Applying Surfactin in the Removal of Blooms of Karlodinium veneficum Increases the Toxic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture and Biosurfactant
2.2. Effect of Surfactin on the Population Growth of K. veneficum
2.3. Acute Effects of Surfactin on K. veneficum
2.4. Antioxidant Activity Variations in K. veneficum under Exposure to Surfactin
2.5. Mortality of Brine Shrimp Nauplii and Clam Juveniles Affected by Surfactin and K. veneficum
2.5.1. Toxicological Experiment on Brine Shrimp Nauplii
2.5.2. Toxicological Experiment on Clam Juveniles
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Effect of Surfactin on the Population Growth of K. veneficum
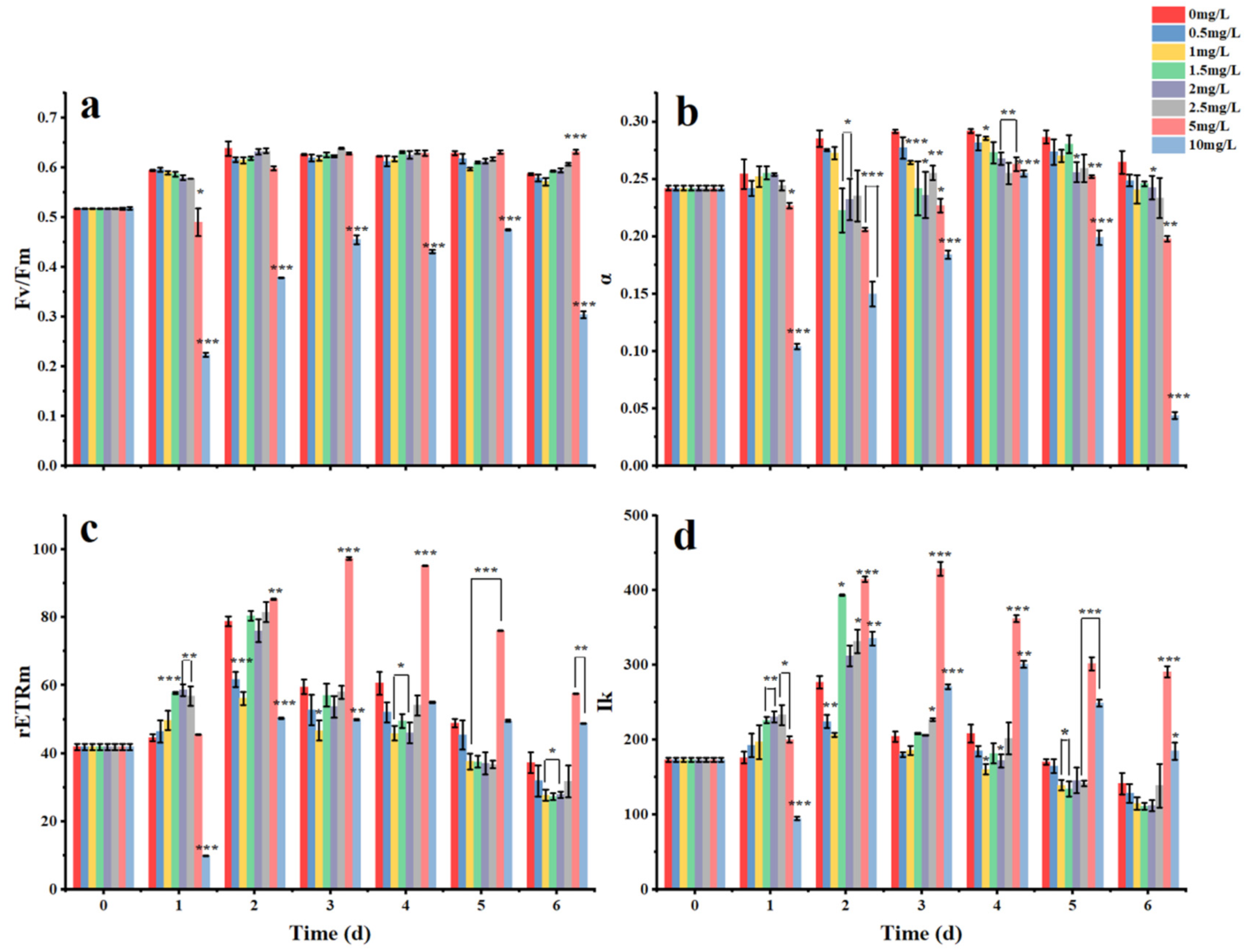
3.2. Acute Effects of Surfactin on K. veneficum
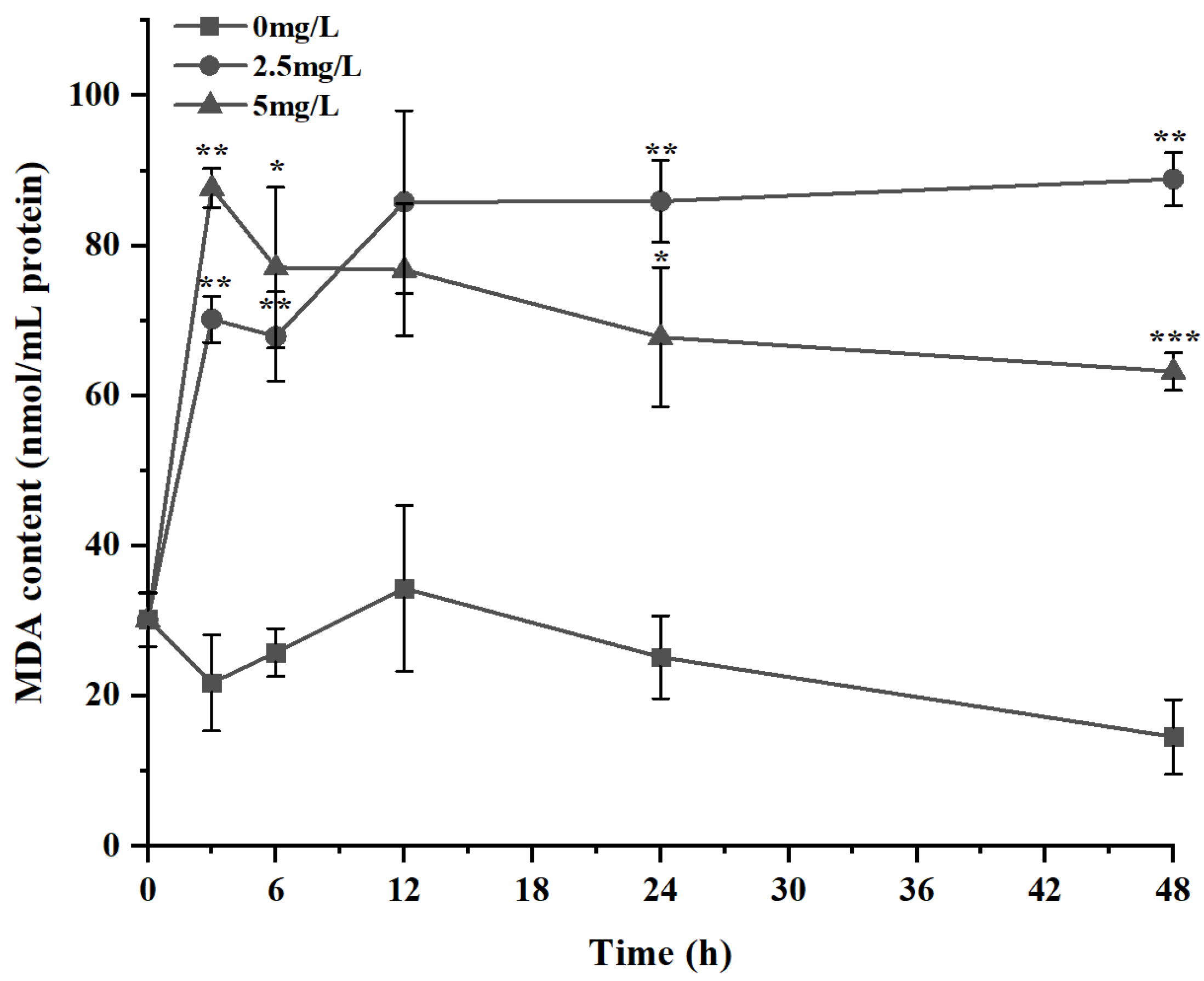
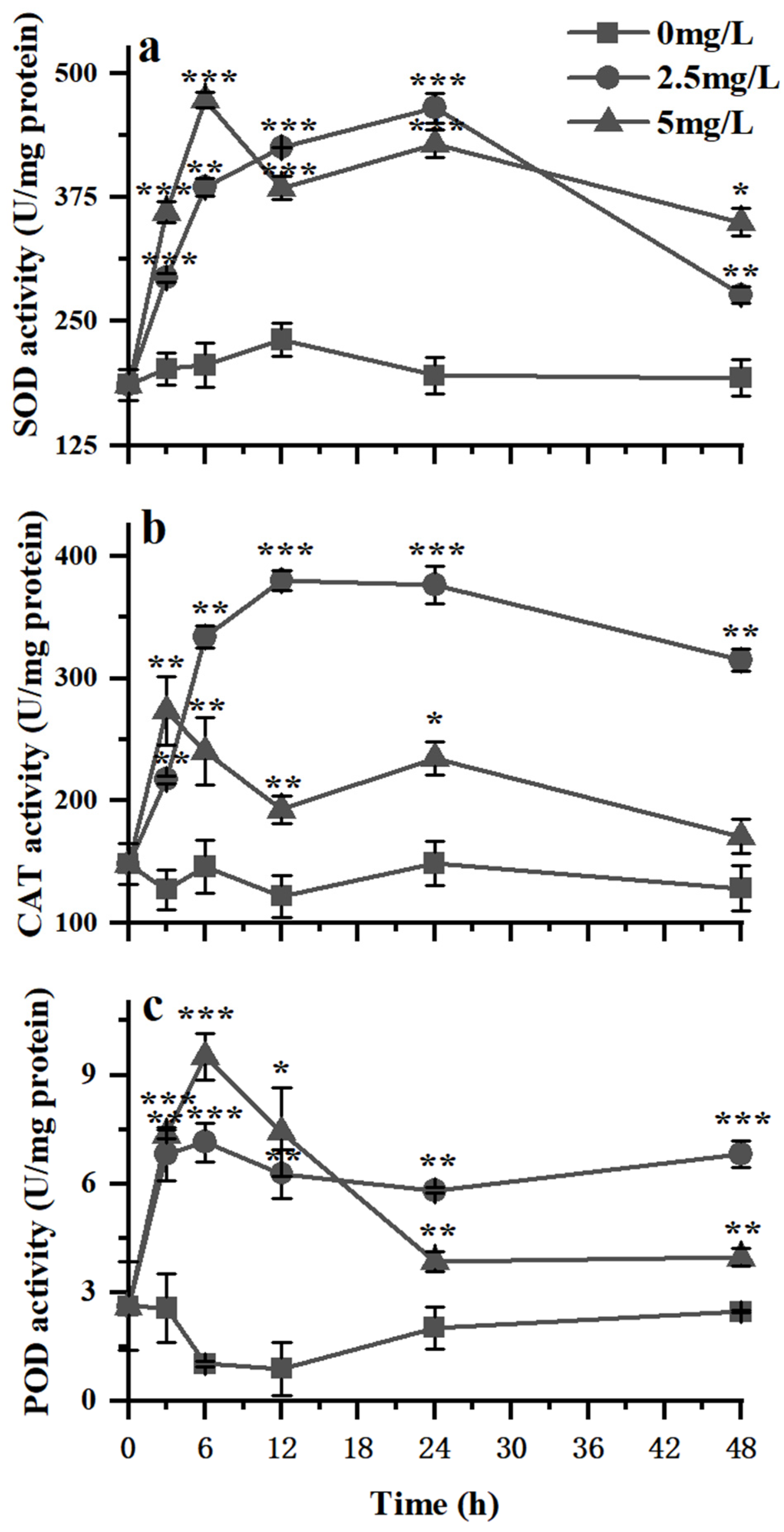
3.3. Variations in the Antioxidant Activity of K. veneficum under Exposure to Surfactin
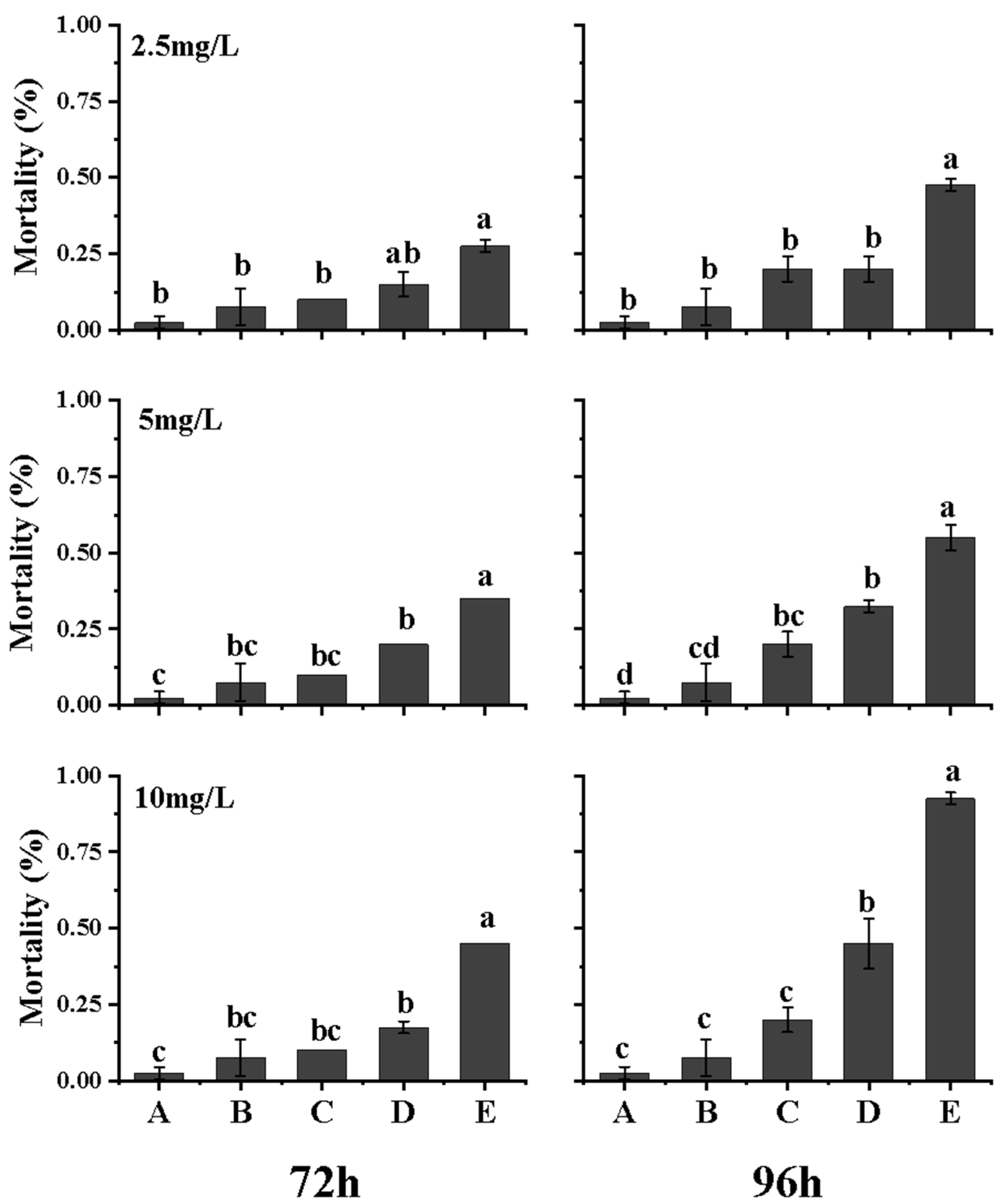
3.4. Synergistic Toxic Effect of Surfactin and K. veneficum on Brine Shrimp Nauplii
3.5. Synergistic Toxic Effect of Surfactin and K. veneficum on Clam Juveniles
4. Discussion
4.1. Population Growth
4.2. Chlorophyll Fluorescence Parameters
4.3. Variations in the Antioxidant Activity
4.4. Synergistic Toxic Effect of Surfactin and K. veneficum
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsen, J.; Moestrup, O. Guide to Toxic and Potentially Toxic Marine Algae; Institut for Sporeplanter, University of Copenhagen. Fish Inspection Service, Ministry of Fisheries: Copenhagen, Denmark, 1989; pp. 33–36. [Google Scholar]
- Nielsen, M.V. Chemical composition of the toxic dinoflagellate Gymnodinium galatheanum in relation to irradiance, temperature and salinity. Mar. Ecol. Prog. Ser. 1996, 136, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Place, A.R.; Bowers, H.A.; Bachvaroff, T.R.; Adolf, J.E.; Deeds, J.R.; Sheng, J. Karlodinium veneficum-the little dinoflagellate with a big bite. Harmful Algae 2012, 14, 179–195. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Deng, Y.; Tang, Y.Z. Evidence for resting cyst production in the cosmopolitan toxic dinoflagellate Karlodinium veneficum and the cyst distribution in the China Seas. Harmful Algae 2020, 93, 76–83. [Google Scholar] [CrossRef]
- Adolf, J.E.; Parrow, M.W.; Place, A.R. Karlodinium veneficum: Still Blooming and Toxic Sixty-Two Years Later; Nova Science Publishers: New York, NY, USA, 2020; Volume 10, pp. 353–403. [Google Scholar]
- Bjornland, T.; Tangen, K. Pigmentation and morphology of a marine Gyrodinium (Dinophycese) with a major carotenoid different from peridinin and fucoxanthin. J. Phycol. 1979, 15, 457–463. [Google Scholar]
- Deeds, J.R.; Terlizzi, D.E.; Adolf, J.E.; Stoecker, D.K.; Place, A.R. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae)—A dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 2002, 1, 169–189. [Google Scholar] [CrossRef]
- Li, A.S.; Stoecker, D.K.; Coats, D.W. Mixotrophy in Gyrodinium galatheanum (Dinophyceae): Grazing responses to light intensity and inorganic nutrients. J. Plankton Res. 2000, 36, 33–45. [Google Scholar] [CrossRef]
- Hall, N.S.; Litaker, R.W.; Fensin, E.; Adolf, J.E.; Bowers, H.A.; Place, A.R.; Paerl, H.W. Environmental factors contributing to the development and demise of a toxic dinoflagellate (Karlodinium veneficum) bloom in a shallow, eutrophic, lagoonal estuary. Estuaries Coasts 2008, 31, 402–418. [Google Scholar] [CrossRef]
- Dai, X.; Lu, D.; Guan, W.; Wang, H.; He, P.; Xia, P.; Yang, H. Newly recorded Karlodinium veneficum dinoflagellate blooms in stratified water of the East China Sea. Deep.-Sea Res. Part II 2014, 101, 237–243. [Google Scholar] [CrossRef]
- Deeds, J.R.; Reimschuessel, R.; Place, A.R. Histopathological effects in fish exposed to the toxins from karlodinium micrum. J. Aquat. Anim. Health 2006, 18, 136–148. [Google Scholar] [CrossRef]
- Wagoner, R.; Deeds, J.R.; Satake, M.; Ribeiro, A.A.; Wright, J. Wright. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 2008, 49, 6457–6461. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; He, S.; Zhou, C.; Place, A.R.; Haq, S.; Ding, L.; Chen, H.; Jiang, Y.; Guo, C.; Xu, Y.; et al. Two new karlotoxins found in Karlodinium veneficum (strain gm2) from the East China Sea. Harmful Algae 2016, 58, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adolf, J.E.; Bachvaroff, T.; Krupatkina, D.N.; Nonogaki, H.; Brown, P.J.P.; Lewitus, A.J. Species specificity and potential roles of Karlodinium micrum toxin. Afr. J. Med. Sci. 2006, 28, 415–421. [Google Scholar]
- Bachvaroff, T.R.; Adolf, J.E.; Place, A.R. Strain variation in Karlodinium veneficum (Dinophyceae): Toxin profiles, pigments, and growth characteristics. J. Phycol. 2009, 45, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fernández, N.; Chen, H.; You, Y.; Yan, X. Toxicological Studies of Karlodinium micrum (Dinophyceae) isolated from East China sea. Toxicon 2011, 57, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, Z.; Shang, L.; Deng, Y.; Tang, Y.Z. A strain of the toxic dinoflagellate karlodinium veneficum isolated from the east China sea is an omnivorous phagotroph. Harmful Algae 2020, 93, 11–27. [Google Scholar] [CrossRef]
- Sheng, J.; Malkiel, E.; Katz, J.; Adolf, J.; Place, A.R. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc. Natl. Acad. Sci. USA 2010, 107, 2082–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.N.; Dorantes-Aranda, J.J.; Seger, A.; Botana, M.T.; Brandini, F.P.; Hallegraeff, G.M. Ichthyotoxicity of the dinoflagellate Karlodinium veneficum in response to changes in seawater pH. Front. Mar. Sci. 2019, 6, 82. [Google Scholar] [CrossRef]
- Sun, P.F.; Lin, H.; Wang, G.; Lu, L.L.; Zhao, Y.H. Preparation of a new-style composite containing a key bioflocculant produced by Pseudomonas aeruginosa ZIU1 and its flocculating effect on harmful algal blooms. J. Hazard. Mater. 2015, 284, 215–221. [Google Scholar] [CrossRef]
- Hou, S.; Shu, W.; Tan, S.; Zhao, L.; Yin, P. Exploration of the antioxidant system and photosynthetic system of a marine algicidal bacillus and its effect on four harmful algal bloom species. Can. J. Microbiol. 2016, 62, 49–59. [Google Scholar] [CrossRef]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid Surfactant produced by Bacillussubtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochemical and Biophysical Research Communications. 1968, 31, 488–494. [Google Scholar] [CrossRef]
- Park, G.; Nam, J.; Kim, J.; Song, J.; Kim, P.I.; Min, H.J.; Lee, C.W. Structure and Mechanism of Surfactin Peptide from Bacillus velezensis Antagonistic to Fungi Plant Pathogens. Bull. Korean Chem. Soc. 2019, 40, 704–709. [Google Scholar] [CrossRef]
- Abdelmawgoud, A.M.; Aboulwafa, M.; Hassouna, N.A. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef]
- De Oliveira, D.W.; Cara, A.B.; Lechuga-Villena, M.; García-Román, M.; Melo, V.M.M.; Gonçalves, L.R.B.; Vaz, D.A. Aquatic toxicity and biodegradability of a surfactant produced by Bacillus subtilis ICA56. Journal of environmental science and health. Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 174–181. [Google Scholar] [CrossRef]
- Mei, Y.; Yang, Z.; Kang, Z.; Yu, F.; Long, X. Enhanced surfactin fermentation via advanced repeated fed-batch fermentation with increased cell density stimulated by EDTA–Fe (II). Food Bioprod. Processing 2021, 127, 288–294. [Google Scholar] [CrossRef]
- Hadia, N.J.; Ottenheim, C.; Li, S.; Hua, N.Q.; Stubbs, L.P.; Lau, H.C. Experimental investigation of biosurfactant mixtures of surfactin produced by Bacillus Subtilis for EOR application. Fuel 2019, 251, 789–799. [Google Scholar] [CrossRef]
- Yang, Z.; Zu, Y.; Zhu, J.; Jin, M.; Cui, T.; Long, X. Application of biosurfactant surfactin as a pH-switchable biodemulsifier for efficient oil recovery from waste crude oil. Chemosphere 2020, 240, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications. Mar. Drugs 2019, 17, 408. [Google Scholar] [CrossRef] [Green Version]
- Fei, D.; Zhou, G.W.; Yu, Z.Q.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Ye, R.Q.; Mu, B.Z. Low-Toxic and Nonirritant Biosurfactant Surfactin and its Performances in Detergent Formulations. J. Surfactants Deterg. 2020, 23, 109–118. [Google Scholar] [CrossRef]
- Ahire, J.J.; Robertson, D.D.; Van Reenen, A.J.; Dicks, L.M.T. Surfactin-loaded polyvinyl alcohol (PVA) nanofibers alters adhesion of Listeria monocytogenes to polystyrene. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 27–33. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhu, X.; Zhao, H.; Lu, Y.; Zhang, C.; Lu, Z. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biotechnol. 2019, 103, 4565–4574. [Google Scholar] [CrossRef]
- Gomes, M.Z.; Nitschke, M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control 2012, 25, 441–447. [Google Scholar] [CrossRef]
- Desmyttere, H.; Deweer, C.; Muchembled, J.; Sahmer, K.; Jacquin, J.; Coutte, F.; Jacques, P. Antifungal Activities of Bacillus subtilis Lipopeptides to Two Venturia inaequalis Strains Possessing Different Tebuconazole Sensitivity. Front. Microbiol. 2019, 10, 2327. [Google Scholar] [CrossRef]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BioMed Central 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Joung, S.; Jeon, J.; Kim, H.; Yoon, B.; Oh, H. Selective control of cyanobacteria by surfactin-containing culture broth of Bacillus subtilis C1. Biotechnol. Lett. 2003, 25, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ahn, C.Y.; Joung, S.H.; Ahn, J.S.; Oh, H.M. Growth inhibition of Microcystis aeruginosa by a glycolipid-type compound from Bacillus subtilis C1. J. Microbiol. Biotechnol. 2010, 20, 1240–1242. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Sun, X.X.; Lee, Y.J.; Wang, S.Y.; Han, K.N.; Choi, J.K.; Noh, J.H.; Kim, E.K. Mitigation of Harmful Algal Blooms by Sophorolipid. J. Microbiol. Biotechnol. 2003, 13, 651–659. [Google Scholar]
- Sun, X.X.; Choi, J.K.; Kim, E.K. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J. Exp. Mar. Biol. Ecol. 2004, 304, 35–49. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Liang, S.; Han, X.; Zhu, C.; Li, Y. Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 2004, 4, 433–443. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Chai, J.; Chen, Y.; Lu, J.; Fang, Y.; Wang, S.; Lu, Z. Inhibitory effect of non ribosomal peptides from Bacillus subtilis fmb60 strain on the growth of Microcystis aeruginosa. Acta Bioeng. Sin. 2021, 37, 625–634. (In Chinese) [Google Scholar]
- Baker, N.R. A possible role for photosystem II in environmental perturbations of photosynthesis. Physiologia Plantarum 1991, 81, 563–570. [Google Scholar] [CrossRef]
- Ryan, K.G.; Ralph, P.J.; McMinn, A. Acclimation of Antarctic bottom-ice algal communities to lowered salinities during melting. Polar Biol. 2004, 27, 679–686. [Google Scholar] [CrossRef]
- Bjrkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.S. Leaf Chlorophyll Fluorescence: Background and Fundamentals for Plant Biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- Tan, S.; Hu, X.; Yin, P.; Zhao, L. Photosynthetic inhibition and oxidative stress to the toxic phaeocystis globosa caused by a diketopiperazine isolated from products of algicidal bacterium metabolism. J. Microbiol. 2016, 54, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sun, S.; Jia, R.; Xu, L.; Hou, L. Effects of sulfamethoxazole exposure on the growth, antioxidant system of Chlorella vulgaris and Microcystis aeruginosa. Bull. Environ. Contam. Toxicol. 2020, 105, 358–365. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, Q. Toxic effects of hydrilla verticillata exposed to toluene, ethylbenzene and xylene and safety assessment for protecting aquatic macrophytes. Chemosphere 2011, 85, 1088–1094. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.; Zhang, J.; Zhu, S.; Shen, Y.; Zhang, R. Effect of fluoride on photosynthetic pigment content and antioxidant system of hydrilla verticillata. Int. J. Phytoremediation 2018, 20, 1257–1263. [Google Scholar] [CrossRef]
- Liao, K.; Chen, W.; Zhang, R.; Zhou, H.; Xu, J.; Zhou, C.; Yan, X. qPCR analysis of bivalve larvae feeding preferences when grazing on mixed microalgal diets. PLoS ONE 2017, 12, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adolf, J.E.; Stoecker, D.K.; Harding, L.W. Autotrophic growth and photoacclimation in Karlodinium micrum (dinophyceae) and Storeatula major (cryptophyceae). J. Phycol. 2010, 39, 1101–1108. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Place, A.R.; Warner, M.E.; Coyne, K.J. Investigation of the algicidal exudate produced by Shewanella sp. iri-160 and its effect on dinoflagellates. Harmful Algae 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Tilney, C.L.; Pokrzywinski, K.L.; Coyne, K.J.; Warner, M.E. Growth, death, and photobiology of dinoflagellates (Dinophyceae) under bacterial-algicide control. J. Appl. Phycol. 2014, 26, 2117–2127. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Liu, H.; Zheng, Z. Effects of Sodium Dodecyl Benzene Sulfonate on Photosynthesis of Microcystis aeruginosa. Dly. Chem. Ind. 2008, 5, 17–21. (In Chinese) [Google Scholar]
- Turner, J.T.; Tester, P.A. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 1997, 42, 1203–1214. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.Z.; Gobler, C.J. Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol. 2009, 156, 2601–2611. [Google Scholar] [CrossRef]
- Yang, X.; Wen, X.; Zhou, C.; Zhu, X.; Meng, R.; Luo, Q.; Yan, X. Comparative study of brine shrimp bioassay-based toxic activities of three harmful microalgal species that frequently blooming in aquaculture ponds. J. Oceanol. Limnol. 2018, 36, 1697–1706. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Meng, R.; Zhou, C.; Pan, Y.; Yan, X. Applying Surfactin in the Removal of Blooms of Karlodinium veneficum Increases the Toxic Potential. J. Mar. Sci. Eng. 2022, 10, 196. https://doi.org/10.3390/jmse10020196
Tian X, Meng R, Zhou C, Pan Y, Yan X. Applying Surfactin in the Removal of Blooms of Karlodinium veneficum Increases the Toxic Potential. Journal of Marine Science and Engineering. 2022; 10(2):196. https://doi.org/10.3390/jmse10020196
Chicago/Turabian StyleTian, Xiaoyu, Ran Meng, Chengxu Zhou, Yuanbo Pan, and Xiaojun Yan. 2022. "Applying Surfactin in the Removal of Blooms of Karlodinium veneficum Increases the Toxic Potential" Journal of Marine Science and Engineering 10, no. 2: 196. https://doi.org/10.3390/jmse10020196





