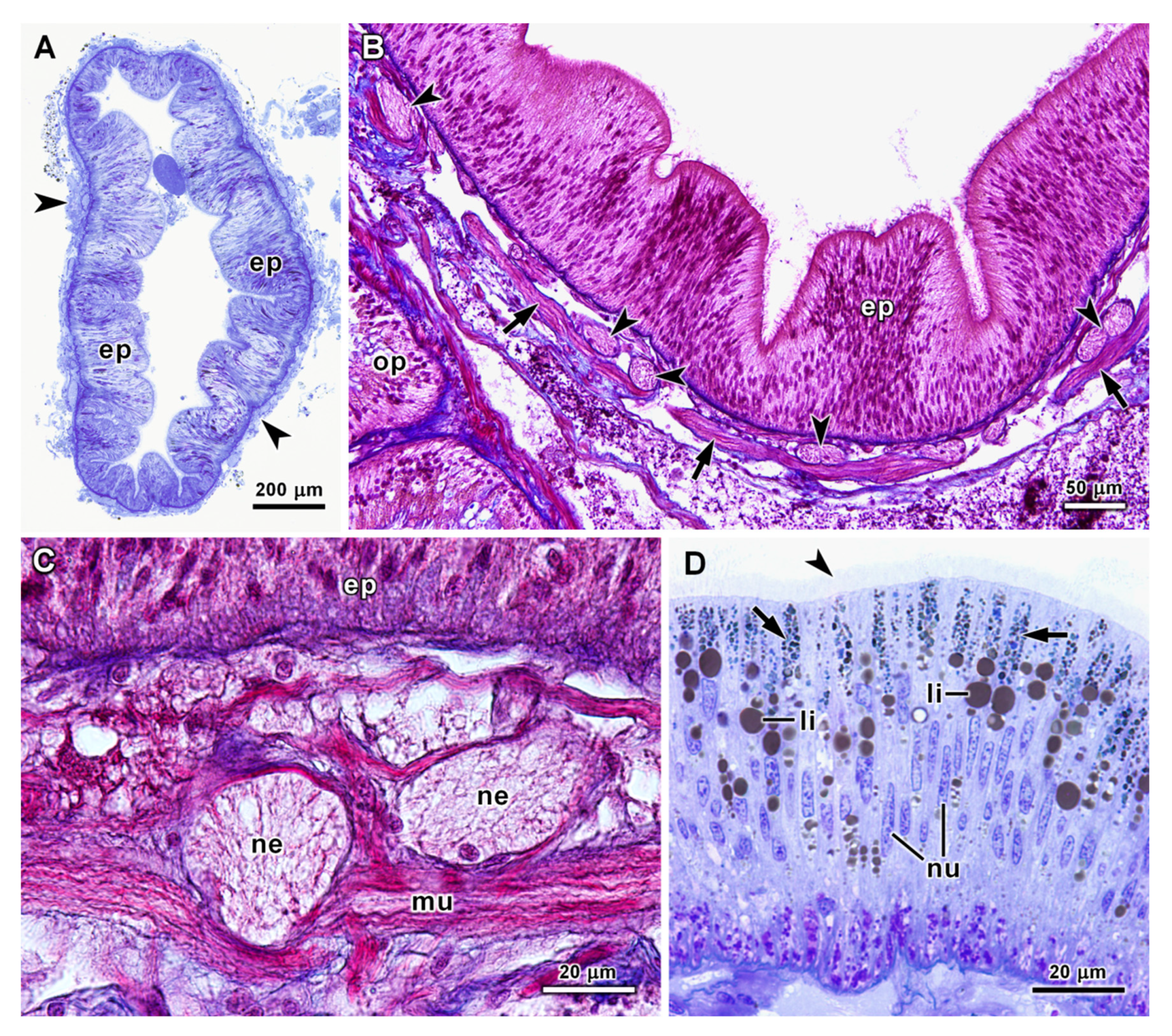
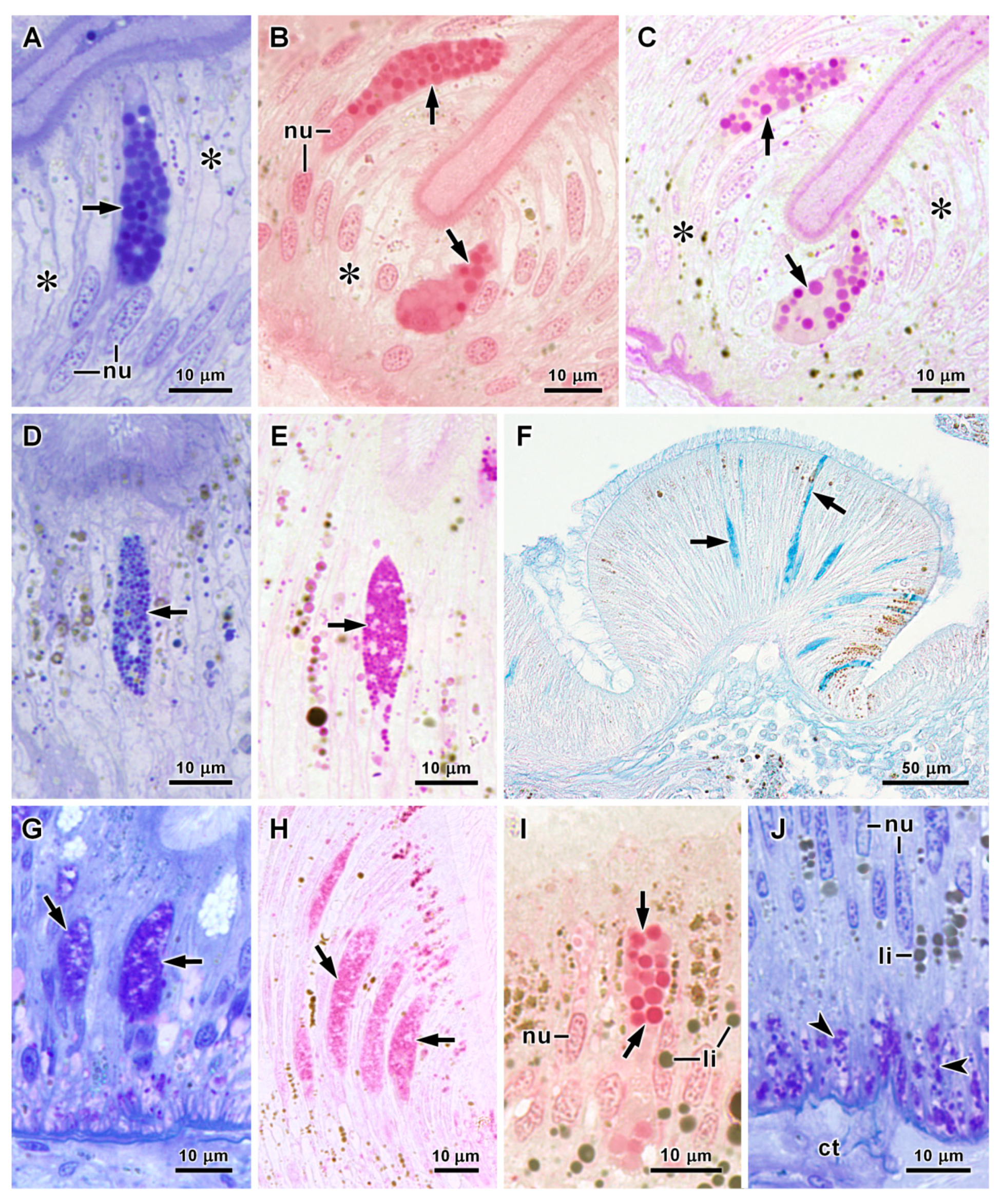
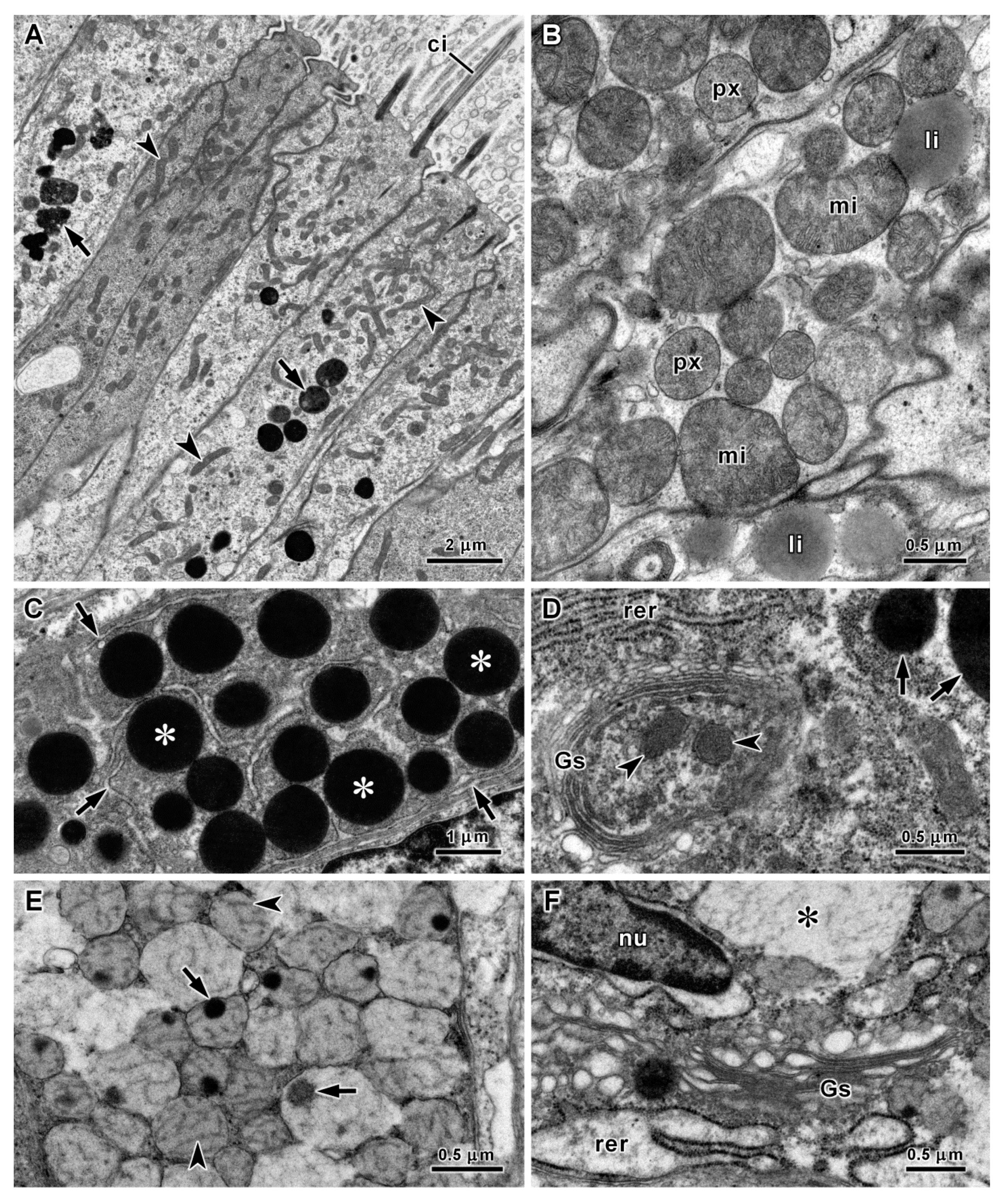
3.2. Histology and Ultrastructure of the Oesophagus
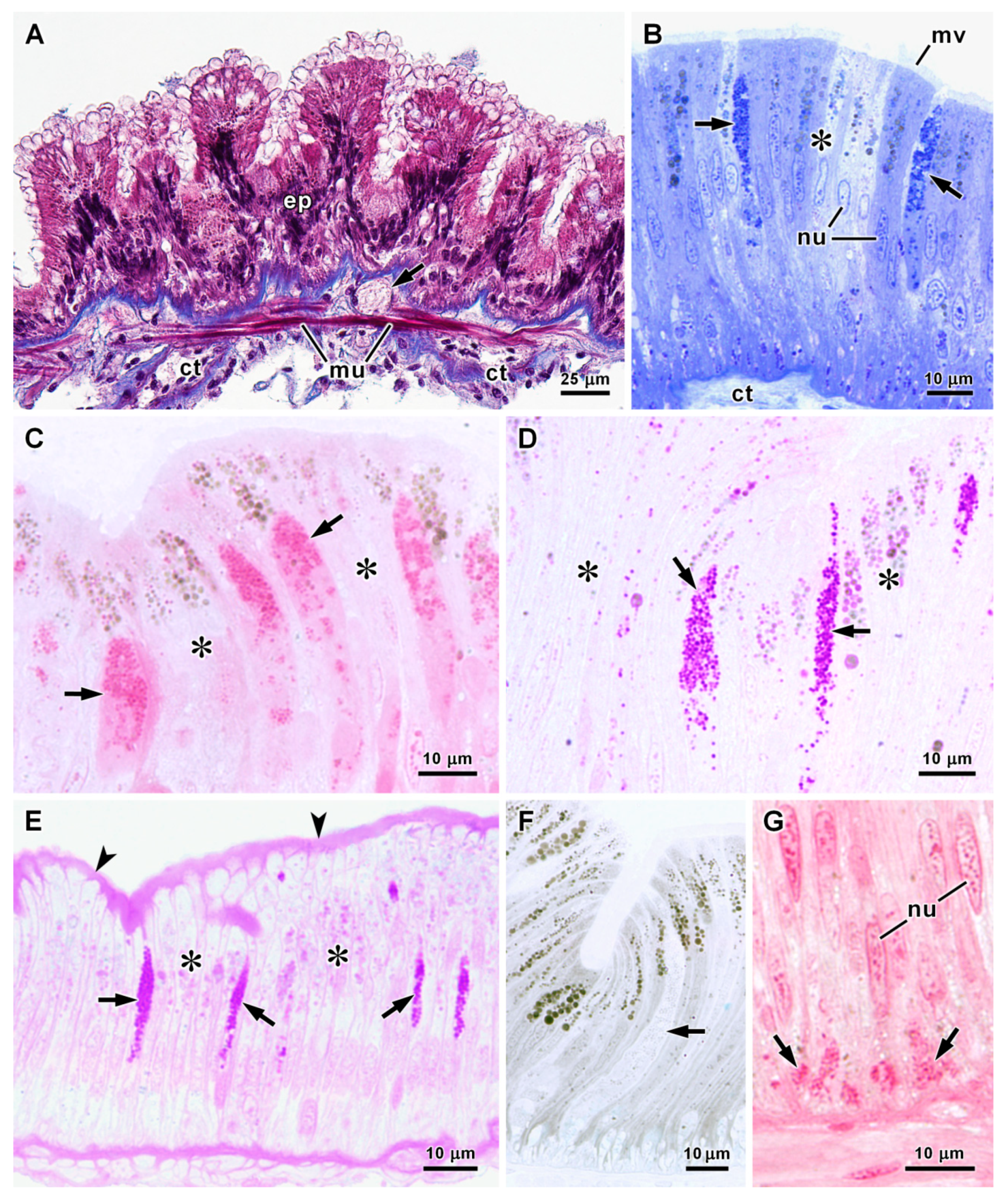
3.3. Histology and Ultrastructure of the Stomach
3.4. Histology and Ultrastructure of the Intestine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eernisse, D.J.; Reynolds, P.D. Polyplacophora. In Microscopic Anatomy of Invertebrates; Harrison, F.W., Kohn, A.J., Mollusca, I., Eds.; Wiley-Liss: New York, NY, USA, 1994; Volume 5, pp. 55–110. [Google Scholar]
- Todt, C.; Okusu, A.; Schander, C.; Schwabe, E. Solenogastres, Caudofoveata, and Polyplacophora. In Phylogeny and Evolution of the Mollusca; Ponder, W.F., Lindberg, D.R., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 71–96. [Google Scholar]
- Sigwart, J.D.; Vermeij, G.J.; Hoyer, P. Why do chitons curl into a ball? Biol. Lett. 2019, 15, 20190429. [Google Scholar] [CrossRef] [PubMed]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2020; Volume 2. [Google Scholar]
- Fretter, V. The structure and function of the alimentary canal of some species of Polyplacophora (Mollusca). Trans. R. Soc. Edin. 1937, 49, 119–164. [Google Scholar] [CrossRef]
- Greenfield, M.L. Feeding and gut physiology in Acanthopleura spinigera (Mollusca). J. Zool. Lond. 1972, 166, 37–47. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A. The peroxisomes of the hepatopancreas in two species of chitons. Cell Tissue Res. 1997, 290, 655–664. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Schwabe, E. Anatomy of the many feeding types in polyplacophoran molluscs. Invert. Zool. 2017, 14, 205–216. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Alves, A.; Oliveira, E.; Calado, G. Functional morphology of the glandular esophageal pouches of chitons (Mollusca, Polyplacophora). J. Morphol. 2021, 282, 355–367. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Khardin, A.S.; Kasyanov, S.P.; Ivanova, M.B. A study on the feeding ecology of chitons using analysis of gut contents and fatty acid markers. J. Moll. Stud. 2004, 70, 225–230. [Google Scholar] [CrossRef]
- Camus, P.; Daroch, K.; Opazo, L. Potential for omnivory and apparent intraguild predation in rocky intertidal herbivore assemblages from northern Chile. Mar. Ecol. Progr. Ser. 2008, 361, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Camus, P.; Arancibia, P.; Ávila-Thieme, M. A trophic characterization of intertidal consumers on Chilean rocky shores. Rev. Biol. Mar. Oceanogr. 2013, 48, 431–450. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Okutani, T. Carnivorous habits of two species of the genus Craspedochiton (Polyplacophora: Acanthochitonidae). J. Malacol. Soc. Aust. 1992, 13, 55–63. [Google Scholar] [CrossRef]
- Duperron, S.; Pottier, M.-A.; Léger, N.; Gaudron, S.M.; Puillandre, N.; Le Prieur, S.; Sigwart, J.D.; Ravaux, J.; Zbinden, M. A tale of two chitons: Is habitat specialisation linked to distinct associated bacterial communities? FEMS Microbiol. Ecol. 2013, 83, 552–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooker, L.R.; Shaw, J. The chiton radula: A unique model for biomineralization studies. In Advanced Topics in Biomineralization; Seto, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 65–84. [Google Scholar] [CrossRef] [Green Version]
- Boyle, P.R. Fine structure of the subradular organ of Lepidochitona cinereus (L), (Mollusca, Polyplacophora). Cell Tissue Res. 1975, 162, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Meeuse, B.J.D.; Fluegel, W. Carbohydrate-digesting enzymes in the sugar gland juice of Cryptochiton stelleri. Arch. Neerl. Zool. 1958, 13, 301–313. [Google Scholar] [CrossRef]
- Saito, H.; Okutani, T. Taxonomy of Japanese species of the genera Mopalia and Plaxiphora (Polyplacophora: Mopaliidae). Veliger 1991, 34, 172–194. [Google Scholar]
- Sannes, P.L.; Katsuyama, T.; Spicer, S. Tannic acid-metal salt sequences for light and electron microscopic localization of complex carbohydrates. J. Histochem. Cytochem. 1978, 26, 55–61. [Google Scholar] [CrossRef]
- Ganter, P.; Jollès, G. Histochimie Normal et Pathologique; Gauthier-Villars: Paris, France, 1970. [Google Scholar]
- Lane, B.P.; Europa, D.L. Differential staining of ultrathin sections of Epon-embedded tissues for light microscopy. J. Histochem. Cytochem. 1965, 13, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Hopsu-Havu, V.K.; Arstila, A.V.; Helminen, H.J.; Kalimo, H.O. Improvements in method for electron microscopic localization of arylsulphatase activity. Histochemie 1967, 8, 54–64. [Google Scholar] [CrossRef]
- Sigwart, J.D.; Stoeger, I.; Knebelsberger, T.; Schwabe, E. Chiton phylogeny (Mollusca: Polyplacophora) and the placement of the enigmatic species Choriplax grayi (H. Adams & Angas). Invertebr. Syst. 2013, 27, 603–621. [Google Scholar] [CrossRef]
- Kaas, P.; Van Belle, R.A. Monograph of living chitons (Mollusca: Polyplacophora). In Suborder Ischnochitonina, Ischnochitonidae: Chaetopleurinae & Ischnochitoninae (pars); E. J. Brill and W. Backhuys: Leiden, The Netherlands, 1987; Volume 3. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2020; Volume 1. [Google Scholar]
- Lobo-da-Cunha, A. Structure and function of the digestive system in molluscs. Cell Tissue Res. 2019, 377, 475–503. [Google Scholar] [CrossRef]
- Klumperman, J.; Raposo, G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a016857. [Google Scholar] [CrossRef] [Green Version]
- Walker, G. The digestive system of the slug, Agriolimax reticulatus (Müller): Experiments on phagocytosis and nutrient absorption. Proc. Malacol. Soc. Lond. 1972, 40, 33–43. [Google Scholar] [CrossRef]
- Bush, M.S. The ultrastructure and function of the oesophagus of Patella vulgata. J. Moll. Stud. 1989, 55, 111–124. [Google Scholar] [CrossRef]
- Bourne, N.B.; Jones, W.; Bowen, I.D. Endocytosis in the crop of the slug, Deroceras reticulatum (Müller) and the effects of the ingested molluscicides, metaldehyde and methiocarb. J. Moll. Stud. 1991, 57, 71–80. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.S. The ultrastructure and function of the intestine of Patella vulgata. J. Zool. 1988, 215, 685–702. [Google Scholar] [CrossRef]
- Schrader, M.; Kamoshita, M.; Islinger, M. Organelle interplay - peroxisome interactions in health and disease. J. Inherit. Metab. Dis. 2020, 43, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Cardoso, M.J.R.; Schrader, M. Be different—The diversity of peroxisomes in the animal kingdom. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 881–897. [Google Scholar] [CrossRef] [Green Version]
- Cajaraville, M.P.; Völkl, A.; Fahimi, H.D. Peroxisomes in the digestive gland cells of the mussel Mytilus galloprovincialis Lmk. Biochemical, ultrastructural and immunocytochemical characterization. Europ. J. Cell Biol. 1992, 56, 255–264. [Google Scholar]
- Cancio, I.; Völkl, A.; Beier, K.; Fahimi, H.D.; Cajaraville, M.P. Peroxisomes in molluscs, characterization by subcellular fractionation combined with western blotting, immunohistochemistry, and immunocytochemistry. Histochem. Cell Biol. 2000, 113, 51–60. [Google Scholar] [CrossRef]
- Owen, G. Lysosomes, peroxisomes and bivalves. Sci. Prog. 1972, 60, 299–318. [Google Scholar]
- Lobo-da-Cunha, A.; Batista, C.; Oliveira, E. The peroxisomes of the hepatopancreas in marine gastropods. Biol. Cell 1994, 82, 67–74. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Oliveira, E.; Alves, A.; Calado, G. Giant peroxisomes revealed by a comparative microscopy study of digestive gland cells of cephalaspidean sea slugs (Gastropoda, Euopisthobranchia). J. Mar. Biol. Assoc. 2019, 99, 197–202. [Google Scholar] [CrossRef]
- Triebskorn, R. Ultrastructural changes in the digestive tract of Deroceras reticulatum (Müller) induced by a carbamate molluscicide and by metaldehyde. Malacologia 1989, 31, 141–156. [Google Scholar]
- Lobo-da-Cunha, A.; Batista-Pinto, C. Stomach cells of Aplysia depilans (Mollusca, Opisthobranchia): A histochemical, ultrastructural, and cytochemical study. J. Morphol. 2003, 256, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Lobo-da-Cunha, A.; Batista-Pinto, C. Light and electron microscopy studies of the oesophagus and crop epithelium in Aplysia depilans (Mollusca, Opisthobranchia). Tissue Cell 2005, 37, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lobo-da-Cunha, A.; Batista-Pinto, C. Ultrastructural, histochemical and cytochemical characterisation of intestinal epithelial cells in Aplysia depilans (Gastropoda, Opisthobranchia). Acta Zool. 2007, 88, 211–221. [Google Scholar] [CrossRef]
- Tandler, B.; Phillips, C.J. Structure of serous cells in salivary glands. Microsc. Res. Tech. 1993, 26, 32–48. [Google Scholar] [CrossRef]
- Boer, H.H.; Kits, K.S. Histochemical and ultrastructural study of the alimentary tract of the freshwater snail Lymnaea stagnalis. J. Morphol. 1990, 205, 97–111. [Google Scholar] [CrossRef]
- Franchini, A.; Ottaviani, E. Intestinal cell types in the freshwater snail Planorbarius corneus: Histochemical, immunocytochemical and ultrastructural observations. Tissue Cell. 1992, 24, 387–396. [Google Scholar] [CrossRef]
- Pfeiffer, C.J. Intestinal ultrastructure of Nerita picea (Mollusca: Gastropoda), an Intertidal marine snail of Hawaii. Acta Zool. 1992, 73, 39–47. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Malheiro, A.R.; Alves, A.; Oliveira, E.; Coelho, R.; Calado, G. Histological and ultrastructural characterisation of the stomach and intestine of the opisthobranch Bulla striata (Heterobranchia: Cephalaspidea). Thalassas 2011, 27, 61–75. [Google Scholar]
- Tandler, B. Structure of mucous cells in salivary glands. Microsc. Res. Tech. 1993, 26, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lobo-da-Cunha, A.; Oliveira, E.; Ferreira, I.; Coelho, R.; Calado, G. Histochemical and ultrastructural characterization of the posterior esophagus of Bulla striata (Mollusca, Opisthobranchia). Microsc. Microanal. 2010, 16, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Leal-Zanchet, A.M. Ultrastructure of the supporting cells and secretory cells of the alimentary canal of the slugs, Lehmannia marginata and Boettgerilla pallens (Pulmonata: Stylommatophora: Limacoidea). Malacologia 2002, 44, 223–239. [Google Scholar]
- Shan, M.M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Portela-Gomes, G.M.; Grimelius, L.; Bergström, R. Enterochromaffin (argentaffin) cells of the rat gastrointestinal tract. An ultrastructural study. Acta Pathol. Microbiol. Immunol. Scand. A 1984, 92, 83–89. [Google Scholar] [CrossRef]
- Wade, P.R.; Westfall, J.A. Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell Tissue Res. 1985, 241, 557–563. [Google Scholar] [CrossRef]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Lv, J.; Xi, R. The specification and function of enteroendocrine cells in Drosophila and mammals: A comparative review. FEBS J. 2021, epublication ahead of print. [Google Scholar] [CrossRef]
- Plisetskaya, E.; Kazakov, V.K.; Soltitskaya, L.; Leibson, L.G. Insulin-producing cells in the gut of freshwater bivalve mollusks Anodonta cygnea and Unio pictorum and the role of insulin in the regulation of their carbohydrate metabolism. Gen. Comp. Endocrinol. 1978, 35, 133–145. [Google Scholar] [CrossRef]
- Marchand, C.R.; Colard, C. Presence of cells and fibers immunoreactive toward antibodies to different peptides or amine in the digestive tract of the snail Helix aspersa. J. Morphol. 1991, 207, 185–190. [Google Scholar] [CrossRef]
- Franchini, A.; Rebecchi, B.; Fantin, A.M. Immunocytochemical detection of endocrine cells in the gut of Viviparus ater (Mollusca, Gastropoda). Eur. J. Histochem. 1994, 38, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cherif-Feildel, M.; Heude Berthelin, C.; Adeline, B.; Rivière, G.; Favrel, P.; Kellner, K. Molecular evolution and functional characterisation of insulin related peptides in molluscs: Contributions of Crassostrea gigas genomic and transcriptomic-wide screening. Gen. Comp. Endocrinol. 2019, 271, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Alba, Y.; Villaro, A.C.; Sesma, P.; Vázquez, J.J.; Abaurrea, A. Gut endocrine cells in the snail Helix aspersa. Gen. Comp. Endocrinol. 1988, 70, 363–373. [Google Scholar] [CrossRef]
- Bohórquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohórquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]




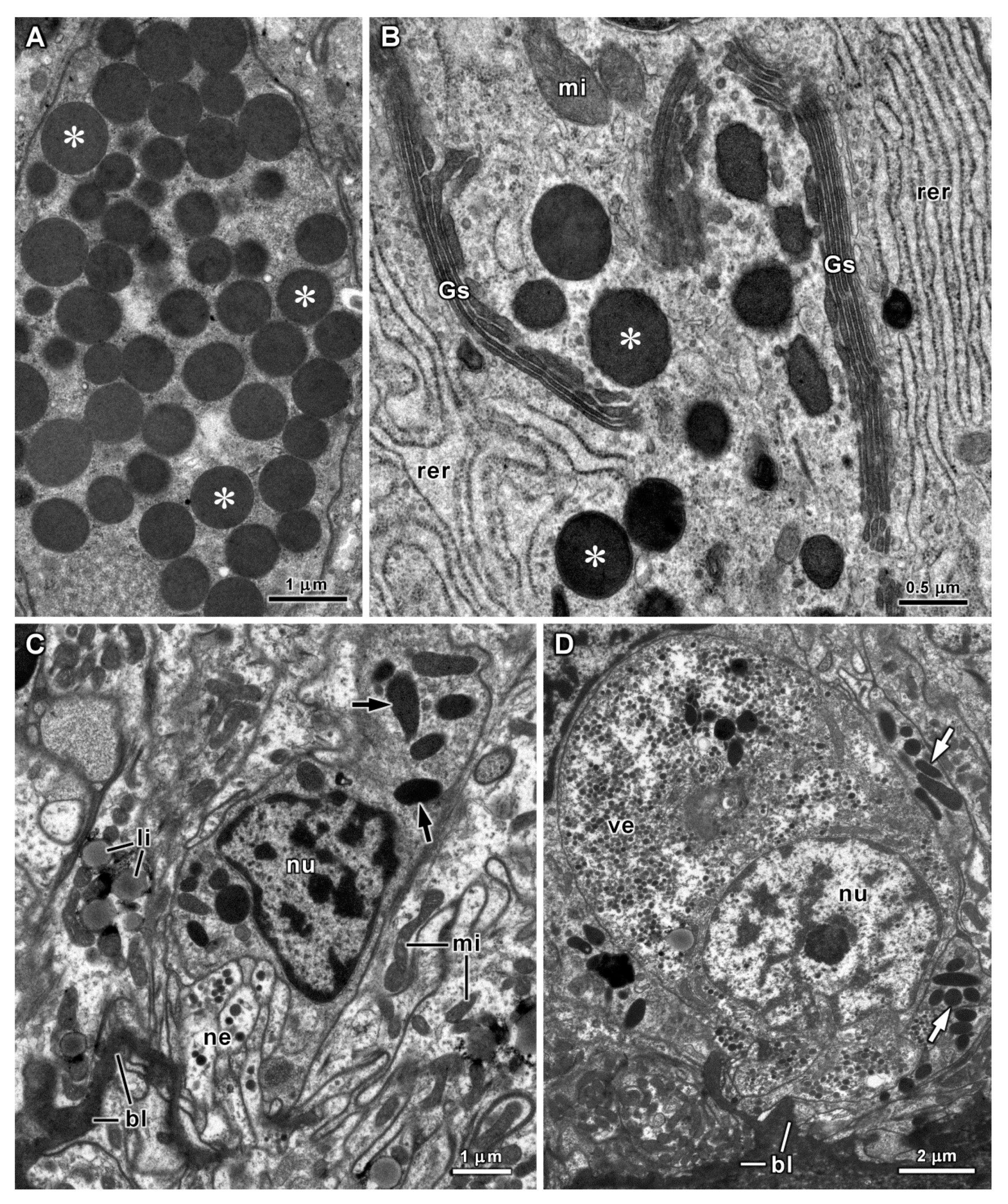

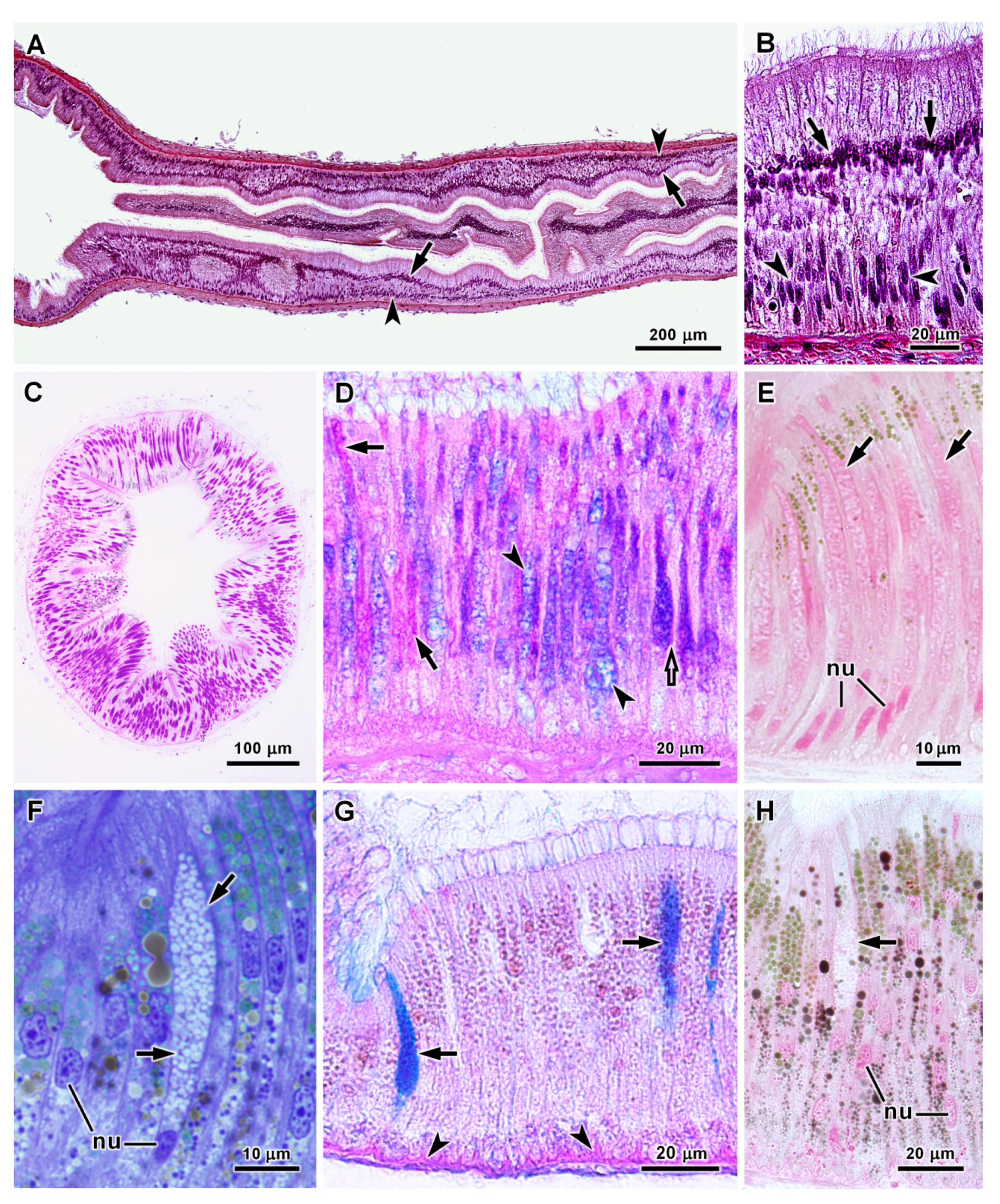
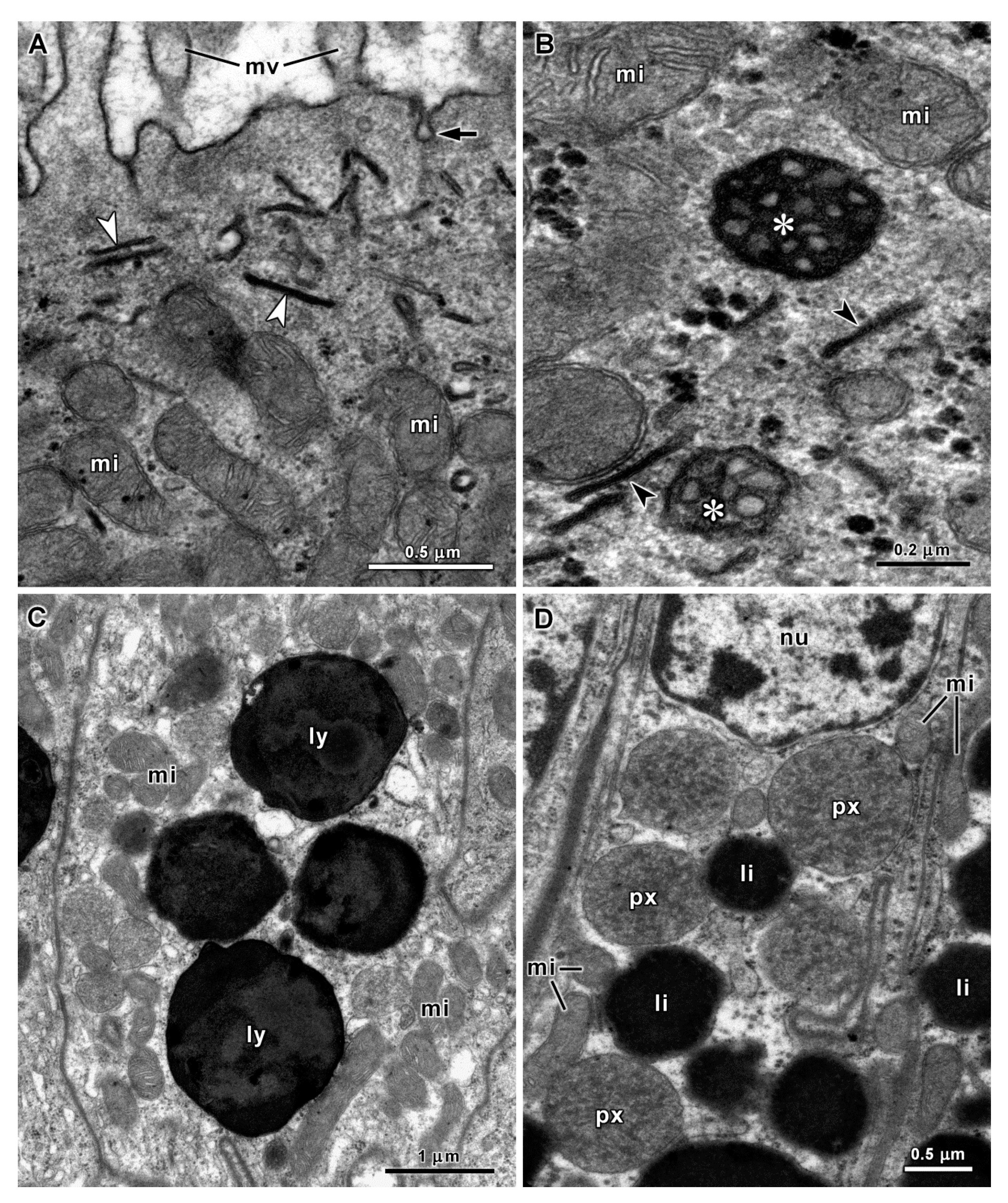
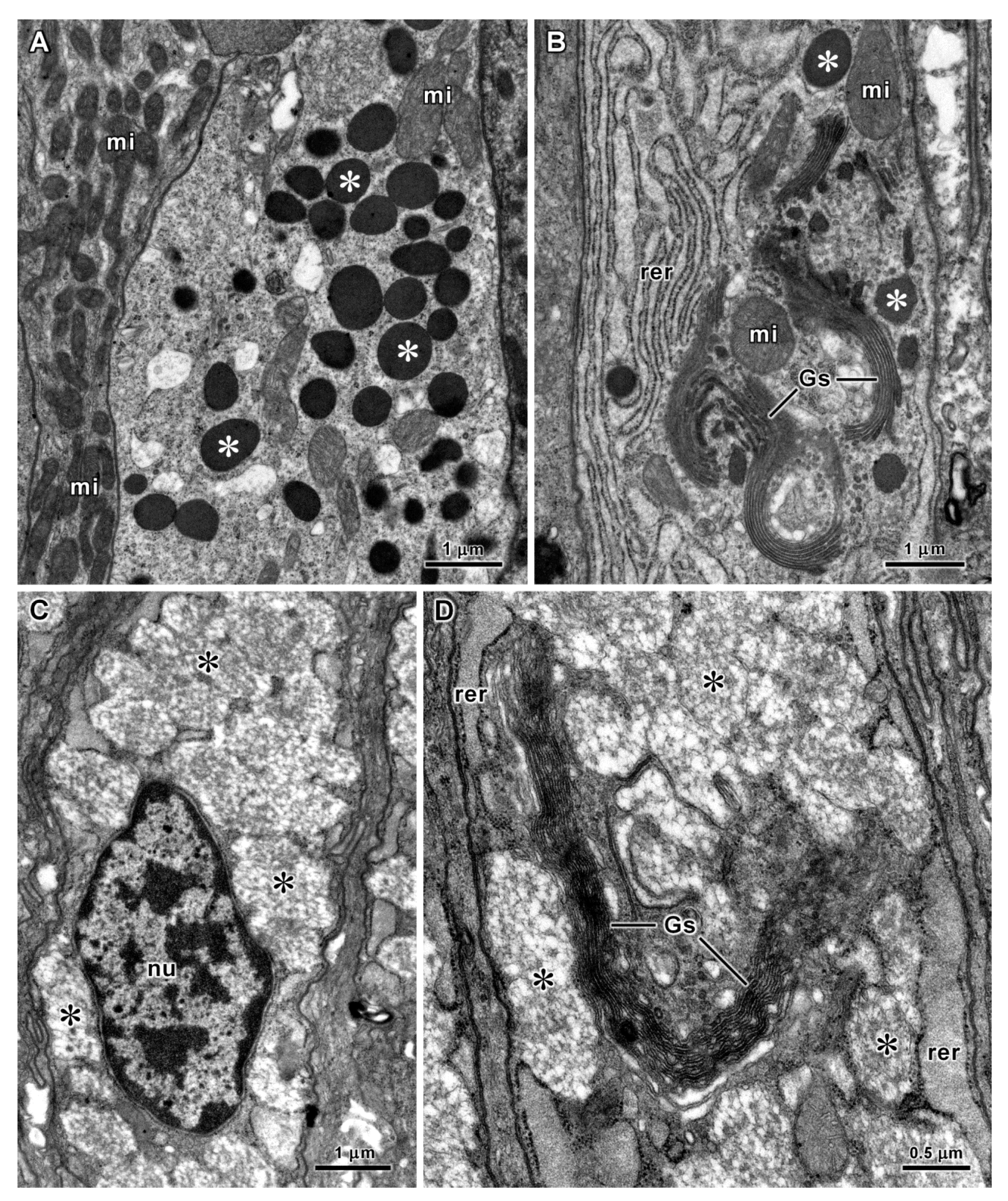

| Organs | Species | Cell Types | Diameters of Secretory Vesicles (μm) (1) | PAS Reaction (Polysaccharides) | Alcian Blue pH 1.0 and 2.5 (Acid Polysaccharides) | Tetrazonium Reaction (Proteins) | Electron Density of Vesicles |
|---|---|---|---|---|---|---|---|
| Oesophagus | C. angulata | Basophilic | 1.0–3.0 | + + + | − | + + + | High |
| Mucous | 0.3–0.7 | + + | + + + | + + | Low | ||
| A. fascicularis | Basophilic with small vesicles | 0.4–0.9 | + + + | + + + | + + | High | |
| Basophilic with large vesicles | 1.0–2.5 | + + + | − | + + + | High | ||
| Stomach | C. angulata | Basophilic | 0.4–0.8 | + + + | − | + + + | High |
| A. fascicularis | Basophilic | 0.6–1.1 | + + + | − | + + + | High | |
| Intestine | C. angulata | Basophilic | 0.4–0.8 | + + + | − | + + + | High |
| Mucous (2) | 0.8–1.8 | − | + + + | − | Low or median | ||
| A. fascicularis | Basophilic | 0.5–1.5 | + + + | + + | + + | High | |
| Mucous (3) | (undetermined due to fusion) | + + + | + + | + | Low | ||
| C. angulata | Basal cells (enteroendocrine) | 0.4–1.0 | − | − | + + + | High | |
| A. fascicularis | Basal cells (enteroendocrine) | 0.2–0.5 | − | − | + + + | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo-da-Cunha, A.; Alves, Â.; Oliveira, E.; Calado, G. Functional Histology and Ultrastructure of the Digestive Tract in Two Species of Chitons (Mollusca, Polyplacophora). J. Mar. Sci. Eng. 2022, 10, 160. https://doi.org/10.3390/jmse10020160
Lobo-da-Cunha A, Alves Â, Oliveira E, Calado G. Functional Histology and Ultrastructure of the Digestive Tract in Two Species of Chitons (Mollusca, Polyplacophora). Journal of Marine Science and Engineering. 2022; 10(2):160. https://doi.org/10.3390/jmse10020160
Chicago/Turabian StyleLobo-da-Cunha, Alexandre, Ângela Alves, Elsa Oliveira, and Gonçalo Calado. 2022. "Functional Histology and Ultrastructure of the Digestive Tract in Two Species of Chitons (Mollusca, Polyplacophora)" Journal of Marine Science and Engineering 10, no. 2: 160. https://doi.org/10.3390/jmse10020160
APA StyleLobo-da-Cunha, A., Alves, Â., Oliveira, E., & Calado, G. (2022). Functional Histology and Ultrastructure of the Digestive Tract in Two Species of Chitons (Mollusca, Polyplacophora). Journal of Marine Science and Engineering, 10(2), 160. https://doi.org/10.3390/jmse10020160






