A Multi-Proxy Approach to Reconstruct Hypoxia on the NW Black Sea Shelf over the Holocene
Abstract
:1. Introduction
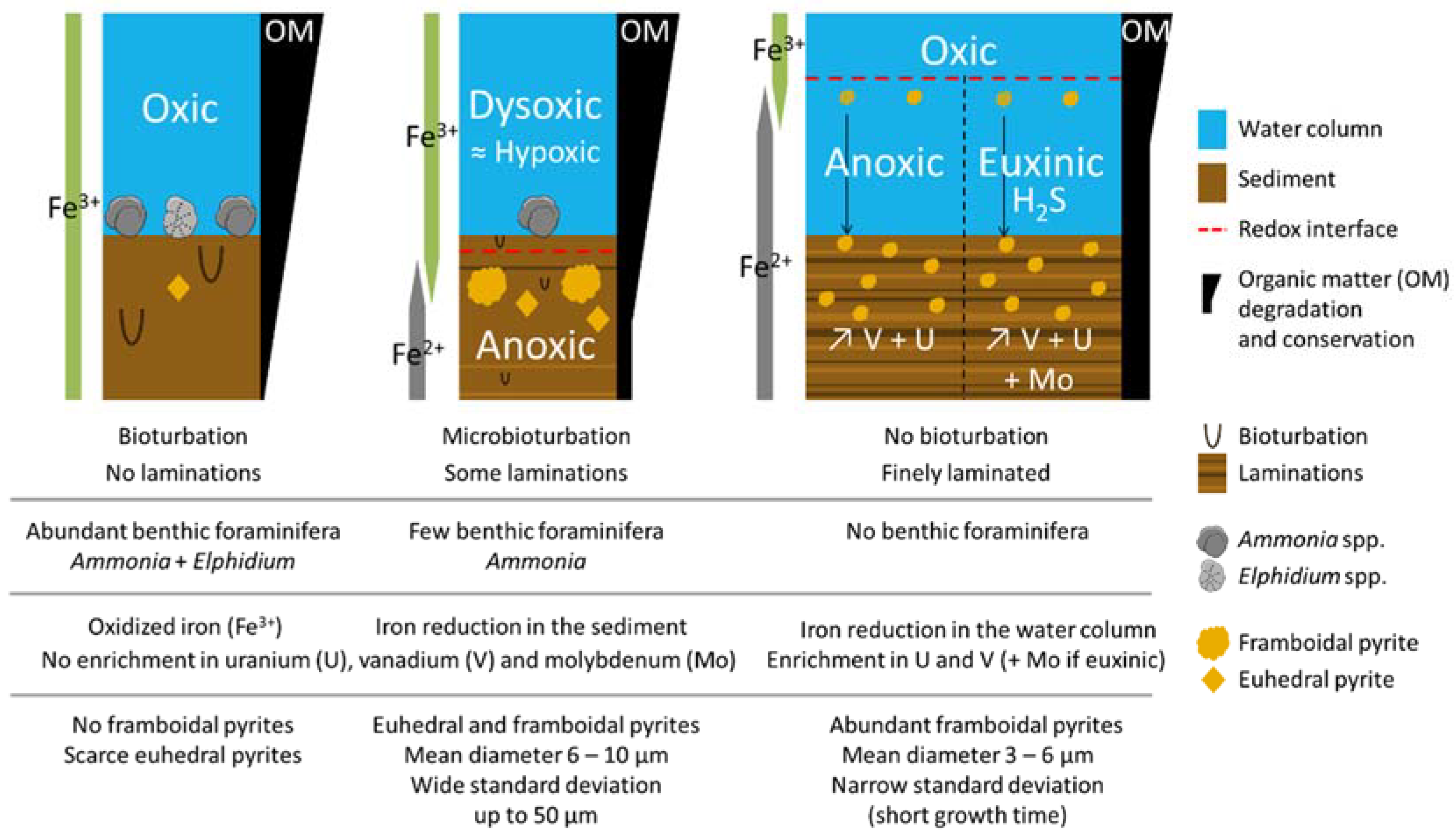
2. Potential Proxies for Bottom Water Oxygen Level
2.1. Biological Proxy: Benthic Foraminifera
2.2. Sedimentological Proxies
2.3. Geochemical Proxies
2.4. Mineralogical Proxy: Framboidal Pyrites
3. Materials and Methods
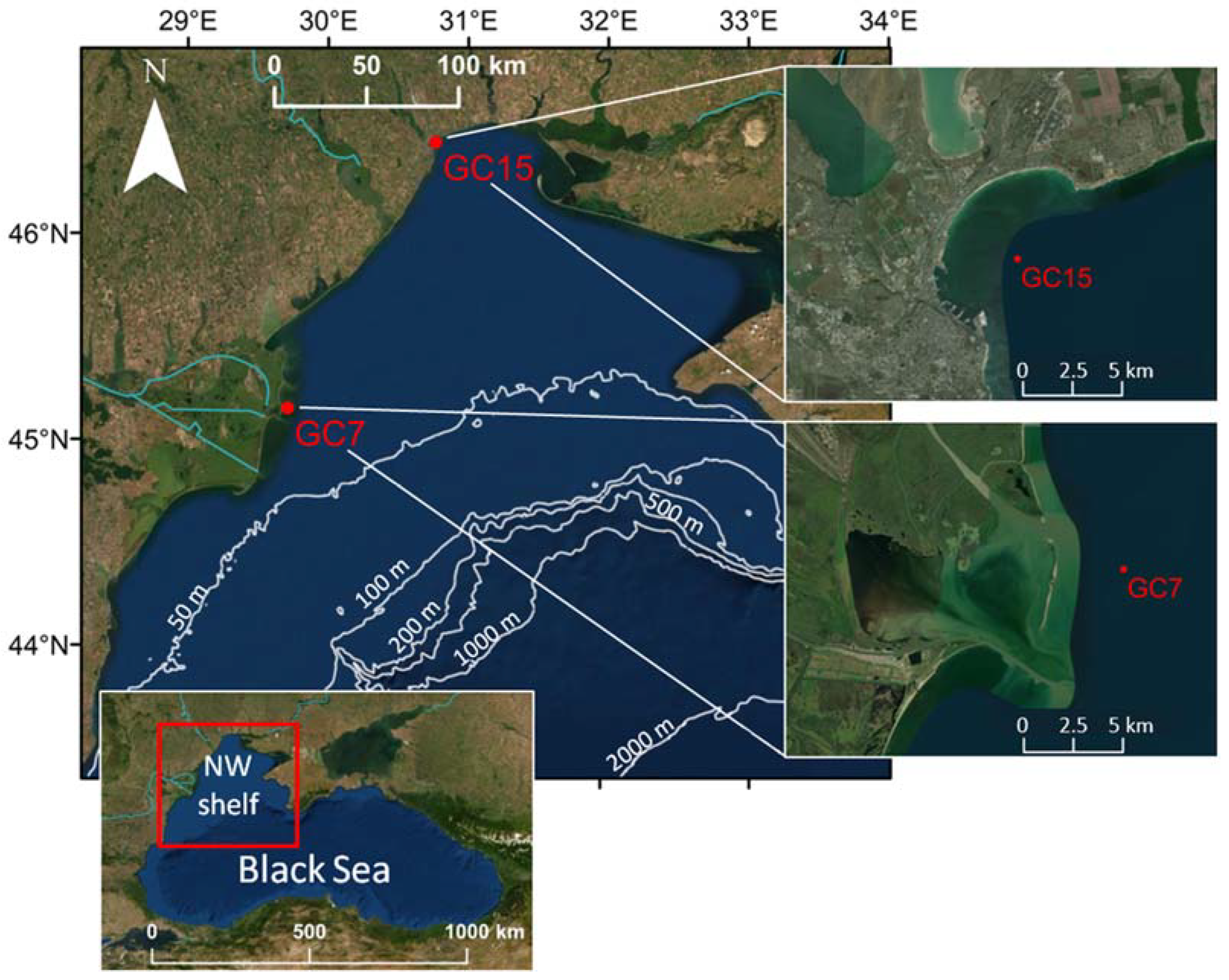
3.1. Study Sites
3.2. Sampling Strategy and Sedimentological Methods
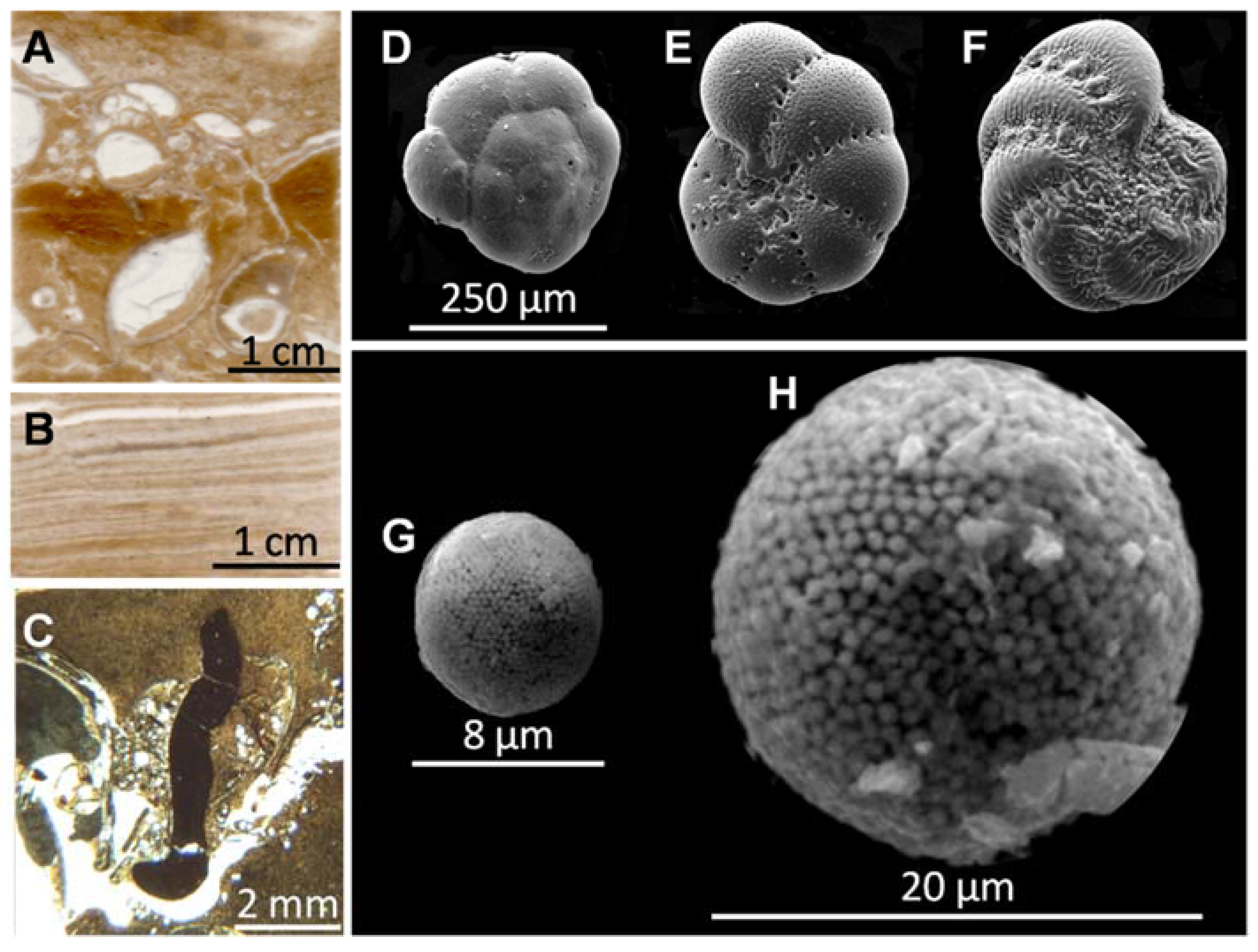
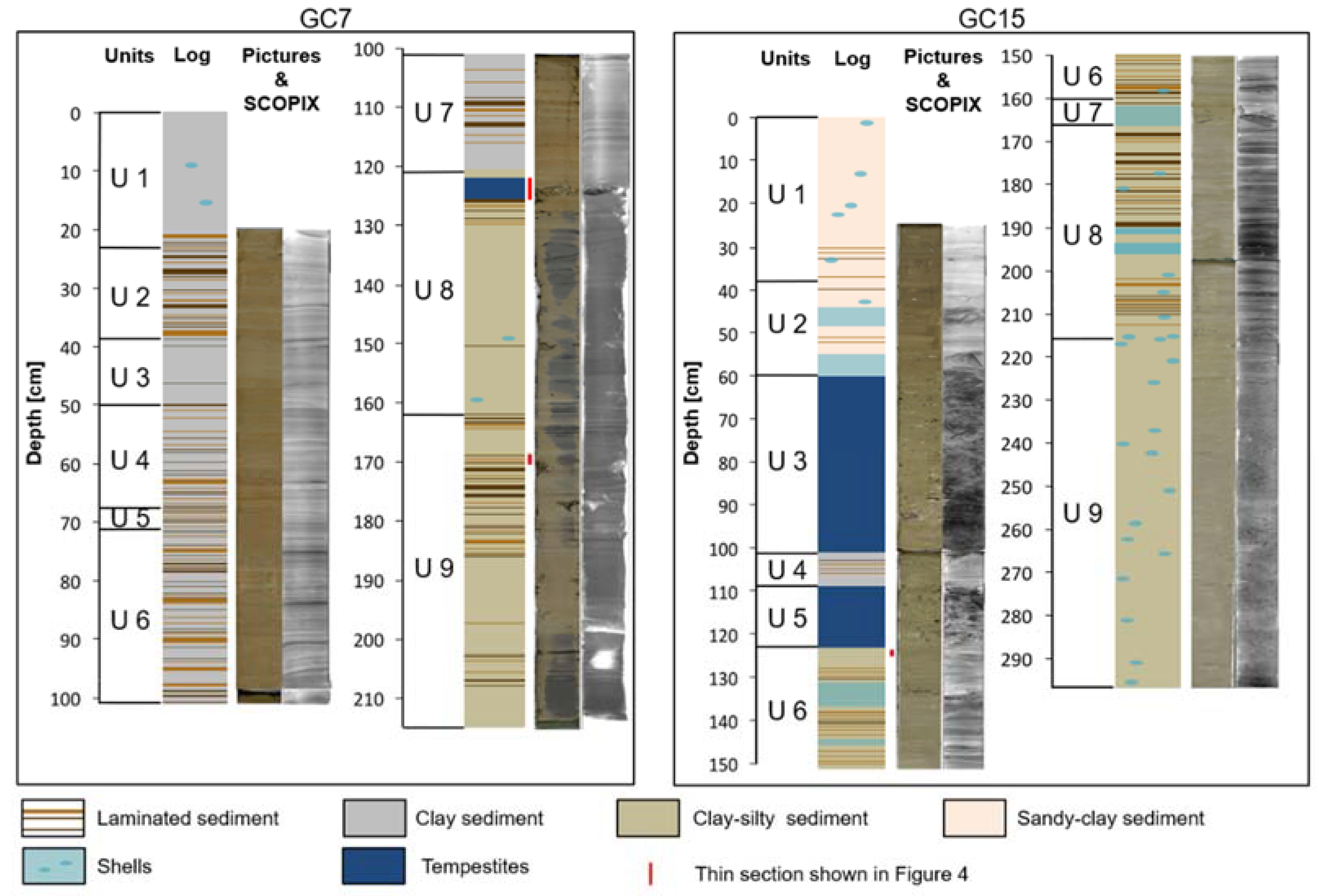
3.3. Description of the Cores and Sediment Characteristics
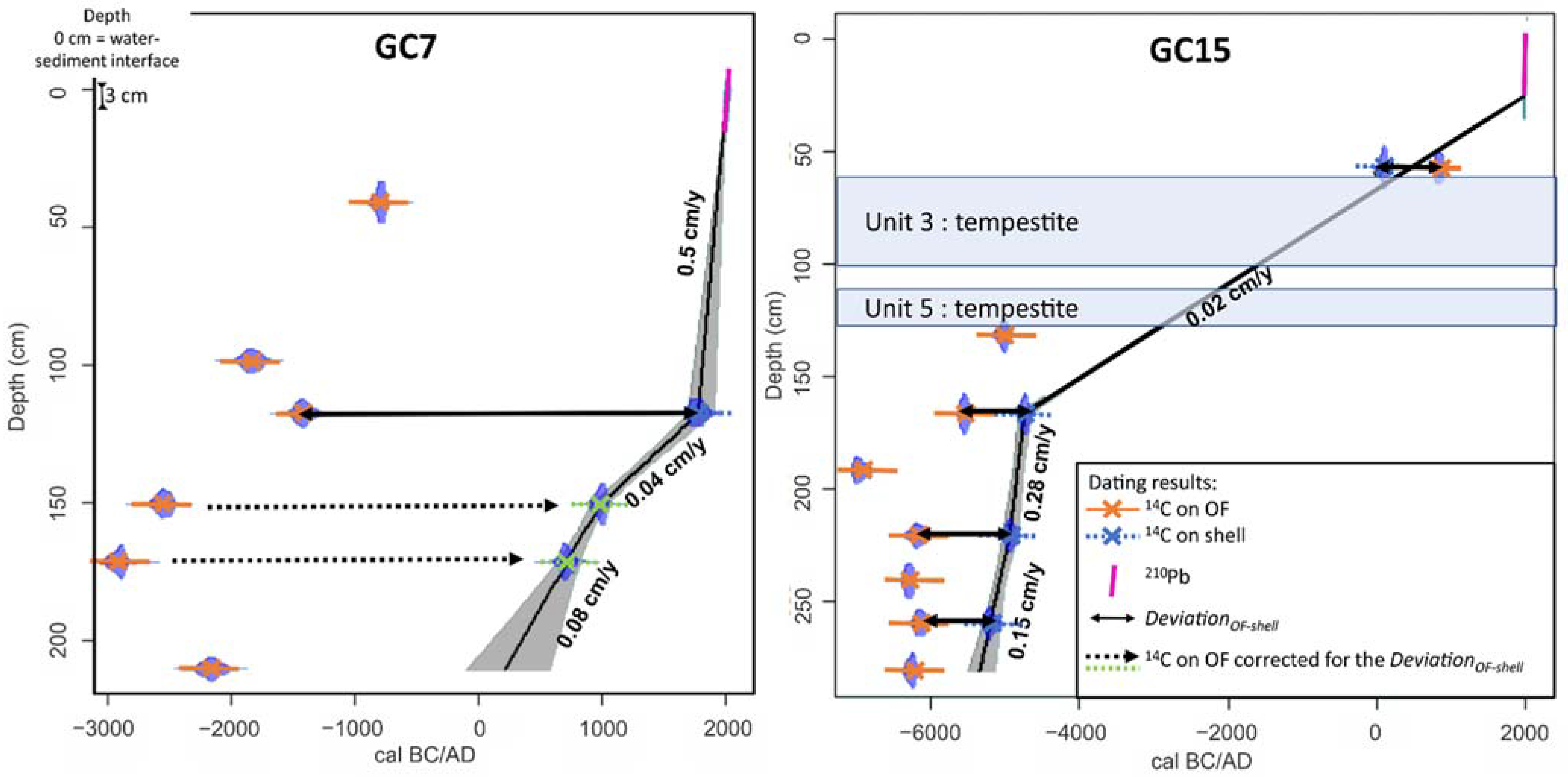
3.4. Core Chronology
3.5. Methods to Investigate Anoxia/Hypoxia Conditions
3.5.1. Biological Proxy: Benthic Foraminifera
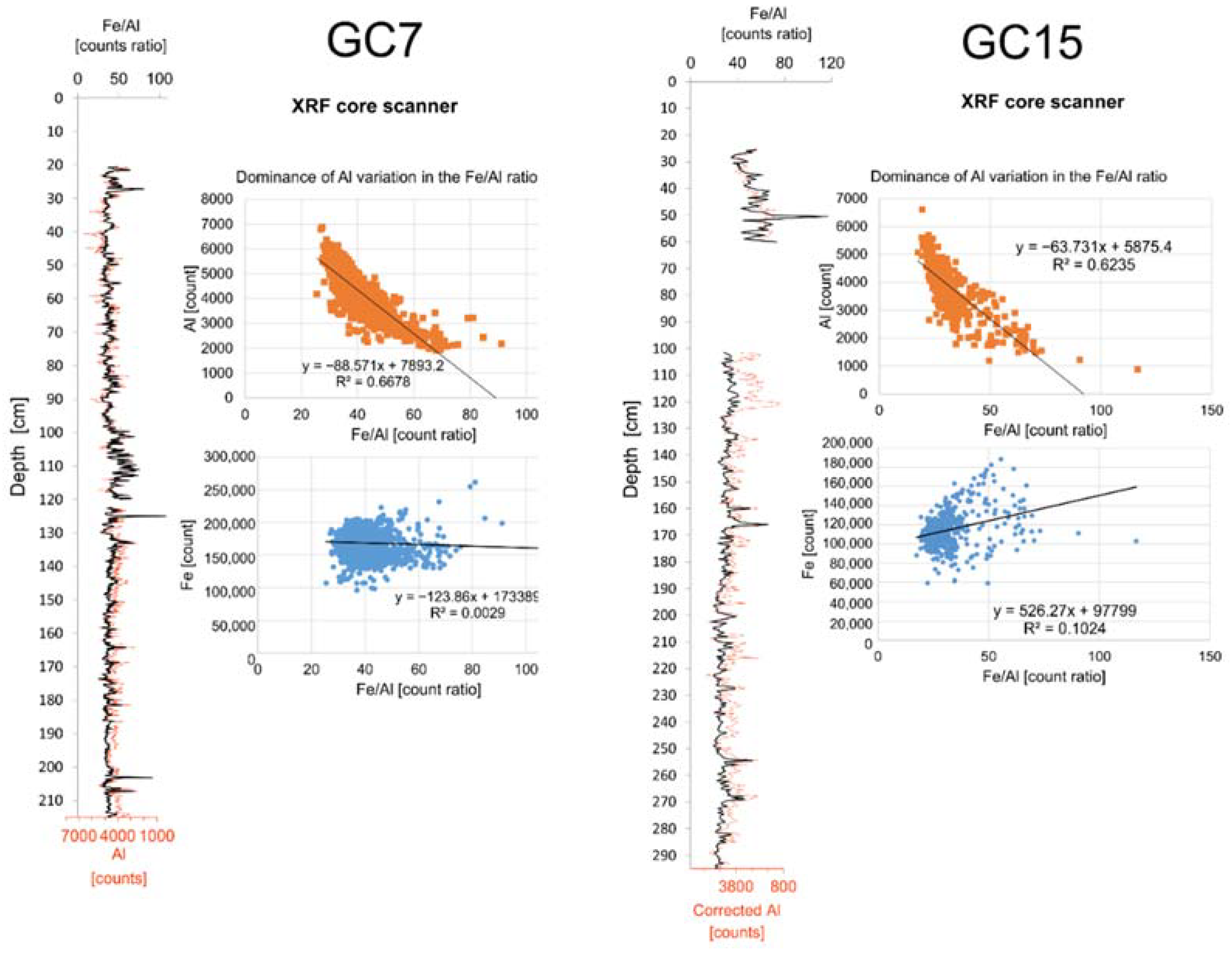
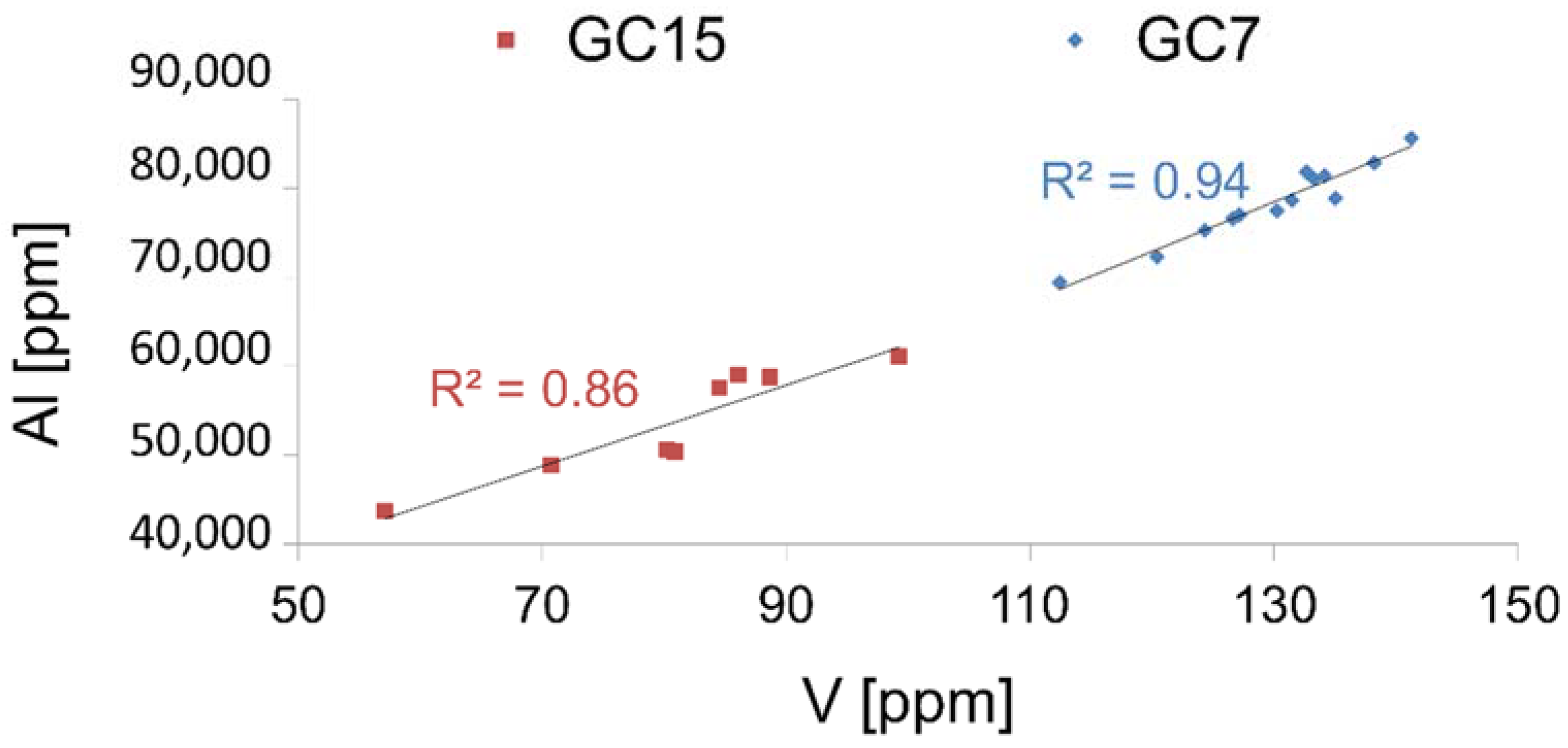
3.5.2. Geochemical Proxies
- It should be possible to distinguish Fe with a detrital signature from Fe remobilised due to changes in redox conditions;
- High Fe/Al ratio in our cores could be used as a proxy for anoxia as in Spofforth et al. [26].
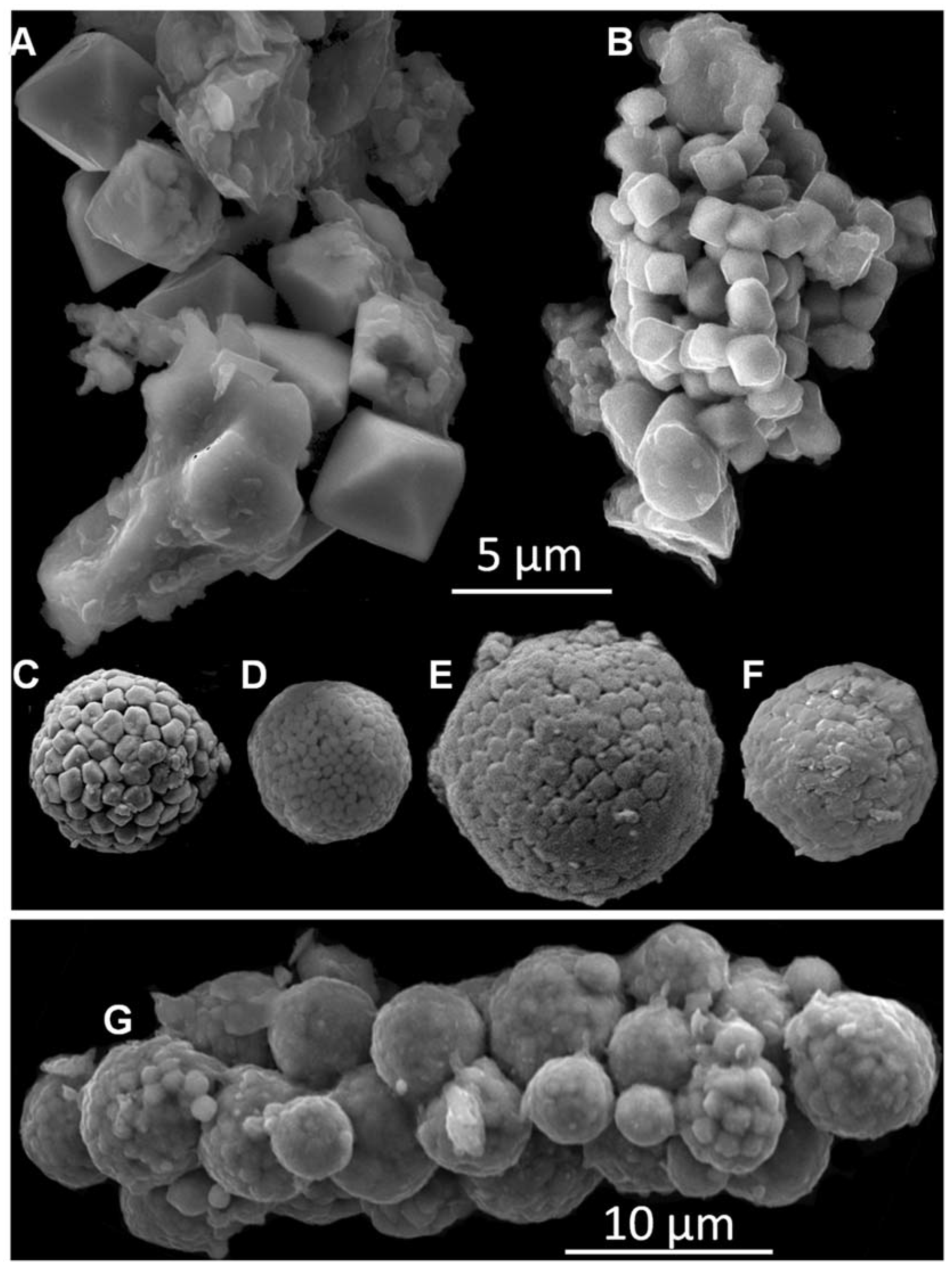
3.5.3. Mineralogical Proxy: Framboidal Pyrites
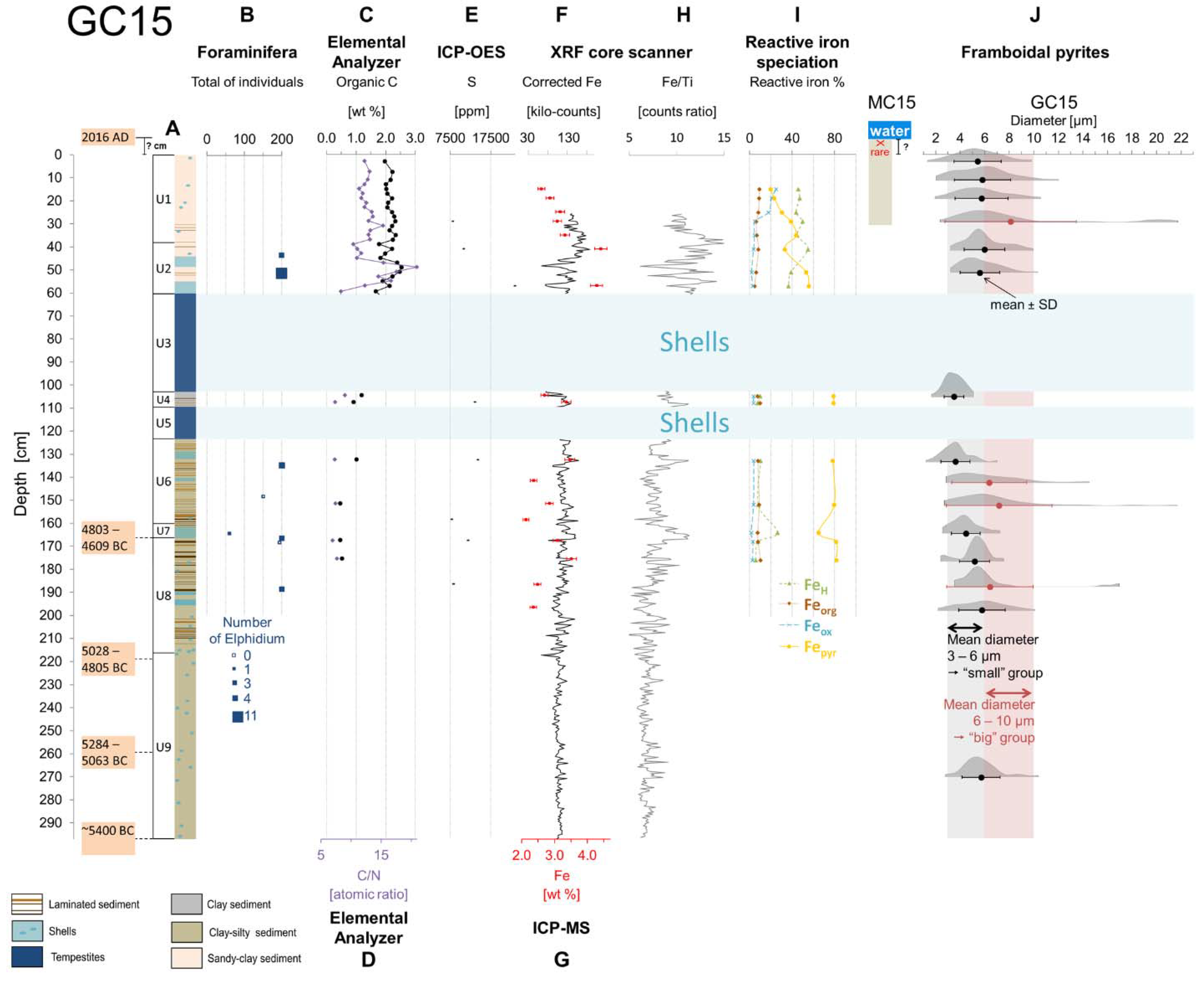
4. Results
4.1. Benthic Foraminifera
4.2. Geochemical Proxies
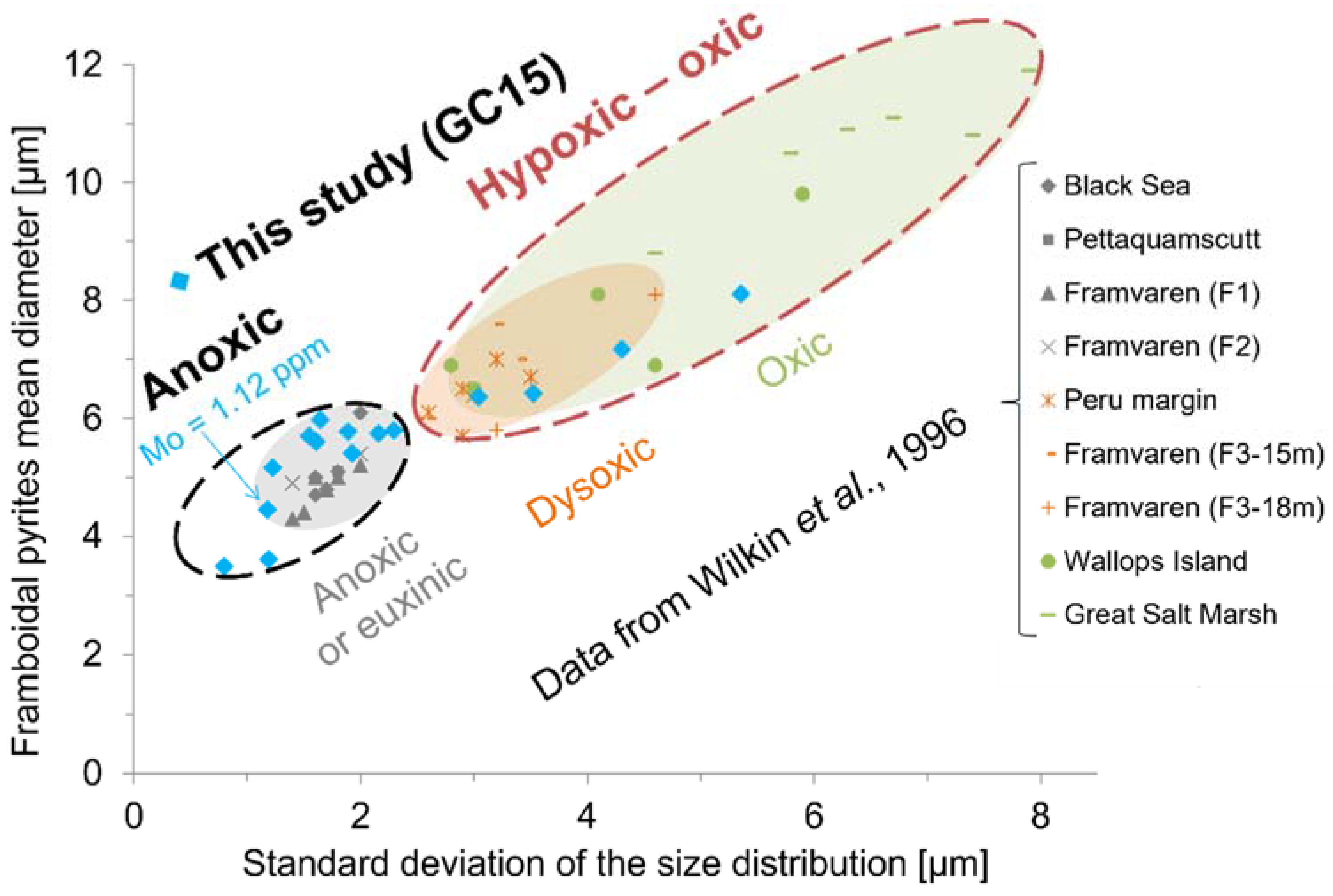
4.3. Mineralogical Proxy: Framboidal Pyrites
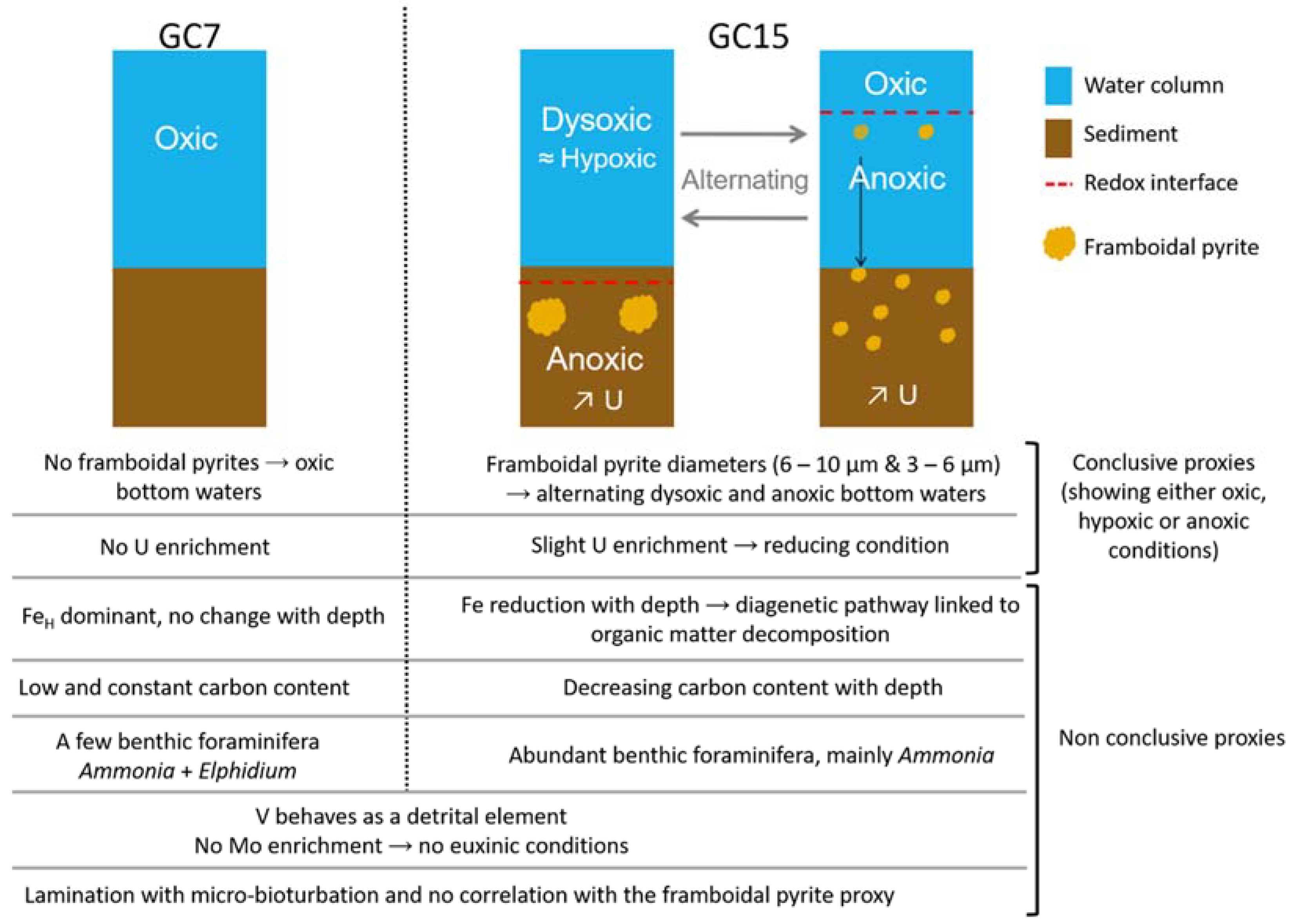
5. Discussion
5.1. Past Oxygenation of the NW Black Sea Shelf Inferred from the Framboidal Pyrite Proxy
5.2. Other Proxies Considered as Inappropriate in the Case of the Black Sea Coastal Environment
5.2.1. Laminations
5.2.2. Foraminifera
5.2.3. Organic Matter
5.2.4. Trace Metals
5.2.5. Iron Behaviour
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Core | Depth (cm) | Material Dated | 210Pb cal BP | Error | Inferred Sedimentation Rate (cm/year) | Included in the Model Age |
|---|---|---|---|---|---|---|
| MC7 | 0.5 | OF | 2015 | 5 | 0.41 | Yes |
| 4 | OF | 2006 | 5 | Yes | ||
| 8 | OF | 1997 | 5 | Yes | ||
| GC15 | 0.5 | OF | 2015 | 5 | 0.65 | No |
| 10.5 | OF | 2000 | 5 | No | ||
| 20.5 | OF | 1984 | 5 | No | ||
| 28 | OF | 1973 | 5 | No | ||
| 32 | OF | 1967 | 5 | No | ||
| 34 | OF | 1963 | 5 | No | ||
| 25 | / | 1977 | 5 | Yes |
| Core | Laboratory ID | Depth (cm) | Material Dated | 14C Age (BP) | Error | WiTi 14C Age (BP) | Ranges of Calendar Age for 95.4% Confidence Level | Local ΔR | DeviationOF-shell |
|---|---|---|---|---|---|---|---|---|---|
| GC7 | GdA-5385 | 41 | OF | 2930 | 45 | 2730 ± 35 | 2704 BP (95.4%) 2361 BP | 126 ± 40 a | / |
| GdA-5812 | OF | 2640 | 30 | 2842 BP (2.5%) 2825 BP–2794 BP (92.9%) 2735 BP | |||||
| GdA-5813 | 98.5 | OF | 3960 | 45 | / | 4526 BP (93.5%) 4284 BP–4272 BP (1.9%) 4256 BP | / | ||
| GdA-5037 | 117.5 | Shell | 665 | 30 | / | 675 BP (50.1%) 630 BP–600 BP (45.3%) 560 BP | 2950 ± 60 | ||
| GdA-5814 | OF | 3615 | 50 | / | 4088 BP (94.1%) 3826 BP–3788 BP (0.9%) 3776 BP–3740 BP (0.4%) 3734 BP | ||||
| GdA-5386 | 150.5 | OF | 4490 | 35 | / | 4697 BP (95.4%) 4360 BP | 2950 ± 60 | ||
| GdA-5815 | 171.5 | OF | 4760 | 50 | / | 5594 BP (75.1%) 5446 BP | 2950 ± 60 | ||
| GdA-5387 | 210.5 | OF | 4200 | 45 | / | 4300 BP (95.4%) 3920 BP | / | ||
| GC15 | GdA-5040 | 57 | Shells | 2110 | 30 | 2260 ± 30 | 1698 BP (7.3%) 1645 BP–1638 BP (88.1%) 1380 BP | 13 ± 60 b | −690 ± 50 |
| GdA-5816A | Shells | 2410 | 30 | 2139 BP (95.4%) 1816 BP | |||||
| GdA-5816 | OF | 1570 | 40 | / | 1548 BP (95.4%) 1378 BP | ||||
| GdA-5388 | 131.5 | OF | 6525 | 35 | / | 7020 BP (95.4%) 6724 BP | 57 ± 85 a | / | |
| GdA-5817A | 166.5 | Shells | 6365 | 35 | / | 6792 BP (95.4%) 6466 BP | 135 ± 60 b | 765 ± 57 | |
| GdA-5817 | OF | 7130 | 45 | / | 8024 BP (79.7%) 7916 BP–7905 BP (15.7%) 7854 BP | ||||
| GdA-5389 | 191.5 | OF | 8385 | 40 | / | 8970 BP (95.4%) 8614 BP | 57 ± 85 a | / | |
| GdA-5818A | 220.5 | Shells | 6505 | 40 | / | 7144 BP (0.9%) 7129 BP–7011 BP (94.5%) 6664 BP | 106 ± 60 b | 1270 ± 72 | |
| GdA-5818 | OF | 7775 | 60 | / | 8700 BP (2.2%) 8669 BP–8658 BP (93.2%) 8415 BP | ||||
| GdA-5819A | 259.5 | Shells | 6745 | 45 | / | 7276 BP (95.4%) 6912 BP | 130 ± 60 b | 1015 ± 64 | |
| GdA-5819 | OF | 7760 | 55 | / | 8632 BP (95.4%) 8422 BP | ||||
| GdA-5390 | 280.5 | OF | 7780 | 40 | / | 8258 BP (95.4%) 7979 BP | 57 ± 85 a | / |
| GC15 | GC7 | ||||||
|---|---|---|---|---|---|---|---|
| Depth [cm] | % N | % C | C/N Atomic Ratio | Depth [cm] | % N | %C | C/N Atomic Ratio |
| 1–5 | 0.19 | 2.0 | 12.3 | 0–1 | 0.14 | 1.5 | 13.1 |
| 5–10 | 0.20 | 2.2 | 13.1 | 1–2 | 0.12 | 1.2 | 11.7 |
| 10–12 | 0.20 | 2.2 | 12.8 | 2–3 | 0.13 | 1.0 | 9.7 |
| 12–14 | 0.19 | 2.0 | 12.2 | 3–4 | 0.12 | 0.9 | 9.4 |
| 14–16 | 0.21 | 2.0 | 11.3 | 4–5 | 0.14 | 1.2 | 10.0 |
| 16–18 | 0.20 | 2.1 | 11.9 | 5–6.5 | 0.14 | 1.3 | 10.9 |
| 18–20 | 0.22 | 2.2 | 11.7 | 6.5–8 | 0.12 | 1.1 | 11.0 |
| 20–22 | 0.19 | 2.1 | 12.6 | 8–9 | 0.12 | 1.0 | 10.2 |
| 22–24 | 0.20 | 2.1 | 12.2 | 9–10 | 0.12 | 1.1 | 10.4 |
| 24–26 | 0.19 | 2.2 | 13.5 | 10–11 | 0.13 | 1.1 | 9.5 |
| 26–28 | 0.20 | 2.3 | 13.7 | 11–12 | 0.15 | 1.2 | 9.7 |
| 28–30 | 0.21 | 2.3 | 12.9 | 12–13 | 0.15 | 1.3 | 10.3 |
| 30–32 | 0.17 | 2.2 | 15.3 | 13–14 | 0.14 | 1.4 | 11.7 |
| 32–34 | 0.19 | 2.1 | 13.2 | 14–15 | 0.15 | 1.3 | 10.1 |
| 34–36 | 0.21 | 2.3 | 12.9 | 15–16 | 0.16 | 1.5 | 10.7 |
| 36–38 | 0.20 | 2.2 | 13.1 | 16–17 | 0.14 | 1.3 | 10.6 |
| 38–40 | 0.20 | 1.8 | 10.3 | 17–18 | 0.16 | 1.6 | 11.2 |
| 40–42 | 0.23 | 2.2 | 11.0 | 18–19 | 0.13 | 1.2 | 11.1 |
| 42–44 | 0.20 | 2.0 | 11.7 | 19–20 | 0.14 | 1.3 | 10.4 |
| 44–46 | 0.19 | 1.8 | 11.0 | 20–21 | 0.15 | 1.7 | 12.9 |
| 46–48 | 0.18 | 2.4 | 15.4 | 21–22 | 0.09 | 0.9 | 11.7 |
| 48–50 | 0.14 | 2.5 | 20.9 | 22–23 | 0.14 | 1.3 | 11.0 |
| 50–52 | 0.16 | 2.5 | 17.6 | 23–24 | 0.12 | 1.0 | 9.8 |
| 52–54 | 0.18 | 2.2 | 14.5 | 24–25 | 0.13 | 1.1 | 9.8 |
| 54–56 | 0.13 | 1.9 | 16.6 | 25–26 | 0.12 | 0.9 | 9.0 |
| 56–58 | 0.20 | 2.1 | 12.3 | 26–28 | 0.11 | 0.8 | 8.6 |
| 104–105 | 0.13 | 1.2 | 9.0 | 28–29 | 0.15 | 1.3 | 10.5 |
| 107–108 | 0.12 | 0.9 | 7.3 | 29–30 | 0.14 | 1.2 | 10.7 |
| 132–133 | 0.14 | 1.0 | 7.3 | 30–31 | 0.14 | 1.2 | 9.9 |
| 151–152 | 0.06 * | 0.5 | 7.4 * | 31–32 | 0.14 | 1.2 | 9.7 |
| 167–168 | 0.07 * | 0.5 | 7.0 * | 32–33 | 0.15 | 1.1 | 9.0 |
| 175–176 | 0.07 * | 0.5 | 7.7 * | 33–34.5 | 0.13 | 1.0 | 9.2 |
| 34.5–35.5 | 0.15 | 1.4 | 10.9 | ||||
| 35.5–36.5 | 0.11 | 1.0 | 10.3 | ||||
| 36.5–37.5 | 0.12 | 1.0 | 9.3 | ||||
| 37.5–38.5 | 0.11 | 0.9 | 9.8 | ||||
| 38.5–39.5 | 0.13 | 1.1 | 9.3 | ||||
| 39.5–40.5 | 0.13 | 1.0 | 8.9 | ||||
| 40.5–41.5 | 0.13 | 1.1 | 9.7 | ||||
| 41.5–42.5 | 0.08 | 0.6 | 8.8 | ||||
| 42.5–43.5 | 0.09 | 0.8 | 10.8 | ||||
| 43.5–44.5 | 0.11 | 0.9 | 8.8 | ||||
| 44.5–45.5 | 0.11 | 0.8 | 8.3 | ||||
| 45.5–46.5 | 0.12 | 1.0 | 9.5 | ||||
| 46.5–47.5 | 0.11 | 0.8 | 9.2 | ||||
| 47.5–48.5 | 0.11 | 0.8 | 8.2 | ||||
| 48.5–49.5 | 0.11 | 0.8 | 8.0 | ||||
| 49.5–50.5 | 0.11 | 0.8 | 8.7 | ||||
| 50.5–51.5 | 0.13 | 1.2 | 10.2 | ||||
| 51.5–52.5 | 0.13 | 1.2 | 10.0 | ||||
| 52.5–53.5 | 0.13 | 1.0 | 9.5 | ||||
| 53.5–54.5 | 0.10 | 1.0 | 11.2 | ||||
| 54.5–55.5 | 0.10 | 0.8 | 9.2 | ||||
| 55.5–56.5 | 0.10 | 0.9 | 9.6 | ||||
| 56.5–57.5 | 0.12 | 1.0 | 10.1 | ||||
| 57.5–58.5 | 0.13 | 1.0 | 8.8 | ||||
| 58.5–59.5 | 0.15 | 1.2 | 9.6 | ||||
| 59.5–60.5 | 0.16 | 1.3 | 9.3 | ||||
| 60.5–61.5 | 0.14 | 1.1 | 9.3 | ||||
| 61.5–62.5 | 0.12 | 1.0 | 9.7 | ||||
| 62.5–63.5 | 0.13 | 0.9 | 8.5 | ||||
| 63.5–64.5 | 0.12 | 1.2 | 11.6 | ||||
| 64.5–65.5 | 0.16 | 1.5 | 10.7 | ||||
| 65.5–66.5 | 0.17 | 1.3 | 9.3 | ||||
| 66.5–67.5 | 0.12 | 0.9 | 8.9 | ||||
| 67.5–68.5 | 0.13 | 1.0 | 9.3 | ||||
| 68.5–69.5 | 0.14 | 1.1 | 9.7 | ||||
| 69.5–70.5 | 0.14 | 1.1 | 9.1 | ||||
| 70.5–71.5 | 0.16 | 1.6 | 11.9 | ||||
| 71.5–72.5 | 0.14 | 1.3 | 10.6 | ||||
| 72.5–73.5 | 0.13 | 1.0 | 9.0 | ||||
| 73.5–74.5 | 0.16 | 1.4 | 10.1 | ||||
| 74.5–75.5 | 0.14 | 1.2 | 10.2 | ||||
| 75.5–76.5 | 0.08 | 0.7 | 9.3 | ||||
| 76.5–77.5 | 0.11 | 1.1 | 10.9 | ||||
| 77.5–78.5 | 0.12 | 1.3 | 12.1 | ||||
| 78.5–79.5 | 0.13 | 1.2 | 11.0 | ||||
| 79.5–81 | 0.14 | 1.2 | 10.5 | ||||
| 81–82 | 0.12 | 1.0 | 9.8 | ||||
| 82–83 | 0.13 | 1.3 | 11.9 | ||||
| 83–84 | 0.13 | 1.1 | 9.4 | ||||
| 84–85 | 0.13 | 1.2 | 10.6 | ||||
| 85–86 | 0.13 | 1.4 | 12.7 | ||||
| 86–87.5 | 0.15 | 1.3 | 10.4 | ||||
| 87.5–88.5 | 0.13 | 1.3 | 11.5 | ||||
| 88.5–89.5 | 0.13 | 1.1 | 9.9 | ||||
| 89.5–90.5 | 0.13 | 1.2 | 10.3 | ||||
| 90.5–91.5 | 0.13 | 1.1 | 9.4 | ||||
| 91.5–92.5 | 0.13 | 0.9 | 8.7 | ||||
| 92.5–93.5 | 0.15 | 1.6 | 12.3 | ||||
| 93.5–94.5 | 0.16 | 1.5 | 11.1 | ||||
| 94.5–97 | 0.14 | 1.3 | 11.1 | ||||
| 107–108 | 0.07 * | 0.5 | 7.9 * | ||||
| 126–127 | 0.09 * | 0.7 | 7.7 * | ||||
| 145–146 | 0.08 * | 0.8 | 10.3 * | ||||
| 179–180 | 0.10 * | 0.8 | 8.2 * | ||||
| Number of Foraminifera | ||||||
|---|---|---|---|---|---|---|
| Core | Depth (cm) | Ammonia NA | Elphidium NE | Nonion | Total | A-E Index (NA/(NA + NE)) × 100 |
| GC7 | 28–29 | 12 | 1 | 0 | 17 | 92 |
| 43.5–44.5 | 6 | 0 | 0 | 9 | 100 | |
| 101–102 | 59 | 1 | 0 | 66 | 98 | |
| 112–113 | 25 | 0 | 1 | 29 | 100 | |
| 126–127 | 13 | 0 | 0 | 13 | 100 | |
| 133–134 | 32 | 0 | 1 | 36 | 100 | |
| 134–135 | 31 | 0 | 0 | 37 | 100 | |
| 151–152 | 49 | 0 | 0 | 49 | 100 | |
| 163–164 | 198 | 1 | 0 | 200 | 99 | |
| 165–166 | 6 | 0 | 0 | 6 | 100 | |
| 179–180 | 57 | 0 | 1 | 58 | 100 | |
| 204–205 | 6 | 0 | 0 | 6 | 100 | |
| GC15 | 42–44 | 184 | 3 | 11 | 199 | 98 |
| 50–52 | 170 | 11 | 19 | 200 | 94 | |
| 134–135 | 193 | 4 | 3 | 200 | 98 | |
| 147–148 | 147 | 0 | 1 | 151 | 100 | |
| 163–164 | 58 | 1 | 1 | 60 | 98 | |
| 165–166 | 182 | 3 | 14 | 200 | 98 | |
| 167–168 | 189 | 0 | 5 | 194 | 100 | |
| 187–188 | 190 | 3 | 7 | 200 | 98 | |
| GC15 | GC7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth | Fereactive | FeH | Feorg | Feox | Fepyr | Depth | Fereactive | FeH | Feorg | Feox | Fepyr |
| [cm] | [% of Fetotal] | [% of Fereactive] | [cm] | [% of Fetotal] | [% of Fereactive] | ||||||
| 14–16 | 32 | 46 | 9 | 25 | 20 | 28–29 | 41 | 86 | 7 | 1 | 7 |
| 18–20 | 34 | 47 | 9 | 21 | 23 | 43.5–44.5 | * | 79 | 10 | 1 | 9 |
| 24–26 | 38 | 44 | 8 | 18 | 30 | 101–102 | * | 85 | 6 | 1 | 7 |
| 28–30 | 33 | 50 | 6 | 5 | 39 | 107–108 | 34 | 83 | 9 | 2 | 6 |
| 34–36 | 37 | 45 | 7 | 5 | 44 | 113–114 | 33 | 87 | 6 | 1 | 6 |
| 40–42 | 36 | 55 | 8 | 4 | 33 | 126–127 | * | 89 | 5 | 1 | 5 |
| 50–52 | * | 39 | 6 | 2 | 53 | 133–134 | * | 91 | 4 | 1 | 4 |
| 56–58 | 55 | 36 | 5 | 3 | 56 | 145–146 | * | 91 | 4 | 1 | 4 |
| 104–105 | 41 | 10 | 8 | 4 | 79 | 151–152 | * | 90 | 4 | 1 | 4 |
| 107–108 | 38 | 7 | 10 | 4 | 79 | 179–180 | * | 86 | 6 | 2 | 7 |
| 132–133 | 36 | 10 | 8 | 4 | 78 | 204–205 | * | 91 | 5 | 1 | 3 |
| 151–152 | 32 | 8 | 9 | 4 | 80 | Mean ± SD | 36 ± 4 | 87 ± 4 | 6 ± 2 | 1 ± 0.4 | 6 ± 2 |
| 163–164 | * | 26 | 8 | 2 | 65 | ||||||
| 167–168 | 37 | 8 | 8 | 3 | 81 | ||||||
| 175–176 | 39 | 6 | 10 | 3 | 81 | ||||||
| Mean ± SD | 38 ± 6 | 26 ± 19 | 8 ± 1 | 5 ± 8 | 61 ± 23 | ||||||
| Depth | Number of Framboidal Pyrites per Classes of Diameters Measured in µm | Mean Diameter ± SD | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [cm] | [1, 2[ | [2, 3[ | [3, 4[ | [4, 5[ | [5, 6[ | [6, 7[ | [7, 8[ | [8, 9[ | [9, 10[ | [10, 11[ | [11, 12[ | [12, 13[ | [13, 14[ | [14, 15[ | [15, 16[ | [16, 17[ | [17, 18[ | [18, 19[ | [19, 20[ | [20, 21[ | [21, 22[ | [µm] |
| 1–5 | 1 | 2 | 3 | 6 | 8 | 4 | 4 | / | 2 | / | / | / | / | / | / | / | / | / | / | / | / | 5.4 ± 1.9 |
| 10–12 | / | 3 | 7 | / | 6 | 8 | 1 | 2 | 2 | / | 1 | / | / | / | / | / | / | / | / | / | / | 5.8 ± 2.3 |
| 18–20 | 1 | 1 | 5 | 4 | 7 | 5 | 1 | 4 | / | 2 | / | / | / | / | / | / | / | / | / | / | / | 5.7 ± 2.2 |
| 28–30 | / | 1 | 1 | 6 | 5 | 7 | 2 | 2 | 1 | / | / | / | / | 1 | / | / | / | / | 2 | 1 | 1 | 8.1 ± 5.4 |
| 40–42 | / | / | 3 | 4 | 13 | 3 | 3 | 3 | 1 | / | / | / | / | / | / | / | / | / | / | / | / | 6.0 ± 1.6 |
| 50–52 | / | / | 3 | 9 | 7 | 5 | 3 | 2 | / | 1 | / | / | / | / | / | / | / | / | / | / | / | 5.6 ± 1.6 |
| 104–105 | 1 | 7 | 14 | 7 | 1 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 3.5 ± 0.8 |
| 132–133 | 2 | 8 | 12 | 4 | 3 | / | 1 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 3.6 ± 1.2 |
| 141–142 | / | 1 | 6 | 4 | 6 | 3 | 3 | 3 | 1 | / | 1 | / | / | 2 | / | / | / | / | / | / | / | 6.4 ± 3.0 |
| 151–152 | / | 1 | 5 | 2 | 7 | 5 | 2 | 2 | 1 | 1 | 1 | / | / | 1 | / | / | / | 1 | / | / | 1 | 7.2 ± 4.3 |
| 163–164 | / | 3 | 8 | 11 | 5 | 2 | 1 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 4.5 ± 1.2 |
| 175–176 | / | 3 | 2 | 4 | 16 | 3 | 2 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 5.2 ± 1.2 |
| 186–187 | / | / | 4 | 5 | 12 | 4 | 2 | / | / | / | / | / | / | / | 1 | 2 | / | / | / | / | / | 6.4 ± 3.5 |
| 196–197 | / | 2 | 3 | 5 | 6 | 6 | 4 | 3 | / | 1 | / | / | / | / | / | / | / | / | / | / | / | 5.8 ± 1.9 |
| 268–269 | / | 1 | 1 | 7 | 12 | 6 | / | 2 | / | 1 | / | / | / | / | / | / | / | / | / | / | / | 5.7 ± 1.5 |
| MC15: 2–4 | / | / | 1 | 1 | 1 | / | / | 2 | 1 | 1 | / | / | / | / | / | / | / | / | / | / | / | not enough data |
References
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, R.; Rosenberg, R. Marine Benthic Hypoxia: A Review of Its Ecological Effects and the Behavioural Response of Benthic Macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 1995, 33, 245–303. [Google Scholar]
- Kemp, W.M.; Testa, J.M.; Conley, D.J.; Gilbert, D.; Hagy, J.D. Temporal Responses of Coastal Hypoxia to Nutrient Loading and Physical Controls. Biogeosciences 2009, 6, 2985–3008. [Google Scholar] [CrossRef] [Green Version]
- Wilkin, R.T.; Barnes, H.L. Formation Processes of Framboidal Pyrite. Geochim. Cosmochim. Acta 1997, 61, 323–339. [Google Scholar] [CrossRef]
- Arthur, M.A.; Dean, W.E. Organic-Matter Production and Preservation and Evolution of Anoxia in the Holocene Black Sea. Paleoceanography 1998, 13, 395–411. [Google Scholar] [CrossRef]
- Wegwerth, A.; Eckert, S.; Dellwig, O.; Schnetger, B.; Severmann, S.; Weyer, S.; Brüske, A.; Kaiser, J.; Köster, J.; Arz, H.W.; et al. Redox Evolution during Eemian and Holocene Sapropel Formation in the Black Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 489, 249–260. [Google Scholar] [CrossRef]
- Konovalov, S.K.; Murray, J.W. Variations in the Chemistry of the Black Sea on a Time Scale of Decades (1960–1995). J. Mar. Syst. 2001, 31, 217–243. [Google Scholar] [CrossRef]
- Lyons, T.W.; Berner, R.A.; Anderson, R.F. Evidence for Large Pre-Industrial Perturbations of the Black Sea Chemocline. Nature 1993, 365, 538–540. [Google Scholar] [CrossRef]
- Huang, Y.; Freeman, K.H.; Wilkin, R.T.; Arthur, M.A.; Jones, A.D. Black Sea Chemocline Oscillations during the Holocene: Molecular and Isotopic Studies of Marginal Sediments. Org. Geochem. 2000, 31, 1525–1531. [Google Scholar] [CrossRef]
- Ukrainskii, V.V.; Popov, Y.I. Climatic and Hydrophysical Conditions of the Development of Hypoxia in Waters of the Northwest Shelf of the Black Sea. Phys. Oceanogr. 2009, 19, 140. [Google Scholar] [CrossRef]
- Panin, N.; Jipa, D. Danube River Sediment Input and Its Interaction with the North-Western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 551–562. [Google Scholar] [CrossRef]
- Friedrich, J.; Dinkel, C.; Friedl, G.; Pimenov, N.; Wijsman, J.; Gomoiu, M.-T.; Cociasu, A.; Popa, L.; Wehrli, B. Benthic Nutrient Cycling and Diagenetic Pathways in the North-Western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Capet, A.; Beckers, J.-M.; Grégoire, M. Drivers, Mechanisms and Long-Term Variability of Seasonal Hypoxia on the Black Sea Northwestern Shelf – Is There Any Recovery after Eutrophication? Biogeosciences 2013, 10, 3943–3962. [Google Scholar] [CrossRef] [Green Version]
- Gooday, A.J.; Jorissen, F.; Levin, L.A.; Middelburg, J.J.; Naqvi, S.W.A.; Rabalais, N.N.; Scranton, M.; Zhang, J. Historical Records of Coastal Eutrophication-Induced Hypoxia. Biogeosciences 2009, 6, 1707–1745. [Google Scholar] [CrossRef] [Green Version]
- Zillén, L.; Conley, D.J.; Andrén, T.; Andrén, E.; Björck, S. Past Occurrences of Hypoxia in the Baltic Sea and the Role of Climate Variability, Environmental Change and Human Impact. Earth-Sci. Rev. 2008, 91, 77–92. [Google Scholar] [CrossRef]
- Sen Gupta, B.K.; Eugene Turner, R.; Rabalais, N.N. Seasonal Oxygen Depletion in Continental-Shelf Waters of Louisiana: Historical Record of Benthic Foraminifers. Geology 1996, 24, 227–230. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Levin, L.A. Coastal Hypoxia and Sediment Biogeochemistry. Biogeosciences 2009, 6, 1273–1293. [Google Scholar] [CrossRef] [Green Version]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace Metals as Paleoredox and Paleoproductivity Proxies: An Update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The Size Distribution of Framboidal Pyrite in Modern Sediments: An Indicator of Redox Conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Bond, D.P.G.; Wignall, P.B. Pyrite Framboid Study of Marine Permian–Triassic Boundary Sections: A Complex Anoxic Event and Its Relationship to Contemporaneous Mass Extinction. GSA Bull. 2010, 122, 1265–1279. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; JustiĆ, D.; Dortch, Q.; Wiseman, W.J.; Sen Gupta, B.K. Nutrient Changes in the Mississippi River and System Responses on the Adjacent Continental Shelf. Estuaries 1996, 19, 386–407. [Google Scholar] [CrossRef]
- Yanko-Hombach, V.V. Controversy over Noah’s Flood in the Black Sea: Geological and Foraminiferal Evidence from the Shelf. In The Black Sea Flood Question: Changes in Coastline, Climate, and Human Settlement; Yanko-Hombach, V., Gilbert, A.S., Panin, N., Dolukhanov, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 149–203. ISBN 978-1-4020-5302-3. [Google Scholar]
- Jilbert, T.; Slomp, C.P. Rapid High-Amplitude Variability in Baltic Sea Hypoxia during the Holocene. Geology 2013, 41, 1183–1186. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, N.; Slomp, C.P.; Behrends, T. Vivianite Is a Key Sink for Phosphorus in Sediments of the Landsort Deep, an Intermittently Anoxic Deep Basin in the Baltic Sea. Chem. Geol. 2016, 438, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Van Helmond, N.A.G.M.; Jilbert, T.; Slomp, C.P. Hypoxia in the Holocene Baltic Sea: Comparing Modern versus Past Intervals Using Sedimentary Trace Metals. Chem. Geol. 2018, 493, 478–490. [Google Scholar] [CrossRef]
- Spofforth, D.J.A.; Pälike, H.; Green, D. Paleogene Record of Elemental Concentrations in Sediments from the Arctic Ocean Obtained by XRF Analyses. Paleoceanography 2008, 23, PA1S09. [Google Scholar] [CrossRef] [Green Version]
- Kraal, P.; Burton, E.D.; Bush, R.T. Iron Monosulfide Accumulation and Pyrite Formation in Eutrophic Estuarine Sediments. Geochim. Cosmochim. Acta 2013, 122, 75–88. [Google Scholar] [CrossRef]
- Kalliokoski, J.; Cathles, L. Morphology, Mode of Formation, and Diagenetic Changes in Framboids. Bull. Geol. Soc. Finl. 1969, 41, 125–133. [Google Scholar] [CrossRef]
- Plante, A. Marine Benthic Hypoxia and Its Consequences for Sediment-Water Exchanges and Early Diagenesis; Université de Liège: Liège, Belgium, 2020. [Google Scholar]
- Krishnaswamy, S.; Lal, D.; Martín, J.; Meybeck, M. Geochronology of Lake Sediments. Earth Planet. Sci. Lett. 1971, 11, 407–414. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0–50,000 Years Cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef] [Green Version]
- Siani, G.; Paterne, M.; Arnold, M.; Bard, E.; Métivier, B.; Tisnerat, N.; Bassinot, F. Radiocarbon Reservoir Ages in the Mediterranean Sea and Black Sea. Radiocarbon 2000, 42, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.A.; Gagnon, A.R. Radiocarbon Chronology of Black Sea Sediments. Deep Sea Res. Part Oceanogr. Res. Pap. 1994, 41, 531–557. [Google Scholar] [CrossRef]
- Soulet, G.; Giosan, L.; Flaux, C.; Galy, V. Using Stable Carbon Isotopes to Quantify Radiocarbon Reservoir Age Offsets in the Coastal Black Sea. Radiocarbon 2019, 61, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Blaauw, M. Methods and Code for ‘Classical’ Age-Modelling of Radiocarbon Sequences. Quat. Geochronol. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the Trace Element Composition of Sedimentary Rocks and Upper Continental Crust. Geochem. Geophys. Geosys. 2001, 2, 1021. [Google Scholar] [CrossRef]
- Claff, S.R.; Sullivan, L.A.; Burton, E.D.; Bush, R.T. A Sequential Extraction Procedure for Acid Sulfate Soils: Partitioning of Iron. Geoderma 2010, 155, 224–230. [Google Scholar] [CrossRef]
- Lloyd, G.E. Atomic Number and Crystallographic Contrast Images with the SEM: A Review of Backscattered Electron Techniques. Mineral. Mag. 1987, 51, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Rickard, D. Sedimentary Pyrite Framboid Size-Frequency Distributions: A Meta-Analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 522, 62–75. [Google Scholar] [CrossRef]
- Leng, M.J.; Lewis, J.P. C/N Ratios and Carbon Isotope Composition of Organic Matter in Estuarine Environments. In Applications of Paleoenvironmental Techniques in Estuarine Studies; Weckström, K., Saunders, K.M., Gell, P.A., Skilbeck, C.G., Eds.; Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2017; pp. 213–237. ISBN 978-94-024-0990-1. [Google Scholar]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A. Fractionation and Extractability of Sulfur, Iron and Trace Elements in Sulfidic Sediments. Chemosphere 2006, 64, 1421–1428. [Google Scholar] [CrossRef]
- Sen Gupta, B.K.; Platon, E. Tracking Past Sedimentary Records of Oxygen Depletion in Coastal Waters: Use of the Ammonia-Elphidium Foraminiferal Index. J. Coast. Res. 2006, 3, 1351–1355. [Google Scholar]
- Cronin, T.; Willard, D.; Karlsen, A.W.; Ishman, S.; Verardo, S.; McGeehin, J.; Kerhin, R.; Holmes, C.; Colman, S.; Zimmerman, A. Climatic Variability in the Eastern United States over the Past Millennium from Chesapeake Bay Sediments. Geology 2000, 28, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, H.A.; Brasier, M.D. Foraminifera. In Microfossils; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 142–187. ISBN 978-1-118-68544-0. [Google Scholar]
- Rothwell, R.G.; Croudace, I.W. Twenty Years of XRF Core Scanning Marine Sediments: What Do Geochemical Proxies Tell Us? In Micro-XRF Studies of Sediment Cores: Applications of a Non-Destructive Tool for the Environmental Sciences; Croudace, I.W., Rothwell, R.G., Eds.; Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2015; pp. 25–102. ISBN 978-94-017-9849-5. [Google Scholar]
- Poulton, S.W.; Canfield, D.E. Development of a Sequential Extraction Procedure for Iron: Implications for Iron Partitioning in Continentally Derived Particulates. Chem. Geol. 2005, 214, 209–221. [Google Scholar] [CrossRef]
- Poulton, S.W.; Canfield, D.E. Ferruginous Conditions: A Dominant Feature of the Ocean through Earth’s History. Elements 2011, 7, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.F.; Raiswell, R. Sources and Mechanisms for the Enrichment of Highly Reactive Iron in Euxinic Black Sea Sediments. Am. J. Sci. 2004, 304, 203–233. [Google Scholar] [CrossRef] [Green Version]
- Van Helmond, N.A.G.M.; Quintana Krupinski, N.B.; Lougheed, B.C.; Obrochta, S.P.; Andrén, T.; Slomp, C.P. Seasonal Hypoxia Was a Natural Feature of the Coastal Zone in the Little Belt, Denmark, during the Past 8ka. Mar. Geol. 2017, 387, 45–57. [Google Scholar] [CrossRef]


| Core | Laboratory ID | Depth (cm) | Material Dated | 14C Age (BP) | Error | WiTi 14C Age (BP) |
|---|---|---|---|---|---|---|
| GC7 | GdA-5385 | 41 | OF | 2930 | 45 | 2730 ± 35 |
| GdA-5812 | OF | 2640 | 30 | |||
| GC15 | GdA-5040 | 57 | shells | 2110 | 30 | 2260 ± 30 |
| GdA-5816A | shells | 2410 | 30 |
| Element | Average Upper Crust Abundance [ppm] | GC7 | GC15 | ||||
|---|---|---|---|---|---|---|---|
| Concentration [ppm] | Enrichment Factor | Concentration [ppm] | Enrichment Factor | ||||
| V a | 107 | 130 ± 8 | 1.3 | 80 ± 10 | 1.1 | ||
| U a | 2.8 | 2.7 ± 0.1 | 1.0 | 2.9 ± 0.2 | 1.5 | ||
| Mo | 1.5 | 0.86 b,1 | 0.6 | 0.96 b,2 | 1.12 b,3 | 1.0 | 1.1 |
| Al c | 80,400 | 78,312 | / | 53,726 | / | ||
| Core | Depth (cm) | Framboidal Pyrites | Oxygenation Level Inferred | Average Concentration (maximum) ppm | ||
|---|---|---|---|---|---|---|
| V | U | Mo | ||||
| GC7 | 106.5–107.6 | none | oxic | 105 (179) | 2.0 (30.5) | 0.86 (10.67) |
| GC15 | 151–151.8 | big group | hypoxic/oxic | 91 (199) | 2.7 (45.8) | 0.96 (6.73) |
| GC15 | 162–164.2 | small group | anoxic | 104 (165) | 3.0 (57.7) | 1.12 (11.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinet, S.; Matossian, A.O.; Capet, A.; Chou, L.; Fontaine, F.; Grégoire, M.; Lepoint, G.; Piotrowska, N.; Plante, A.; Román Romín, O.; et al. A Multi-Proxy Approach to Reconstruct Hypoxia on the NW Black Sea Shelf over the Holocene. J. Mar. Sci. Eng. 2022, 10, 319. https://doi.org/10.3390/jmse10030319
Robinet S, Matossian AO, Capet A, Chou L, Fontaine F, Grégoire M, Lepoint G, Piotrowska N, Plante A, Román Romín O, et al. A Multi-Proxy Approach to Reconstruct Hypoxia on the NW Black Sea Shelf over the Holocene. Journal of Marine Science and Engineering. 2022; 10(3):319. https://doi.org/10.3390/jmse10030319
Chicago/Turabian StyleRobinet, Sarah, Alice Ofélia Matossian, Arthur Capet, Lei Chou, François Fontaine, Marilaure Grégoire, Gilles Lepoint, Natalia Piotrowska, Audrey Plante, Olaya Román Romín, and et al. 2022. "A Multi-Proxy Approach to Reconstruct Hypoxia on the NW Black Sea Shelf over the Holocene" Journal of Marine Science and Engineering 10, no. 3: 319. https://doi.org/10.3390/jmse10030319
APA StyleRobinet, S., Matossian, A. O., Capet, A., Chou, L., Fontaine, F., Grégoire, M., Lepoint, G., Piotrowska, N., Plante, A., Román Romín, O., & Fagel, N. (2022). A Multi-Proxy Approach to Reconstruct Hypoxia on the NW Black Sea Shelf over the Holocene. Journal of Marine Science and Engineering, 10(3), 319. https://doi.org/10.3390/jmse10030319






