Abstract
The expanding human activities in coastal areas increase the need for developing solutions to limit impacts on the marine environment. Sea disposal affects the marine environment, but despite the growing knowledge of potential impacts, there are still no standardized leaching tests for sea disposal. The aim of this study was to contribute to the development of leaching tests, exemplified using mine tailings, planned for submarine disposal in the Repparfjord, Norway. The mine tailings had elevated concentrations of Ba, Cr, Cu, Mn and Ni compared to background concentrations in the Repparfjord. Variables known to affect metal leaching in marine environments (DOC, pH, salinity, temperature, aerated/anoxic) were studied, as was the effect of flocculant (Magnafloc10), planned to be added prior to discharge. Stirred/non-stirred setups simulated the resuspension and disposal phases. Leaching of metals was below 2% in all experiments, with the highest rate observed for Cu and Mn. Multivariate analysis revealed a different variable importance for metals depending on their association with minerals. Higher leaching during resuspension than disposal, and lower leaching with the addition of Magnafloc10, especially for Cu and Mn, was observed. The leaching tests performed in this study are transferable to other materials for sea disposal.
1. Introduction
The anthropogenic impact on the marine environment has increased over the past 75 years due to the rise in human activities in offshore and coastal areas [1]. There is a growing international concern for the management of the marine environment and strong focus on measures to reduce the impacts of anthropogenic pressures [2]. One of these pressures is sea disposal as part of national waste management practices. Despite the increased focus on using waste as a resource in a circular economy [3,4], and the potential for reuse in construction materials [5,6], the practice of reuse is only implemented on a smaller scale; accordingly, there will still be need for disposal options for many years to come. Historically, the uncontrolled discharge, disposal and dumping of waste at sea has had negative impacts on the marine environment [7]. The past 40–50 years has caught up with the ‘out of sight, out of mind’ practice and through international conventions and national environmental regulations, sea disposal is presently conducted under more controlled conditions. There is still an international consensus of reducing sea disposal [8] and thereby pressure on the aquatic environment. However, in some cases sea disposal is considered a more environmentally friendly option (e.g., disposal of dredged sediments) than the alternatives (e.g., disposal on land) [9]. The local impacts of sea disposal are mainly biological and physical alterations to the seabed, including local effects on biodiversity [10], while dewatering and disposal on land can increase contaminant transport to neighboring areas and increase the risk of exposure of flora, fauna and humans [11].
Norway is one of the countries that continues to include sea disposal as a waste management option for dredged sediments and mine tailings. Since the mid-2000s, confined aquatic disposal has played an important part in management of dredged polluted sediments from clean-up of harbors or dredging to increase navigational depth. Mine tailings disposal at sea has been a common practice in Norway since the emergence of the mining industry in the 19th century. Harbor sediments and mine tailings differ in characteristics. Harbor sediments are fine-coarse grained with a complex composition of pollutants [12], while mine tailings are fine grained (clay-silt texture) with elevated concentration of metals and process chemicals [13]. Despite these differences in characteristics, the type of environmental impacts encountered during placement and disposal are comparable. During release through a pipe or from a split hull barge over/in the confined disposal area, increased turbidity in the water column due to dispersion of particles, and leaching of pollutants, is expected [14]. Technical solutions for pipe placement and release of particles can minimize these effects and monitoring is applied to ensure that the dispersion of particles and pollutants are within acceptable limits, set by environmental authorities. During disposal on the seabed, the environmental impacts include smothering of benthic organisms [15,16], physical/geochemical alterations to the seabed [17], reduced levels of organic carbon and reduced biodiversity of marine communities [18] with potential long-term effects [19]. Environmental impacts are accepted in the area regulated for disposal, but not expected outside the disposal area. There are, however, historical examples of particle dispersion [20], pollutant leaching [21,22] and bioaccumulation [23] outside the disposal areas. In order to limit these effects, actions are often taken to stabilize pollutants and particles in confined aquatic disposal of polluted dredge sediment, e.g., by capping with a clean material [24]. Upon cessation of mine tailings disposal, actions to stabilize particles and pollutants have not been a common practice.
There are strict regulations and guidelines for sea disposal, both with regards to mapping the seabed in the designated disposal area to assess potential for dispersal (current conditions, salinity) environmental impacts, as well as monitoring programs to assess dispersion of particles and pollutants during and after placement of waste [25]. As opposed to disposal on land, there are no standardized leaching tests for characterization of marine sediments [26]. Leaching tests do not only provide information as to whether waste can be considered inert or hazardous [27], but also provide knowledge on the availability of pollutants and quantification of leaching on a short-term basis. In addition, this provides foundation for assessment of actions to be taken if leaching is at levels with high risk for adverse effects for the marine environment. In some cases, leaching tests based on waste disposal have been used (e.g., EN-14405, EN-12457-2, USEPA SW-846 [28]), and although these can be conducted using sea water or varying pH [29] they do not simultaneously take into account conditions that prevail and vary in marine environments, such as pH, temperature, salinity and dissolved organic carbon (DOC), nor the use of flocculants during discharge of mine tailings. These are all factors known to influence the availability and leaching of pollutants [29,30,31,32,33,34,35]. Flocculants are used in mineral processing to separate minerals, dewatering and as agents for enhancing sedimentation of submarine tailings [36,37]. Flocculation increases the settling rate, decreases turbidity and decreases the risk of resuspension upon settling. Adsorption of the flocculant polymer onto the surface of the target molecule occurs through hydrogen bonding, electrostatic interaction, van der Waals forces, or chemical bonding [38]. In the mineral processing industry, polyacrylamide-based flocculants are most commonly used (>90%) and the advantage of polyacrylamide is it can be designed according to solid–liquid separation conditions, such as differing contents of suspended solids, dissolved solids, pH, temperature and mineral/metal composition [39].
The Repparfjord is located in the arctic region of Norway and was used for submarine mine tailings disposal during mining operations in the 1970s. There are plans to reopen the copper mine in the near future. Several studies have been conducted to assess the long-term effects of the historic tailings disposal and these have shown that dispersion of particles and metals outside the designated disposal area has taken place, mainly during the mine operating period in the 1970s [40,41]. Dispersion continues to this day, but is confined to the disposal area in the inner part of the fjord [42]. The future discharge of mine tailings will take place in another part of the fjord [43] and in 2015 the mining company, Nussir ASA, received a permit for annual discharge of up to 2 million tonnes of mine tailings in a 30-year operational period. The mining company is planning to use Magnafloc10, a polyacramide flocculant, due to its properties to dewater after flotation processing and enhance the sedimentation rate of discharged tailings. According to the zoning plan, the boundary for the new disposal area is 8.5 km2 and the majority of the discharged tailings (approximately 98%) is expected to settle in an area of 2.4 km2 [44]. Studies have shown that the historic and new mine tailings have different mineral composition [42,45], due to mining of a new ore body at Nussir, in addition to the ore body at Ulveryggen, mined in the 1970s. The new mine tailings have a higher content of carbonate, known to function as a buffer with the potential of limiting metal leaching from the tailings. Leaching tests can give indications of whether there is risk of similar leaching and dispersion of metals from the mine tailings as observed in the 1970s and whether actions are needed to limit dispersion of metals outside the disposal area.
The main objective of this study was to provide a foundation for assessment of metal leaching, exemplified by leaching tests of new mine tailings planned for disposal in the Repparfjord. The leaching tests included different settings of parameters known to affect metal desorption in fjords (salinity, pH, DOC, temperature, air/nitrogen) as well as conditions specific to the mine tailings. The resuspension and disposal phases were represented by stirred/non-stirred conditions and the effect of adding the flocculant, Magnafloc10, planned to be used prior to discharge of tailings to increase the sedimentation rate, was also tested. The mine tailings were mixed with natural sediments from the disposal area in the leaching tests, to simulate effects of resuspension. The leaching tests were conducted in an experimental domain representative of Arctic conditions, resulting in low leaching of metals (<2%) with the highest leaching levels for Cu and Mn. The influence of variables on the leaching varied between the metals. Generally higher leaching of metals is expected in the resuspension phase (stirred setup) and ensuring sedimentation can limit the leaching. Magnafloc10 was also shown to limit the leaching, especially for Cu and Mn.
2. Materials and Methods
2.1. Mine Tailings and Sediments Used in the Leaching Experiments
Mine tailings used in the leaching experiments were purchased from Nussir ASA and consisted of rock sourced from the Nussir (90%) and Ulveryggen (10%) ores in Kvalsund, Norway. The rock was processed at SGS Mineral Services, Canada, to simulate the commercial mining metal extraction processes planned to take place at the mine in 2022. To separate the copper minerals, the ore is processed by crushing into a fine material (clay/silt texture) followed by flotation. After flotation and removal of copper minerals, the remaining mine tailings are dewatered by flocculation and planned to be directly discharged to the Repparfjord. XRF analysis showed difference in the mineral composition of the two ores. The main mineral in the Ulveryggen ore was quartz (53.8%), and in the Nussir ore the content was 25.8%. The main mineral in the Nussir ore was calcite (28.4%), while in the Ulveryggen ore the content of calcite was 0.04%. The copper minerals in the two ores were associated with chalcopyrite, bornite, covellite and chalcocite, however, varying in composition. In the Nussir ore, the content of Cu minerals was in the order bornite (1.14–1.53%) > chalcopyrite (0.11–1%) > chalcocite (0.05–0.5) > covellite (0.02%), while the order of Cu minerals in the Ulveryggen ore was chalcocite (0.88%) > bornite (0.87%) > chalcopyrite (0.27%) > covellite (0.02%). [46]. In a previous study [31], the 90:10 mixture of mine tailings was characterized as calcareous (20% carbonate), with a high pH (9.2) and a low content of organic matter (<0.5%). The content of fine particles (<63 µm) was 72%.
Sediments used in the experiments were sampled from the area planned for submarine mine tailings disposal. Description of core sampling in 2017, location of core stations and sampling depth are given in Sternal et al. 2017 [40]. In this study, the surface sediment (0–10 cm) of core HH13-005 (sediment surface depth 53 m) was used in the leaching experiments. The sediments used had low content of carbonate (1.7–2.4%), alkaline pH (8.0–8.2), 1.1–1.5% total organic carbon content and a fine particles (<63 µm) fraction of 41–49%.
2.2. Chemical Analysis
For metal analysis (aluminum (Al), barium (Ba), calcium (Ca), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn)), tailings were digested (Norwegian standard NS4770); dry mine tailings (1.0 g) and HNO3 (9 M, 20 mL) were autoclaved (200 kPa, 120 °C, 30 min). Solid particles were removed by vacuum filtration through a 0.45 µm filter and the liquid was diluted with distilled water to 100 mL. Metal concentrations in the liquid were measured by inductively coupled plasma–optical emission spectrometry (ICP-OES) on a Varian 720-ES with standards and internal controls PlasmaCAL from SCP Science (Courtaboeuf, France).
Metal partitioning analysis was conducted by sequential extraction in four steps based on the improvement of the three-step method [47] described by the Standards, Measurements and Testing Program of the European Union [48]. In the first step, acetic acid (0.11 M, 20 mL, pH 3) was agitated with dry mine tailings (0.5 g) for 16 h. In the second step, the solid particles were agitated with hydroxylammonium chloride (0.1 M, 20 mL; pH 2) for 16 h. In the third step, the solid particles were agitated with hydrogen peroxide (8.8 M, 5 mL) for 1 h, subsequently heated and kept at 85 °C for 1 h, then the lid was removed to allow evaporation of liquid at 85 °C, followed by agitation of the cooled solid fraction with ammonium acetate (1 M, 25 mL, pH 2) for 16 h. In the fourth step, the remaining solid particles were digested as described above. The liquids from each step represented the exchangeable, reducible, oxidizable and residual fractions, respectively, and were analyzed for metals by ICP–OES.
2.3. Leaching Experiments
Leaching experiments were made to investigate the influence of fjord conditions, addition of Magnafloc10, and resuspension/disposal on the leaching and partitioning of metals in the mine tailings/sediment slurries. The range of the fjord conditions parameters investigated was based on the variance in Arctic and sub-Arctic climates: pH (6–9), salinity (0.5–40 ppt), dissolved organic carbon (DOC) (0.5–20 mg/L), temperature (4–20 °C) and aerated/anoxic conditions (air/nitrogen). In addition, resuspension was investigated by stirred setups. The mining company has a discharge permit for Magnafloc10 concentration of 30 µg/g [49] and the range of Magnafloc10 concentration in the experiments was 0–60 µg/g.
For testing the comparative influence of the seven experimental variables, multivariate experimental design was employed. The continuous variables were pH, salinity, DOC, temperature and Magnafloc10. The discrete variables were aerated/anoxic conditions (air/nitrogen) and stirring. By assuming that the interaction effects compared to the main effects were negligible, the number of experiments could be reduced from a complete 27 factorial design to a 27−4 fractional factorial design consisting of 8 experiments (1–8) and 3 replicate experiments (9–11) representing the center of the continuous experimental domain to validate the multivariate models. The experimental design is given in Table 1.

Table 1.
Experimental design of the leaching experiments.
In the experiments, 10 g of Repparfjorden sediments and 10 g of mine tailings were transferred to a 100 mL Pyrex© glass bottle and 50 mL of liquid with concentrations of salinity and DOC in accordance with the experimental design were transferred to the bottles. Salinity was based on 40 ppt stock solution of artificial seawater (27.5 g NaCl, 5.0 g MgCl2, 2.0 g MgSO4, 1.0 g KCl, 0.5 g CaCl2, 0.001 g FeSO4 per 1000 L of distilled water). DOC was based on 1 g/L stock solution (0.25 g humic acid per 250 mL of distilled water). All stock solutions were prepared in volumetric flasks. To achieve salinity of 0.5 ppt and 20 ppt, respectively, 12.5 mL and 500 mL of the 40 ppt stock solution were used per litre. To achieve DOC of 0.5 mg/L, 10 mg/L and 20 mg/L, respectively, 0.5 mL, 10 mL and 20 mL of the 1 g/L stock solution were used per liter. Magnafloc10 was added to the sediment–tailings slurries in solution (1.2 g/L by 0.3 g Magnafloc10 per 250 mL distilled water), prepared in a volumetric flask and left for 3–4 h to achieve a viscous liquid. In order to achieve 30 µg/g tailings and 60 µg/g tailings, respectively, 0.5 mL and 1.0 mL were added to the sediment–tailings slurries in accordance with the experimental design. Between 0.5 and 2.5 mL of 1 M HCl were added to the slurry in increments of 0.5mL, and the pH was measured after each addition using a radiometer analytical electrode, to ensure final pH values of 6 or 7.5. After addition of stock solutions and acid to the mine tailings, silicone rubber stopper plugs (Verneret) were applied to the glass bottles. In the aerated experiments, needle holes were made in the plugs. In the anoxic bottles, nitrogen gas was added every 24 h. To ensure low temperature settings, the experiments were placed in one of two fridges with temperatures of 4 and 12 °C, or on a laboratory bench at an ambient temperature of 21 °C. Stirring was achieved by stirring magnets at 1000 rpm.
The duration of the experiments was 3 weeks, assuming chemical equilibrium as is the foundation of leaching tests developed for waste for land disposal (standard EN-12457-2). Twenty milliliters of the water in the slurries was sampled at the end of the experiments, through a syringe with a 0.45 µm filter and kept for later metal analysis. Solid particles were subsequently separated from the slurries by vacuum filtration through a 0.45 µm filter. The solid particles were used in analysis of metal and sequential extraction.
2.4. Multivariate Analysis
SimcaP11 software was used for the multivariate modeling, by projection onto latent structures (PLS), based on reducing the number of dimensions to facilitate interpretation of the results. In PLS, the quantitative relation between a descriptor matrix, X, and a response matrix, Y, is used to assess the comparative influence of descriptors on the responses, and to predict responses at given descriptor settings within the studied experimental domain [50]. The X-matrix consisted of the experimental variables and discrete variables were included by arbitrarily setting them to 1 or −1. The Y-matrix consisted of the concentrations of leached metals (mg/kg dw). To assess viability and stability of the calculated PLS models, correlation coefficients, R2Y (the fraction of the Y-matrix explained by the model) and predictive powers, Q2 (an estimate of the reliability of the model calculated by cross-validation) were used. In order to obtain a high predictive power, R2Y should be high. A value of R2Y > 0.9 is excellent, while a value above 0.5 is good. The difference between R2Y and Q2 should be as low as possible and a difference larger than 0.2–0.3 may indicate outliers or the presence of irrelevant variables in the X block [51].
Influence of variables on leaching. Variable importance in the projection (VIP) values present the absolute importance of each parameter in the PLS model with respect to its correlation to all the responses (Y) and to the projection (X). The VIP values are calculated for each X variable by summing the squares of the PLS loading weights, weighted by the amount of sum of squares explained in each model component. The sum of squares of all VIPs is equal to the number of terms in the model; accordingly, the average VIP is equal to 1. High VIP values (>1) represent high influence of the variable(s) in the model, and VIP values < 0.5 indicate low influence of the variable(s) on the model. VIP plots were used to assess the variable importance in the calculated models. To evaluate if the variables had positive or negative impacts on the model responses, coefficient plots were used.
Quantification of leaching. The experimental domain of this study provides good representation of variable ranges in Arctic and sub-Arctic fjords, and the PLS models can be used to estimate leaching at specific conditions in a given fjord. For the quantification of leaching at specific variable settings, the model calculations were based on the 11 experiments as prediction set, and predictions included all components of the PLS models.
3. Results
3.1. Metal Concentrations
The sediments used in the leaching experiments represent background concentrations in the Repparfjord [40]. The mine tailings had concentrations of Ba, Ca, Mg, Mn, Cr, Cu and Ni, exceeding the background concentrations in the Repparfjord sediments by 2 (Ni) to 100 (Cu) times (Table 2). The higher concentration of Ca (×35) was associated with the high content of carbonate in the mine tailings. The Repparfjord sediments had higher concentrations of Al, Fe, As, Pb and Zn (Table 2), from 1.5–9 times the concentrations in the mine tailings. Cd and K had similar concentrations in the mine tailings and Repparfjord sediments. In the 1:1 tailings and sediment mixture used in the leaching experiments, the concentration of metals was within the ranges of the original materials, apart from Ba, Cr, K and Ni. For these four metals, the concentrations in the mixture exceeded concentrations of the original materials and may have been due to inhomogeneity in the mine tailings.

Table 2.
Metal concentrations (mg/kg TS) in the mine tailings with data from [31], Repparfjord sediment, and the mixture of mine tailings and the Repparfjord sediment, used in the leaching experiments.
Seven of the analyzed metals (As, Cd, Cr, Cu, Ni, Pb and Zn) are included in the Norwegian environmental quality standards (EQS) [52], in line with the water framework of the European Union (EU) and applied in many countries outside the EU (e.g., Australia, Canada, New Zealand, Russia and the United States). The Norwegian EQS values for pollutants are based on technical guidelines for the calculation of EQS from the EU [53]. Apart from Cu, all the priority metals in the mine tailings were well below the Norwegian threshold values for the annual average EQS (AA-EQS). The concentration of Cu in the mine tailings and in the mixture of mine tailings and Repparfjord sediment exceeded the threshold values for maximum acceptable concentration under EQS (MAC-EQS) for acute toxicity. Although there are no EQS for Ba and Mn, there has recently been increased focus on their effects on the aquatic environment with suggestions of implementing EQS for Ba in the EU water framework [54], and EQS for Mn in guidelines for Australia and New Zealand [55].
3.2. Leaching and Metal Partitioning
The focus of the leaching tests was Cu, due to the high concentrations in the mixture of mine tailings and Repparfjord sediment with potential toxic effect on the marine environment. The toxicity of metals is not related to the total concentrations; rather it is related to how available the metals are for mobilization and uptake in marine organisms. For this reason, priority metals with elevated concentrations compared to background levels in the Repparfjord were included in the leaching tests (Cr and Ni) as well as Ba and Mn due to elevated concentrations and potential inclusion in priority metals in the future. Al and Fe, known to be bound in stable minerals in the mine tailings and Repparfjord sediments [41], were included in the leaching tests as reference metals.
In the leaching experiments, low percentages of Al, Ba, Fe, Mn, Cr, Cu and Ni were leached, <2% of the original content of metals in the tailings–sediment slurry (Table 3). The highest leaching percentages were observed for Cu and Mn, while the lowest were observed for Al and Fe. The trends in leached amount in the different experiments appeared to vary depending on the metal. The highest leaching was for instance observed in different experiments: Al and Fe (exp. 7), Ba (exp. 3), Cr (exp. 11), Cu (exp. 8), and Mn and Ni (exp. 4). This is an indication of different associations of the metals with minerals and different influences of variables on the mobilization of metals.

Table 3.
Leaching of metals (%) from the mine tailing–sediment mixture calculated as the quantity of metals in the liquid phase compared to the total content of metals in mine tailings and liquid at the end of the leaching experiments.
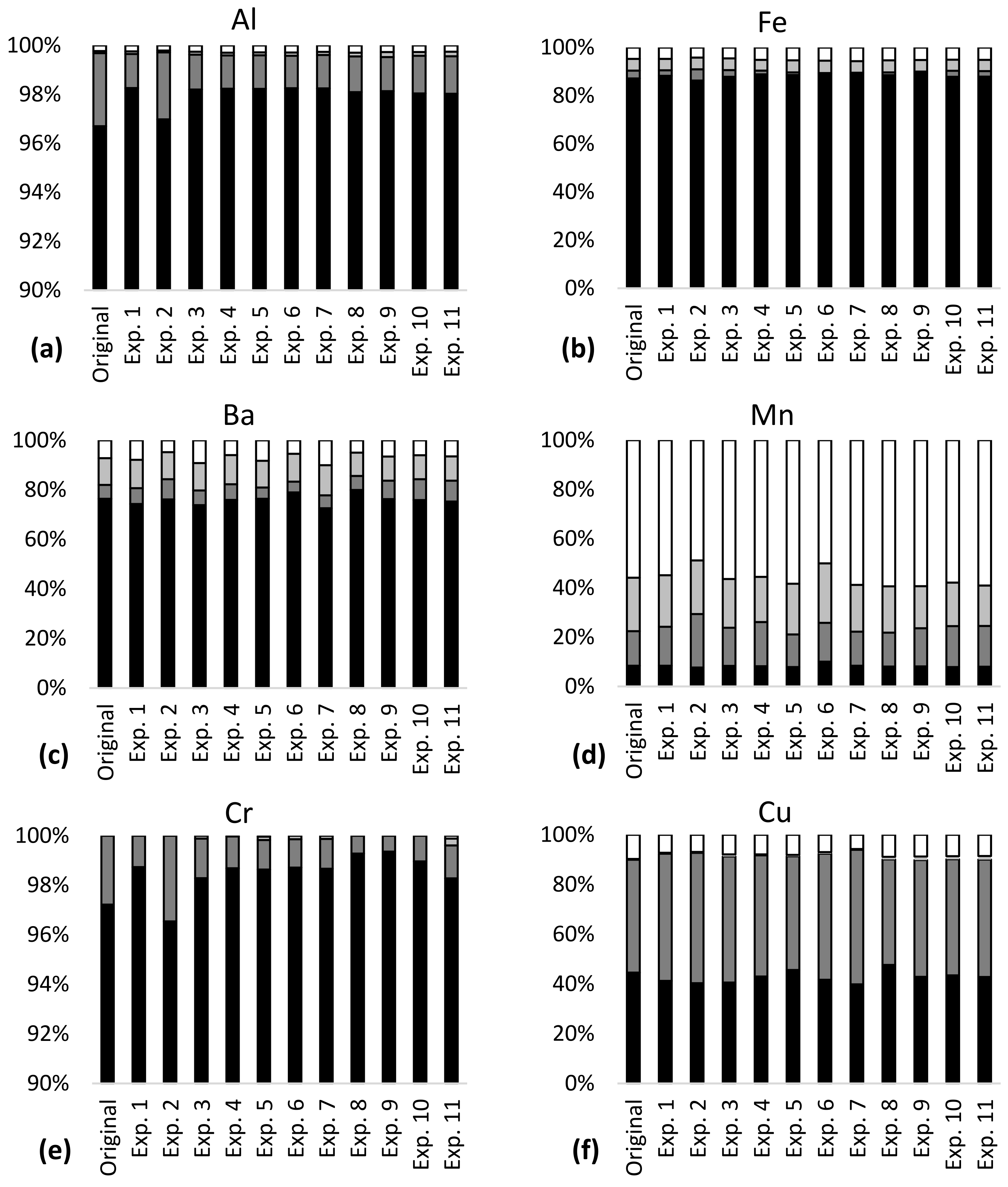
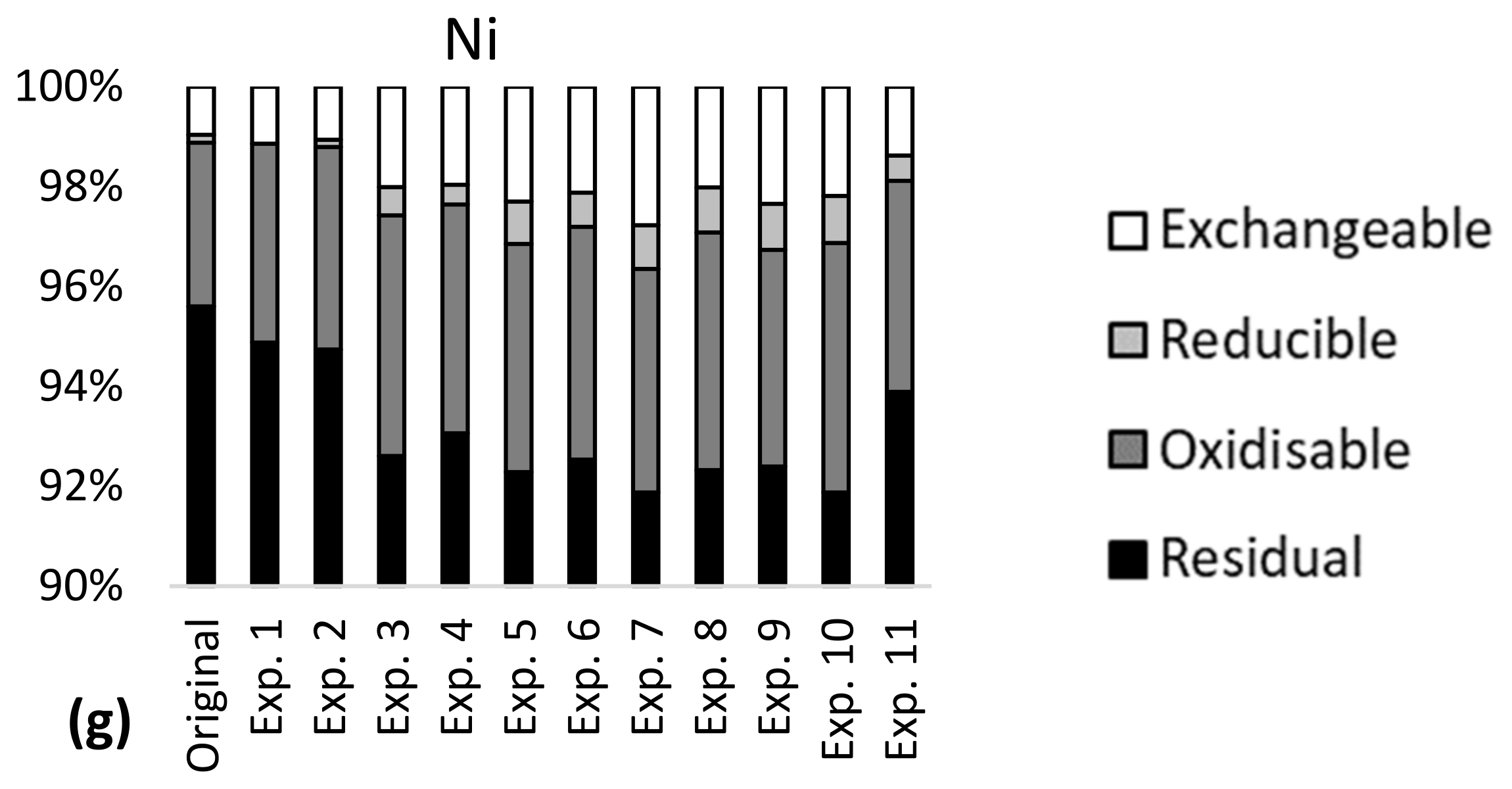
More than 85% of the total content of Al, Fe, Cr and Ni was bound in stable minerals in the tailings–sediment slurry particles (residual fraction, Figure 1), meaning that a large amount of these metals was not available for leaching in the studied pH range 6–9. Approximately 20% of Ba, 55% of Cu and 90% of Mn were bound in the more available fraction in the particles, with the potential of higher leaching via ion exchange and carbonate dissolution (exchangeable fraction), reducing conditions (reducible fraction) and oxidizing conditions (oxidizable fraction). Seven percent of Ba, 10% of Cu, 60% of Mn and less than 5% of Al, Fe, Cr and Ni were bound in the most available fraction (exchangeable). Metal partitioning indicated metal availability for leaching in the order Mn > Cu > Ba >> Al~Fe~Cr~Ni and this was largely in line with the results of the leaching tests (Table 3).
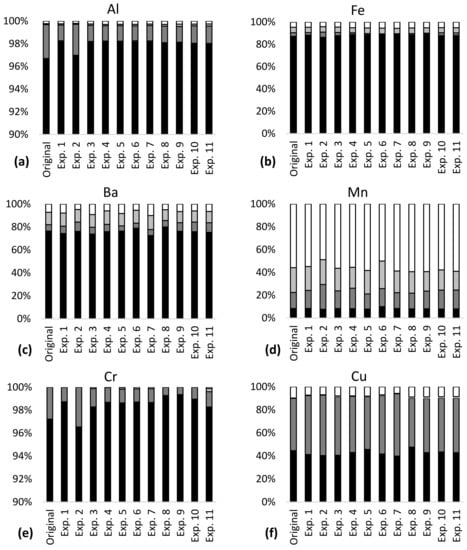
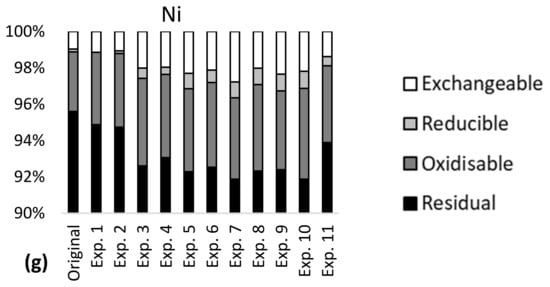
Figure 1.
Metal partitioning before (original) and after leaching experiments 1–11 in the mine tailings–sediment particles for (a) Al, (b) Fe, (c) Ba, (d) Mn, (e) Cr, (f) Cu and (g) Ni.
Metal partitioning was largely the same in the tailings–sediment particles before and after the leaching experiments (Figure 1). Confidence intervals of the metal concentrations for each of the partitioning fractions were narrow (Table 4), indicating low variability in metal partitioning in the experiments. The confidence intervals were generally lower than 20% of the mean concentrations, except for oxidizable Cr and iron, and reducible Cu, which had confidence intervals of 36–50% of the mean concentrations. The confidence intervals of leaching (Table 4) showed high variability for Ba, Mn, Cu and Ni. The widest confidence interval was observed for Cu and was almost equal to the mean concentration. In 10 of the 11 experiments, Al and Fe had leaching concentrations below the detection limit; the same was the case for Cr in 8 experiments.

Table 4.
The range and mean metal concentrations (mg/kg TS), and confidence interval in the 11 leaching experiments, for the mine tailing–sediment slurry fractions: leaching, exchangeable, reducible, oxidizable and residual.
3.3. Variable Importance for Leaching
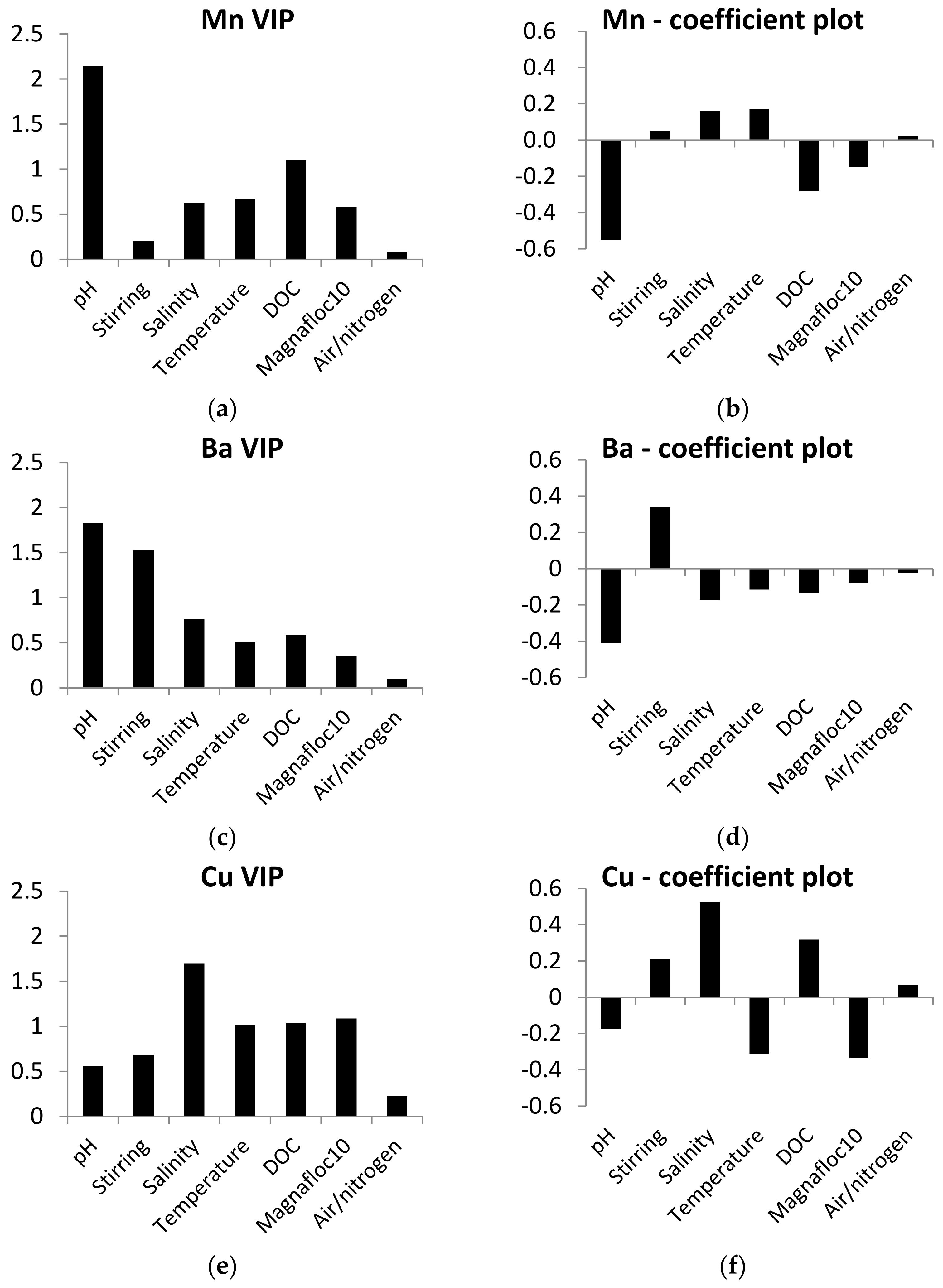
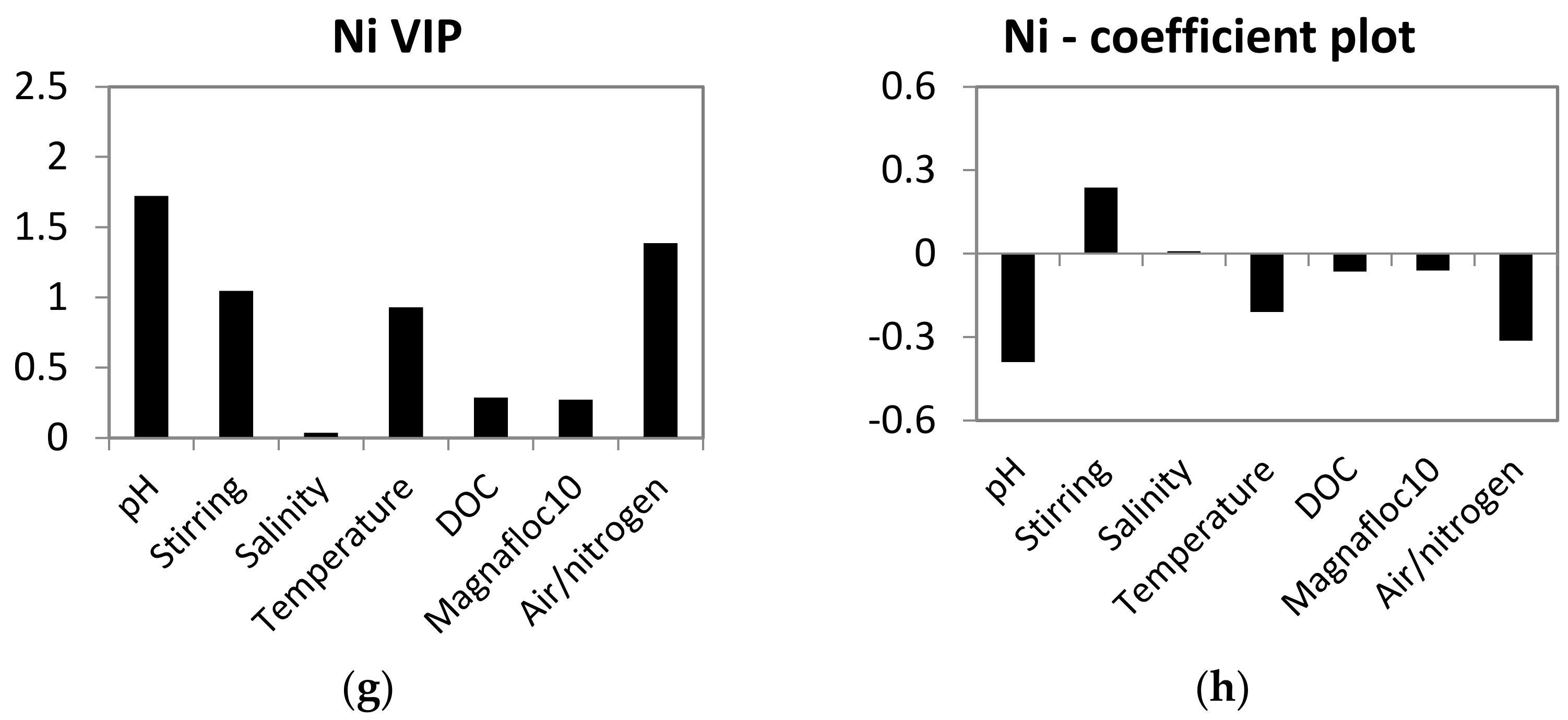
PLS models for leaching of Mn, Ba, Cu and Ni were calculated to assess the influence of experimental variables on leaching. Al, Fe and Cr were not included in the models due to limited results above the detection limit. The PLS models were good (R2Y 0.57–0.84), had low Q2 values (0.1) for Mn and Ba and good Q2 values (0.65–0.68) for Cu and Ni. Despite some instability in the PLS models for Mn and Ba, they can still be used as indications of the influence of experimental variables on leaching of metals. The variable importance calculated in the models was different depending on the metal (Figure 2). All the experimental variables showed high influence (VIP > 1) of one or several of the metals.
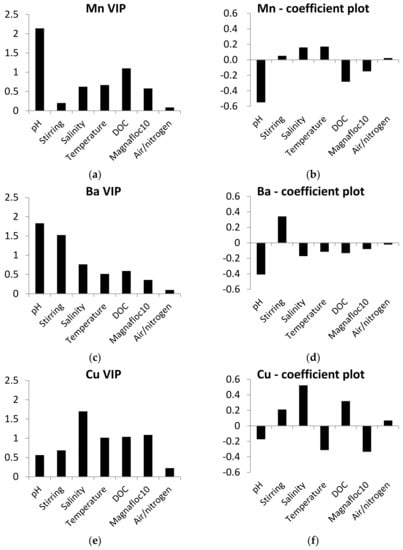
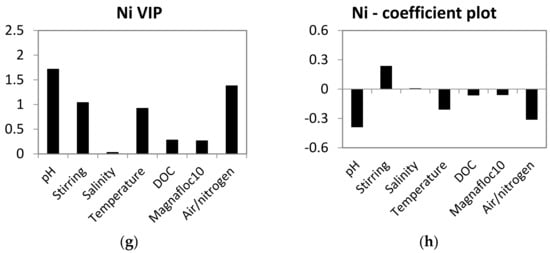
Figure 2.
Variable importance in the projection (VIP) and coefficient plots for the PLS models of metal leaching. Parameters with VIP values > 1 have high, 0.5–1 have moderate and <0.5 have low influence on the desorption of metals from the mine tailings. Parameters with coefficient values > 0 have positive correlation and coefficient values < 0 have opposite correlation with desorption of metals. (a) VIP plot of Mn, (b) coefficient plot of Mn, (c) VIP plot of Ba, (d) coefficient plot of Ba, (e) VIP plot of Cr, (f) coefficient plot of Cr, (g) VIP plot of Cu and (h) coefficient plot of Cu.
pH was the most important variable for Mn, Ba and Ni, and had a low influence on the leaching of Cu (VIP < 0.5). The coefficient plots of Mn, Ba and Ni (Figure 2) show that the low setting of pH increased the leaching. Salinity was the most important variable for Cu and had a moderate influence on Ba and Mn (VIP 0.5–1), and a low influence on leaching of Ni (VIP < 0.5). The coefficient plots for Cu, Ba and Mn show effects of salinity. For Cu and Mn, high salinity increased the leaching, while low salinity increased the leaching of Ba.
Temperature was an important variable for Cu (VIP > 1) and had a moderate influence on the leaching of the three other metals (VIP 0.5–1). The influence of temperature varied in that high temperature increased the leaching of Mn, while low temperature increased the leaching of Ba, Cu and Ni. DOC was an important variable for Cu and Mn (VIP > 1) and had a moderate influence on Ba (VIP 0.5–1) and a low influence on Ni (VIP < 1). The coefficient plots show different trends in the influence of DOC on the leaching of the metals. High DOC increased the leaching of Cu, while low concentrations of DOC increased leaching of Ba and Mn. Aerated/anoxic conditions had a high influence on Ni (VIP > 1), but a low influence on the other three metals (VIP < 0.5). Anoxic conditions caused higher leaching of Ni, compared to aerated conditions.
Stirring was used to simulate resuspension and was important for leaching of Ba and Ni (VIP > 1) and had a moderate influence on Cu (VIP 0.5–1) and a low influence on Mn (VIP < 0.5). Magnafloc10 was an important variable for Cu (VIP > 1) and had a moderate influence on Mn (VIP 0.5–1) and a low influence on Ba and Ni (VIP < 0.5). The addition of Magnafloc10 to the tailings–sediment slurries decreased the leaching of metals.
3.4. Model Prediction for Leaching of Metals from Mine Tailings Sediments
In the fractional factorial design used for the experimental design in this study, experiments did not cover all corners of the experimental domain. Experiments with variable settings that ensured minimum or maximum leaching also differed depending on the metal, as presented in the previous section and seen in Figure 2. Even though experiments were not conducted in all the corners of the experimental domain, the PLS models were able to predict leaching ranges in the entire experimental domain. By applying settings of the variables (high/low) representing the leaching range in the experimental domain for each metal, the minimum and maximum leaching of each metal was predicted. The setting of variables at high/low setting (Table 5) was made in accordance with coefficient plots (Figure 2). The prediction set was the 11 experiments, and the predicted leaching range is summarized in Table 5. The metal with the lowest leaching concentrations and narrow range was Ni (0.01–0.17 mg/kg). Ba had a leaching range in the experimental domain of 0.1–0.65 mg/kg. Cu had a wider leaching range (0.03–1.5 mg/kg), and Mn had the widest range and highest leaching concentrations of 0.06–98 mg/kg.

Table 5.
Predicted leaching of metals in the experimental domain of the leaching experiments. Minimum leaching was predicted in the PLS models by using the experimental settings of each variable that gave the lowest leaching for each metal. Maximum leaching was predicted in the PLS models by using experimental settings of each variable that gave the highest leaching for each metal.
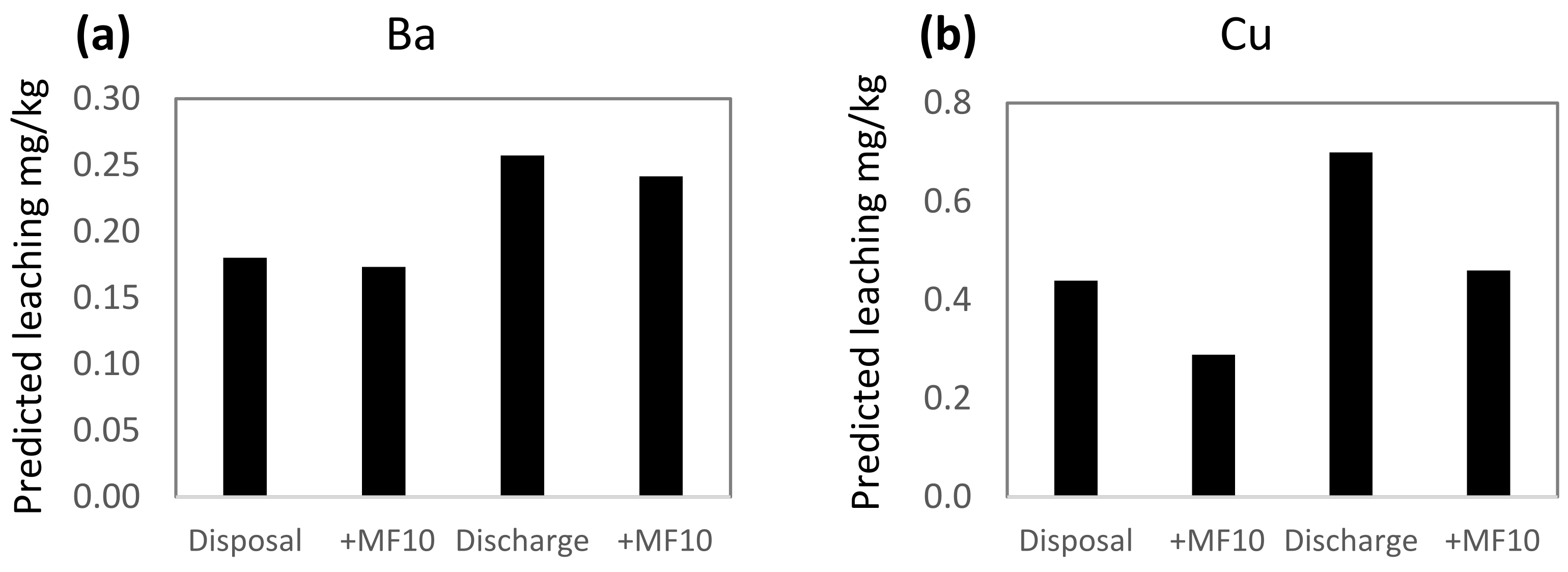
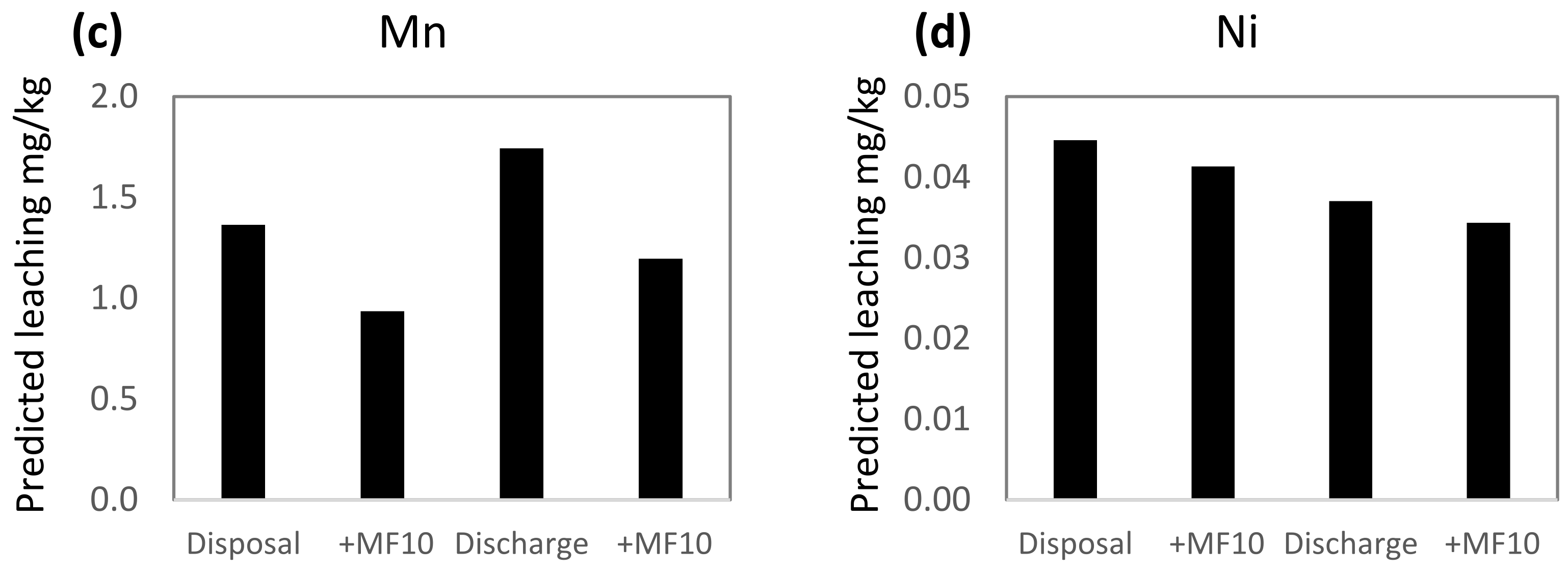
Conditions in the Repparfjord are within the ranges used in the leaching experiments and the PLS models were used to predict leaching from mine tailings and sediment under natural fjord conditions. This was done by using average values of seasonal variations of DOC, pH, salinity and temperature, measured in the fjord 2010–11. The average salinity was 33.0 ppt, the temperature 4.5 °C, pH 8.02 and DOC 1.6 mg/L [56]. Two scenarios were used in the prediction calculations: disposal (i.e., no stirring, anoxic) and resuspension (stirring, aerated). The calculated leaching predictions show 30–60% higher leaching of Ba, Cu and Mn, and 20% lower leaching of Ni during resuspension compared to disposal (Figure 3). The lower leaching of Ni during resuspension is related to aerated conditions, found to have the highest influence of all variables on the leaching of Ni (Figure 2) rather than to the positively correlated influence of stirring.
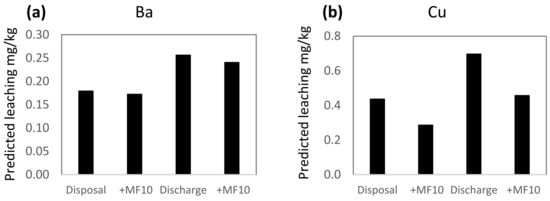
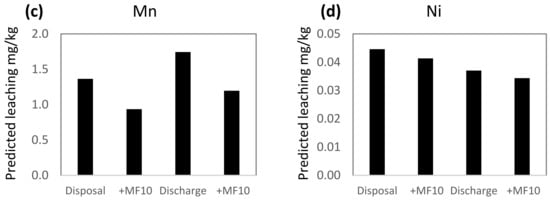
Figure 3.
PLS predicted leaching of (a) Ba, (b) Cu, (c) Mn and (d) Ni in two different scenarios: disposal (no stirring, anoxic) and resuspension (stirring, aerated) in the Repparfjord. The prediction of metal leaching with the addition of Magnafloc10 is given for each of the scenarios (+MF10). For the prediction of leaching, the following settings were used: salinity 33.0 ppt, pH 8.02, temperature 4.5 °C and DOC 1.6 mg/L, representing average conditions in the Repparfjord.
The addition of Magnafloc10 to the mine tailings prior to discharge is to ensure fast sedimentation and minimize the dispersion of mine tailing particles in the fjord. The addition of Magnafloc10 also influences leaching (Figure 3). For Cu and Mn, leaching is reduced by 31–34% in both the disposal and resuspension phase, while the leaching of Ba and Ni is reduced by 3–7%. The difference in the effect of Magnafloc10 on the metals is reflected in the VIP plots (Figure 2), where it was found that Magnafloc10 had moderate–high influence on Cu and Mn, and a low influence on Ba and Ni.
4. Discussion
4.1. Metal Concentration Discrepancy
For the metals Ba, Cr, K and Ni there was a discrepancy between the concentrations in the mine tailings and the Repparfjord sediments, and the analyzed concentrations of the mixture of the two (1:1, Table 2), used in the leaching experiments. The concentrations of the Repparfjord sediment found in this study are similar to previous findings from the same core and within the variation of metal concentrations found for each cm in the 20 cm core (005, [40]). The discrepancy found in the mixture of sediment and tailings is likely caused by inhomogeneity in the mine tailings. The inhomogeneity in the finer fractions (<104 µm) of the mine tailings, making up more than 80% of the tailings, was partly studied previously [46] in different grain size fractions, and generally metal concentrations were highest in the finest fraction (<37 µm). The concentrations of Ba, Cr, K and Ni were generally higher in all the analyzed fine fractions than in the concentrations in Table 2. The total concentrations of these four metals based on summary of the sequential extraction fractions in this study also revealed higher concentrations for Ba (211 mg/kg dw), Cr (145 mg/kg dw), K (6240 mg/kg dw) and Ni (44 mg/kg dw), while other analyzed metals were within concentrations (+/− standard deviation) of those in the mine tailings in Table 2.
The inhomogeneity of the mine tailings illustrates the need for leaching tests of several mine tailings samples to include the variation in metal concentrations. This will provide a better estimation of the variations in leaching.
4.2. Variable Importance
The variability in variable importance for metal leaching, as determined by the PLS models (Figure 2), between the different metals is related to how the metals are bound in the sediment–tailings particles. Other studies have shown that the influence of variables on leaching can differ for the same metal, depending on which minerals the metals are associated with [57,58,59]. In the timeframe of this study, significant changes to metal partitioning were not observed. The low leaching percentages and limited effect on metal partitioning indicated low dissolution of minerals as well as low impact of experimental variables on reactions between different fractions (exchangeable, reducible, oxidizable, residual). In other studies, effects on metal partitioning were observed for the variables included in this study, but at more extreme settings, such as pH < 2 [60], or similar experimental domains but higher initial content of metals in some of the more available fractions [61].
For the purpose of evaluating the influence of variables on metal leaching, the focus remains for variables with the highest impact on the PLS models (VIP values > 1) of the four metals (Ba, Cu, Mn and Ni). pH had a high influence on all metals and the trend of higher leaching at the low setting (pH 6) is in line with other studies that have included Ba, Cu, Ni and/or Mn [31,62,63]. The highest reported metal leaching was at pH < 2 [64,65], which is outside the experimental domain of this study. The reason for keeping the lowest setting at pH 6 in this study is that lower pH levels would represent an extreme marine environment and would not be relevant for sea disposal of metal polluted sediment/tailings. Leaching caused by changes in pH is most likely predominantly from the exchangeable fraction, since pH in the range 6–9 is known to impact ion exchange and dissolution of carbonates, while dissolution of other minerals occurs at lower pH [66]. The effect of pH in ion exchange is related to charge surfaces and at neutral and high pH, the surface charge is negative on clay minerals and organic matter, enabling adsorption of cations. With decreasing pH, there are more cations in solution to compete for the negative charged sites, and in addition surfaces become more positively charged due to protonation of functional groups [67]. The sediment–tailing mixture had a high content of calcite (approximately 10%) and the total concentration of Ca (Table 2) indicates that most of it was bound in calcite; accordingly, Ca can be used as measure for dissolution of carbonates in the sediment–tailings slurry. In the experiments, 4–10% Ca was leached at the low pH setting and 0.3–1.1% was leached at the high pH setting (Table 3). These levels of calcite dissolution are in line with calcite dissolution kinetics [68,69]; complete dissolution of carbonates would occur at pH levels below 4.
The high influence of pH, compared to salinity and DOC, was confirmed in a recent study of metal adsorption–desorption in sediments [70]. The higher influence of salinity on Cu in this study indicates difference in Cu availability between the sediments in the previous study and the sediment–tailings mixture used in this study. The higher levels of leaching with increasing salinity are related to strong influence of ion exchange on the leaching of Cu from the sediment–tailings mixture.
Temperature had a high influence on Cu leaching, with the highest leaching levels found at the low setting (4 °C). It is well known that desorption of Cu from clay minerals increases with increasing temperatures [71], and the opposite correlation in this study indicates that there are other temperature-dependent reactions that dominate the adsorption–desorption of Cu in the sediment–tailings slurries. One of these could be the adsorption of Cu to organic material, e.g., humic substances, as it has been shown that adsorption of Cu increases with increasing temperature [72]. Thus, the lower Cu leaching observed at the high temperature could be related to higher adsorption to humic substances. The high influence of DOC on the leaching of Cu, with lowest leaching concentrations at high settings of DOC (20 mg/L), underlines the importance of adsorption to humic substances for Cu in the sediment–tailings slurry. DOC is also important for leaching of Mn, however, with an opposite correlation than Cu; in fact, this is also the case for temperature (although moderate influence). This suggests that adsorption of Mn to humic substances is not a dominating process and confirms that Cu has a higher affinity for humic substances than Mn [73].
Anoxic conditions (nitrogen) increased the leaching of Ni, indicating the effect of reducing conditions. The highest leaching of Ni was observed in experiments 2 and 4, which also had low setting of pH and salinity. In these experiments the highest concentration of Ca and Mn was observed. In addition to anoxic conditions, Ni leaching is most likely influenced by which minerals it is associated with and the water composition. It is uncertain whether the higher concentrations of Ca and Mn have an impact on Ni leaching from the sediment–tailings, as was the case in other studies for Ni mobilization from degradation of pyrite in groundwater aquifers [74,75].
Stirring of the sediment–tailings slurry increases the contact between water and solids, and the governing leaching reactions of Ba and Ni from the suspended solids. For Cu and Mn other variables are more important for leaching. The addition of the flocculant chemical Magnafloc10 had high influence on Cu and decreased leaching. Magnafloc10 has previously been shown to have high affinity for Cu, compared to the other metals included in this study [31,76].
The influence of mixing the tailings with sediment was not included as a variable in this study. However, previous leaching experiments of the same mine tailings, conducted within the same experimental domain, showed lower leaching of Cu, Mn and Ni and higher leaching of Ba than was the case in this study [61]. It is not clear whether leaching of Cu, Mn and Ni is higher in the Repparfjord sediments, or whether leaching from the mine tailings is affected by interactions with the sediment. This warrants further investigations in the future.
The results of variable importance have shown that leaching of metals is largely related to how metals are associated with minerals in the sediment. Multivariate analysis can provide a powerful tool for interpretation of variable importance and correlation between variables and leaching. The variation in the influence of variables dependent on the metal has demonstrated the importance of including more variables than is usual in standard leaching tests for disposal on land, as well as testing different settings to better understand the effect of conditions specific to the disposal area. Design of leaching tests will depend on sediment characteristics, local fjord/sea conditions as well as the properties of the pollutant(s) in question.
4.3. Leaching Tests—Implications for the Repparfjord and Future Outlook
The application of stirred and non-stirred setups in the leaching tests represents upwhirling and mixing with sea water, and stationary disposal on the seafloor. Based on the leaching tests, leaching estimations can be made. Since they are on lab scale, it is important to supplement with monitoring measurements. In addition to estimations of leaching, the tests can be used to screen and identify important pollutants for monitoring, and which variable settings trigger higher leaching, thereby providing a foundation for potential actions to limit leaching.
In the case of submarine mine tailings disposal in the Repparfjord, metal leaching during resuspension was estimated from the experimental settings of stirred setup and aeration. The estimate of metal leaching represents the total potential leaching from a short-term perspective, in line with the frames of standard leaching tests for disposal on land [77,78]. The PLS prediction of metal leaching using average seasonal measurements of salinity, temperature and DOC in the Repparfjord showed leaching concentrations in the order Mn > Cu > Ba > Ni (Figure 3).
Metal leaching during disposal was estimated from the experimental settings of non-stirred setup and anoxic conditions. In the center of the disposal area, the mine tailings are expected to be continuously covered. In the boundary of the disposal, the seawater and currents may provide aerated conditions. Based on the leaching tests, it is possible to estimate metal leaching for both scenarios. The estimations represent a short-term perspective. The PLS prediction of metal leaching in the Repparfjord during disposal showed the same order of metal leaching as during resuspension, namely Mn > Cu > Ba > Ni (Figure 3). Based on these results, it would be recommended to include all four metals in the monitoring program of the submarine mine tailings, with focus on Mn and Cu.
Based on the variable importance analysis, the leaching tests can also be used as a foundation for suggesting actions to limit metal leaching. Leaching can be limited by adjusting controllable variables with high influence, by ensuring fast sedimentation (low stirring) and addition of flocculant. Magnafloc10 is planned to be used for dewatering and to ensure fast sedimentation and in this study, it was shown to have an added value to limit leaching, especially for the two metals with highest leaching levels, Cu and Mn (Figure 3). The implications of using Magnafloc10 for the marine environment must also be taken into consideration, and other studies have investigated the effects of Magnafloc10 on selected marine species [79,80,81]. These studies show limited effects of Magnafloc10 for the planned use of Magnafloc10 concentrations (30 µg/kg tailings).
The leaching tests indicate low influence of aeration on leaching and for this reason covering the mine tailings with clean material upon cessation will not influence the availability of metals for leaching. Capping the mine tailings would primarily be for other reasons, e.g., stabilization or recultivation.
In this study, the beneficial use of leaching tests prior to initiating physical actions that interfere with sediments was exemplified through the study of planned submarine mine tailings disposal in the Repparfjord. The results and knowledge gained are easily transferable to other sites by adjusting the variable settings according to local conditions, and expanding the experimental domain with other variables, if necessary. The stirred leaching tests can for instance be applied to dredging operations to assess the potential leaching and the influence of aeration, mixing and changes in salinity, temperature and DOC during the operation. Application of silt curtains can limit leaching outside the dredging area; however, dredging can lead to higher concentrations of suspended solids with the risk of dispersion and leaching after dredging of an area [8]. Application of non-stirred leaching tests can be applied for sea disposal, when this is assessed as the best available technology for disposal. Leaching tests can assist in assessment of sea disposal and actions to limit leaching. In this study, there was a positive effect of Magnafloc10 on the leaching of Cu and Mn. In the establishment of sea disposal sites, flocculants can not only enhance sedimentation but also limit leaching during disposal. Different flocculants target different pollutants [82] and mapping flocculants to match pollutants is important prior to the use.
Leaching tests are valuable tools for assessing leaching from polluted sediments and, by including different variables at realistic settings, they can be used to better understand leaching and conditions that trigger leaching for a specific site and for specific pollutants. In this way they can contribute to better understanding of environmental risks, coupled with environmental studies of effects of sea disposal on the marine environment.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources and data curation: K.B.P.; writing—original draft preparation and review and editing: K.B.P., T.L. and A.E.; visualization and project administration: K.B.P.; funding acquisition: K.B.P., A.E. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FRAM—High North Research Centre for Climate and Environment in the flagship MIKON (Environmental Impacts of Industrial Development in the North).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data will be part of a database to be developed by Akvaplan-niva (public at the time of publishing).
Acknowledgments
The authors wish to acknowledge laboratory technician Ebba Schnell at the Technical University of Denmark for assistance in metal analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Moullec, F.; Asselot, R.; Sguotti, C.; Steidle, L.; Tams, V.; Pellerin, F.; Auch, D.; Blöcker, A.M.; Börner, G.; Färber, L.; et al. Identifying and addressing the anthropogenic drivers of global change in the North Sea: A systematic map protocol. Environ. Evid. 2021, 10, 19. [Google Scholar] [CrossRef]
- Halpern, B.S.; Longo, C.; Lowndes, J.S.S.; Best, B.D.; Frazier, M.; Katona, S.K.; Kleisner, K.M.; Rosenberg, A.A.; Scarborough, C.; Selig, E.R. Patterns and Emerging Trends in Global Ocean Health. PLoS ONE 2015, 10, e0117863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacho, K.O.; Mosgaard, M.; Riisgaard, H. Capturing uncaptured values—A Danish case study on municipal preparation for reuse and recycling of waste. Resour. Conserv. Recycl. 2018, 136, 297–305. [Google Scholar] [CrossRef]
- Weißenbach, T.; Graf, J.; Pomberger, R.; Sarc, R. Calculation of the additional recycling potential in the European Union by implementing the Circular Economy Package. Environ. Waste Manag. Recycl. 2020, 3, 1–9. [Google Scholar]
- Junakova, N.; Junak, J. Alternative reuse of bottom sediments in construction materials: Overview. IOP Conf. Ser. Mater. Sci. Eng. 2019, 549, 012038. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bizzigotti, G.O.; Castelly, H.; Hafez, A.M.; Smith, W.H.B.; Whitmire, M.T. Parameters for Evaluation of the Fate, Transport, and Environmental Impacts of Chemical Agents in Marine Environments. Chem. Rev. 2009, 109, 236–256. [Google Scholar] [CrossRef]
- Bianchini, A.; Cento, F.; Guzzini, A.; Pellegrini, M.; Saccani, C. Sediment management in coastal infrastructures: Techno-economic and environmental impact assessment of alternative technologies to dredging. J. Environ. Manag. 2019, 248, 109332. [Google Scholar] [CrossRef]
- Lonsdale, J.-A.; Blake, S.; Griffith, A. A novel systematic, risk based approach to support the designation of aquatic disposal sites. Mar. Pollut. Bull. 2021, 162, 111874. [Google Scholar] [CrossRef]
- Bolam, S.G.; Rees, H.L. Minimizing impacts of maintenance dredged material disposal in the coastal environment: A habitat approach. Environ. Manag. 2003, 32, 171–188. [Google Scholar] [CrossRef]
- Fredette, T.J. Why confined aquatic disposal cells often make sense. Integr. Environ. Assess. Manag. Int. J. 2006, 2, 35–38. [Google Scholar] [CrossRef]
- Ausili, A.; Mecozzi, M.; Gabellini, M.; Ciuffa, G.; Mellara, F. Physico chemical characteristics and multivariate analysis of contaminated harbour sediments. Water Sci. Technol. 1998, 37, 131–139. [Google Scholar] [CrossRef]
- Lindsay, M.; Moncur, M.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Fredette, T.; French, G. Understanding the physical and environmental consequences of dredged material disposal: History in New England and current perspectives. Mar. Pollut. Bull. 2004, 49, 93–102. [Google Scholar] [CrossRef]
- Brooks, S.J.; Escudero-Oñate, C.; Lillicrap, A.D. An ecotoxicological assessment of mine tailings from three Norwegian mines. Chemosphere 2019, 233, 818–827. [Google Scholar] [CrossRef]
- Vogt, C.; Peck, E.; Hartman, G. Dredging for Navigation, for Environmental Cleanup, and for Sand/Aggregates. In Handbook on Marine Environment Protection: Science, Impacts and Sustainable Management; Salomon, M., Markus, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 189–213. [Google Scholar]
- Ramirez-Llodra, E.; Trannum, H.C.; Evenset, A.; Levin, L.A.; Andersson, M.; Finne, T.E.; Hilario, A.; Flem, B.; Christensen, G.; Schaanning, M.; et al. Submarine and deep-sea mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Mar. Pollut. Bull. 2015, 97, 13–35. [Google Scholar] [CrossRef]
- Bolam, S.G. Impacts of dredged material disposal on macrobenthic invertebrate communities: A comparison of structural and functional (secondary production) changes at disposal sites around England and Wales. Mar. Pollut. Bull. 2012, 64, 2199–2210. [Google Scholar] [CrossRef]
- Gambi, C.; Canals, M.; Corinaldesi, C.; Dell’Anno, A.; Manea, E.; Pusceddu, A.; Sanchez-Vidal, A.; Danovaro, R. Impact of historical sulfide mine tailings discharge on meiofaunal assemblages (Portmán Bay, Mediterranean Sea). Sci. Total Environ. 2020, 736, 139641. [Google Scholar] [CrossRef]
- Perner, K.; Leipe, T.; Dellwig, O.; Kuijpers, A.; Mikkelsen, N.; Andersen, T.; Harff, J. Contamination of arctic Fjord sediments by Pb–Zn mining at Maarmorilik in central West Greenland. Mar. Pollut. Bull. 2010, 60, 1065–1073. [Google Scholar] [CrossRef]
- Søndergaard, J.; Asmund, G.; Johansen, P.; Rigét, F. Long-term response of an arctic fiord system to lead–zinc mining and submarine disposal of mine waste (Maarmorilik, West Greenland). Mar. Environ. Res. 2011, 71, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.; Andrade, S.; Faugeron, S.; Lagos, N.; Mella, D.; Correa, J. Biodiversity of rocky intertidal benthic communities associated with copper mine tailing discharges in northern Chile. Mar. Pollut. Bull. 2005, 50, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Kristensen, J.; Asmund, G.; Bjerregaard, P. Lead and zinc in sediments and biota from Maarmorilik, West Greenland: An assessment of the environmental impact of mining wastes on an Arctic fjord system. Environ. Pollut. 2001, 114, 275–283. [Google Scholar] [CrossRef]
- Oen, A.M.; Pettersen, A.; Eek, E.; Glette, T.; Brooks, L.; Breedveld, G.D. Monitoring chemical and biological recovery at a confined aquatic disposal site, Oslofjord, Norway. Environ. Toxicol. Chem. 2017, 36, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- OSPAR. OSPAR Guidelines for the Management of Dredged Material at Sea, Agreement 2014-06; OSPAR: London, UK, 2014. [Google Scholar]
- Kvassnes, A.J.S.; Iversen, E. Waste sites from mines in Norwegian Fjords. Mineralproduksjon 2013, 3, A27–A38. [Google Scholar]
- Côtè, P.; Constable, T. Evaluation of experimental conditions in batch leaching procedures. Resour. Conserv. 1982, 9, 59–73. [Google Scholar] [CrossRef]
- USEPA. Test Methods for Evaluating Solid Waste: Physical/Chemical Methods Compendium. SW-846; USEPA: Washington, DC, USA, 2014.
- Cappuyns, V.; Swennen, R. The application of pHstat leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste materials. J. Hazard. Mater. 2008, 158, 185–195. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Reinardy, H.C.; Jensen, P.E.; Ottosen, L.M.; Junttila, J.; Frantzen, M. The influence of Magnafloc10 on the acidic, alkaline, and electrodialytic desorption of metals from mine tailings. J. Environ. Manag. 2018, 224, 130–139. [Google Scholar] [CrossRef]
- Du Laing, G.; De Vos, R.; Vandecasteele, B.; Lesage, E.; Tack, F.M.G.; Verloo, M. Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuar. Coast. Shelf Sci. 2008, 77, 589–602. [Google Scholar] [CrossRef]
- Ghosh, U.; Talley, J.W.; Luthy, R.G. Particle-Scale Investigation of PAH Desorption Kinetics and Thermodynamics from Sediment. Environ. Sci. Technol. 2001, 35, 3468–3475. [Google Scholar] [CrossRef]
- Badea, S.-L.; Mustafa, M.; Lundstedt, S.; Tysklind, M. Leachability and desorption of PCBs from soil and their dependency on pH and dissolved organic matter. Sci. Total Environ. 2014, 499, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Benamar, A.; Tian, Y.; Portet-Koltalo, F.; Ammami, M.; Giusti-Petrucciani, N.; Song, Y.; Boulangé-Lecomte, C. Enhanced electrokinetic remediation of multi-contaminated dredged sediments and induced effect on their toxicity. Chemosphere 2019, 228, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Skei, J.M.; Syvitski, J.P. Natural flocculation of mineral particles in seawater-influence on mine tailings sea disposal and particle dispersal. Mineralproduksjon 2013, 3, 1–10. [Google Scholar]
- Taylor, M.L.; Morris, G.E.; Self, P.G.; Smart, R.S. Kinetics of Adsorption of High Molecular Weight Anionic Polyacrylamide onto Kaolinite: The Flocculation Process. J. Colloid Interface Sci. 2002, 250, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M. An overview of the use of chemical reagents in mineral processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Sternal, B.; Junttila, J.; Skirbekk, K.; Forwick, M.; Carroll, J.; Pedersen, K.B. The impact of submarine copper mine tailing disposal from the 1970s on Repparfjorden, northern Norway. Mar. Pollut. Bull. 2017, 120, 136–153. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.B.; Jensen, P.E.; Sternal, B.; Ottosen, L.M.; Henning, M.V.; Kudahl, M.M.; Junttila, J.; Skirbekk, K.; Frantzen, M. Long-term dispersion and availability of metals from submarine mine tailing disposal in a fjord in Arctic Norway. Environ. Sci. Pollut. Res. 2018, 25, 32901–32912. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Finne, T.; Jensen, L.; Eggen, O. Geochemistry of a copper mine tailings deposit in Repparfjorden, northern Norway. Sci. Total Environ. 2018, 644, 1219–1231. [Google Scholar] [CrossRef]
- Guttorm, N.; Christensen, A.J.S.K.; Tjomsland, T.; Leikvin, Ø.; Kempa, M.; Kolluru, V.; Velvin, R.; Dahl-Hansen, G.A.P.; Jørgensen, N.M. Consequences of Establishing Submarine or Landbased Disposal for Nussir and Ulveryggen Mine Tailings for the Marine Environment in Repparfjorden, Kvalsund Municipality, Norway (in Norwegian); 5249-01; Akvaplan-niva AS: Tromsø, Norway, 2011; pp. 1–214. [Google Scholar]
- Didriksen, T.-A.; Wilersrud, Ø. Zoning and Environmental Impact of Planned Mining of Nussir and Ulveryggen in Kvalsund Municipality (in Norwegian) Reguleringsplan med Konsekvensutredning for Planlagt Gruvedrift i Nussir og Ulveryggen i Kvalsund Kommune; SWECO: Alta, Norway, 2010. [Google Scholar]
- Pedersen, K.B.; Jensen, P.E.; Ottosen, L.M.; Evenset, A.; Christensen, G.N.; Frantzen, M. Metal speciation of historic and new copper mine tailings from Repparfjorden, Northern Norway, before and after acid, base and electrodialytic extraction. Miner. Eng. 2017, 107, 100–111. [Google Scholar] [CrossRef]
- Kleiv, R.A. Physical and Chemical Properties of Flotation Tailings from Nussir- and Ulveryggen Ores (in Norwegian); M-RAK 2011:7; NTNU: Trondheim, Norway, 2011; p. 25. [Google Scholar]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Quevauviller, P.; Rauret, G.; Muntau, H.; Ure, A.M.; Rubio, R.; Fiedler, H.D.; Griepink, B. Evaluation of a sequential extraction procedure for the determination of extractable trace metal contents in sediments. Fresenius' J. Anal. Chem. 1994, 349, 808–814. [Google Scholar] [CrossRef]
- The Norwegian Environment Agency. Permission to Operate under the Pollution Control Act for Nussir ASA (in Norwegian); The Norwegian Environment Agency: Oslo, Norway, 2015; pp. 1–40. [Google Scholar]
- Eriksson, L.; Trygg, J.; Wold, S. A chemometrics toolbox based on projections and latent variables. J. Chemom. 2014, 28, 332–346. [Google Scholar] [CrossRef]
- Carlson, R.; Carlson, J.E. Design and Optimization in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- The Norwegian Environment Agency. Quality Standards for Water, Sediment and Biota (in Norwegian) Updated 30.10.2020; The Norwegian Environment Agency: Oslo, Norway, 2016; 13. [Google Scholar]
- European Commission. Technical Guidance for Deriving Environmental Quality Standards. Common Implementation Strategy for the Water Framework Directive (2000/60/EC), Guidance Document No. 27; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Verbruggen, E.; Smit, C.; van Vlaardingen, P. Environmental Quality Standards for Barium in Surface Water: Proposal for an Update According to the Methodology of the Water Framework Directive; National Institute for Public Health and the Environment, RIVM: Bilthoven, The Netherlands, 2020. [Google Scholar]
- Summer, K.; Reichelt-Brushett, A.; Howe, P. Toxicity of manganese to various life stages of selected marine cnidarian species. Ecotoxicol. Environ. Saf. 2019, 167, 83–94. [Google Scholar] [CrossRef]
- Christensen, G.N.; Dahl-Hansen, G.A.P.; Gaardsted, F.; Leikvin, Ø.; Palerud, R.; Velvin, R.; Vögele, B. Marine Baseline Survey of Repparfjorden in Finnmark, 2010–2011 (in Norwegian); APN 4973-01; Akvaplan-niva AS: Tromsø, Norway, 2011; pp. 1–100. [Google Scholar]
- Chen, M.; Lu, G.; Wu, J.; Sun, J.; Yang, C.; Xie, Y.; Wang, K.; Deng, F.; Yi, X.; Dang, Z. Acidity and metallic elements release from AMD-affected river sediments: Effect of AMD standstill and dilution. Environ. Res. 2020, 186, 109490. [Google Scholar] [CrossRef]
- Gurung, B.; Race, M.; Fabbricino, M.; Kominkova, D.; Libralato, G.; Siciliano, A.; Guida, M. Assessment of metal pollution in the Lambro Creek (Italy). Ecotoxicol. Environ. Saf. 2018, 148, 754–762. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Influence of biogeochemical interactions on metal bioleaching performance in contaminated marine sediment. Water Res. 2013, 47, 5139–5152. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Xiong, W.; Xu, Y.; Chi, R.-A. A two-step leaching method designed based on chemical fraction distribution of the heavy metals for selective leaching of Cd, Zn, Cu, and Pb from metallurgical sludge. Environ. Sci. Pollut. Res. 2018, 25, 1752–1765. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Lejon, T.; Jensen, P.E.; Ottosen, L.M.; Evenset, A.; Frantzen, M. Impacts of climate change on metal leaching and partitioning for submarine mine tailings disposal. 2022; To be submitted. [Google Scholar]
- Atkinson, C.A.; Jolley, D.; Simpson, S. Effect of overlying water pH, dissolved oxygen, salinity and sediment disturbances on metal release and sequestration from metal contaminated marine sediments. Chemosphere 2007, 69, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Keshavarzifard, M.; Moore, F.; Sharifi, R. The influence of physicochemical parameters on bioavailability and bioaccessibility of heavy metals in sediments of the intertidal zone of Asaluyeh region, Persian Gulf, Iran. Geochemistry 2019, 79, 178–187. [Google Scholar] [CrossRef]
- Ho, H.H.; Swennen, R.; Cappuyns, V.; Vassilieva, E.; Van Gerven, T.; Van Tran, T. Potential release of selected trace elements (As, Cd, Cu, Mn, Pb and Zn) from sediments in Cam River-mouth (Vietnam) under influence of pH and oxidation. Sci. Total Environ. 2012, 435–436, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Beolchini, F.; Fonti, V.; Rocchetti, L.; Saraceni, G.; Pietrangeli, B.; Dell’Anno, A. Chemical and biological strategies for the mobilisation of metals/semi-metals in contaminated dredged sediments: Experimental analysis and environmental impact assessment. Chem. Ecol. 2013, 29, 415–426. [Google Scholar] [CrossRef]
- Davidson, C.; Thomas, R.P.; McVey, S.E.; Perala, R.; Littlejohn, D.; Ure, A.M. Evaluation of a sequential extraction procedure for the speciation of heavy metals in sediments. Anal. Chim. Acta 1994, 291, 277–286. [Google Scholar] [CrossRef]
- Sparks, D.L. (Ed.) 6—Ion Exchange Processes. In Environmental Soil Chemistry, 2nd ed.; Academic Press: Burlington, VT, USA, 2003; pp. 187–205. [Google Scholar]
- Sjöberg, E.; Rickard, D. Temperature dependence of calcite dissolution kinetics between 1 and 62 °C at pH 2.7 to 8.4 in aqueous solutions. Geochim. Cosmochim. Acta 1984, 48, 485–493. [Google Scholar] [CrossRef]
- Sulpis, O. Calcite Dissolution Kinetics at the Sediment-Water Interface in an Acidifying Ocean; McGill University (Canada): Montreal, QC, Canada, 2019. [Google Scholar]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-desorption behavior of heavy metals in aquatic environments: Influence of sediment, water and metal ionic properties. J. Hazard. Mater. 2021, 421, 126743. [Google Scholar] [CrossRef]
- Doula, M.; Ioannou, A.; Dimirkou, A. Thermodynamics of Copper Adsorption-Desorption by Ca-Kaolinite. Adsorption 2000, 6, 325–335. [Google Scholar] [CrossRef]
- Li, Y.; Yue, Q.; Gao, B. Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid. J. Hazard. Mater. 2010, 178, 455–461. [Google Scholar] [CrossRef]
- Hsu, J.H.; Lo, S.L. Characterization and Extractability of Copper, Manganese, and Zinc in Swine Manure Composts; 0047-2425; Wiley Online Library: Hoboken, NJ, USA, 2000. [Google Scholar]
- Larsen, F.; Postma, D. Nickel Mobilization in a Groundwater Well Field: Release by Pyrite Oxidation and Desorption from Manganese Oxides. Environ. Sci. Technol. 1997, 31, 2589–2595. [Google Scholar] [CrossRef]
- Matern, K.; Lux, C.; Ufer, K.; Kaufhold, S.; Mansfeldt, T. Removal of nickel from groundwater by iron and manganese oxides. Int. J. Environ. Sci. Technol. 2019, 16, 2895–2904. [Google Scholar] [CrossRef]
- Bailey, S.E.; Hwang, S.; Brooks, M.C.; Schroeder, P.R. Evaluation of Chemical Clarification Polymers and Methods for Removal of Dissolved Metals from CDF Effluent. 2006. Available online: https://apps.dtic.mil/sti/pdfs/ADA452680.pdf (accessed on 9 March 2022).
- Hage, J.; Mulder, E. Preliminary assessment of three new European leaching tests. Waste Manag. 2004, 24, 165–172. [Google Scholar] [CrossRef]
- Townsend, T.; Jang, Y.-C.; Tolaymat, T. A Guide to the Use of Leaching Tests in Solid Waste Management Decision Making; University of Florida, The Florida Center for Solid and Hazardous Waste: Gainesville, FL, USA, 2003. [Google Scholar]
- Lillicrap, A.; Sweetman, A.; Macrae, K.; Heiaas, H. Determination of the Acute Toxicity of Mine Tailings from Nussir. ASA to the Marine Alga Skeletonema Costatum, the Marine Copepod Tisbe Battagliai and the Polychaete Arenicola Marina; NIVA: Oslo, Norway, 2011; Available online: https://niva.brage.unit.no/niva-xmlui/handle/11250/215449 (accessed on 9 March 2022).
- Farkas, J.; Altin, D.; Hansen, B.H.; Øverjordet, I.B.; Nordtug, T. Acute and long-term effects of anionic polyacrylamide (APAM) on different developmental stages of two marine copepod species. Chemosphere 2020, 257, 127259. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.; Beylich, B.; Brooks, S.; Jaccard, P.; Tobiesen, A.; Øxnevad, S. Monitoring of Bøkfjorden 2011 and Toxicity Tests of Process Chemicals Magnafloc LT 38 and Magnafloc 10 (in Norwegian); NIVA: Oslo, Norway, 2012; Available online: https://niva.brage.unit.no/niva-xmlui/bitstream/handle/11250/215821/6310-2012_72dpi.pdf?sequence=1&isAllowed=y (accessed on 9 March 2022).
- Othmani, B.; Rasteiro, M.G.; Khadhraoui, M. Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation. Clean Technol. Environ. Policy 2020, 22, 1025–1040. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).