Antioxidant and Anti-Colorectal Cancer Properties in Methanolic Extract of Mangrove-Derived Schizochytrium sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture Conditions of Thraustochytrids from Mangroves
2.2. Morphological Identification of Thraustochytrids
2.3. DNA Extraction and Amplification of the 18S rDNA Gene
2.4. Sequencing and Phylogenetic Analyses of 18S rDNA Gene
2.5. Biomass Production and Extraction of Intracellular Specialized Metabolites
2.6. Antioxidant Assays
2.7. GC-MS Analysis (Gas Chromatography-Mass Spectroscopy)
2.8. Preparation of Protein and Ligand Structures
2.9. Molecular Docking Analysis
2.10. In Silico ADME Predictions for Pharmacokinetic Properties
2.11. Determination of the In Vitro Anti-Proliferative Effect of Thraustochytrid Extracts on Colon Cell Lines
2.12. Preparation of Thraustochytrid Extracts
2.13. MTT Cytotoxicity Assay
3. Results
3.1. Morphological and Molecular Identification of Thraustochytrids
3.2. Antioxidant Assay
3.3. Molecular Docking and ADME Predictions
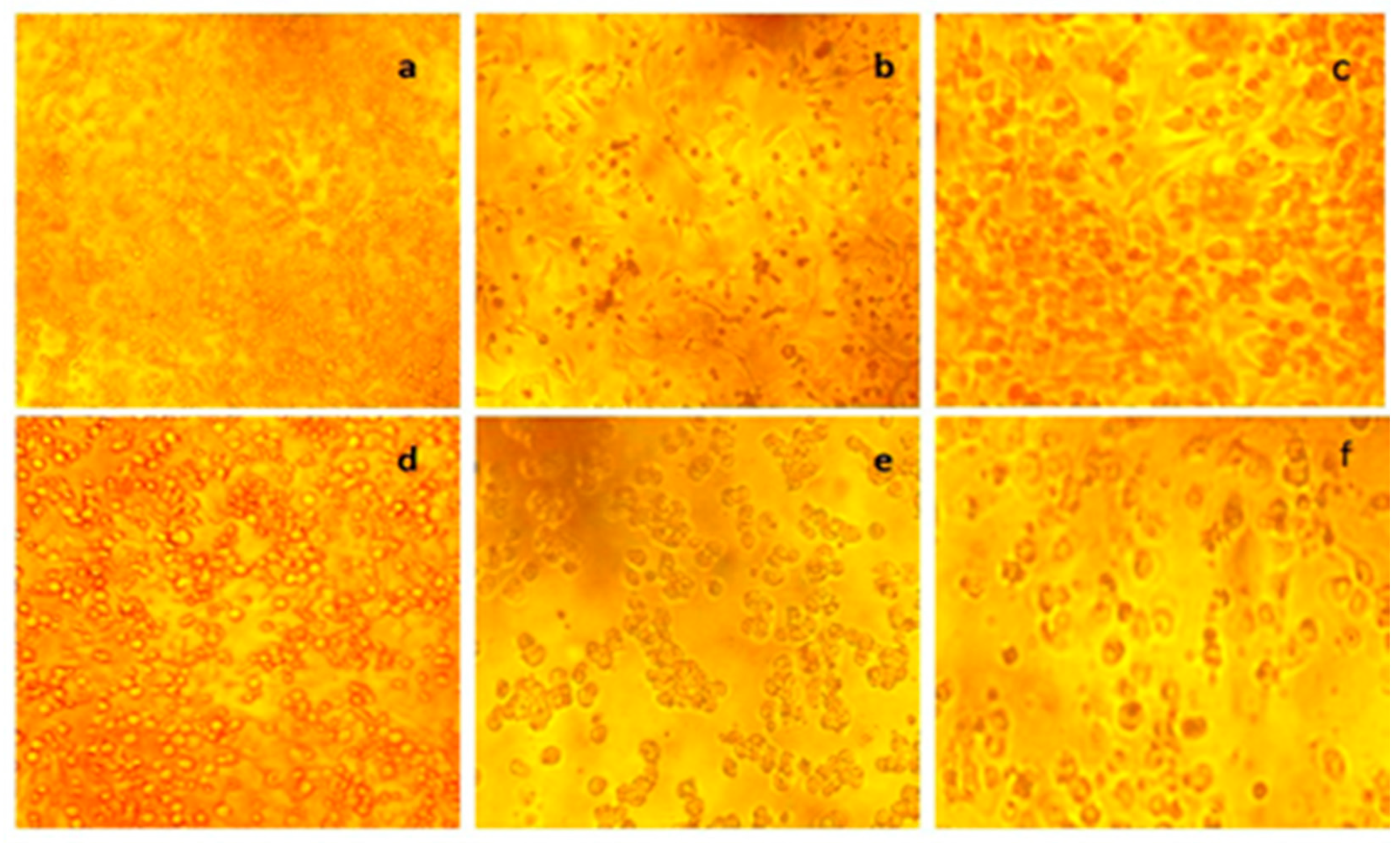
3.4. In Vitro Colon Cancer Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopnin, B.P. Targets of oncogenes and tumor suppressors: Key for understanding basic mechanisms of carcinogenesis. Biochemistry 2000, 65, 2–27. [Google Scholar] [PubMed]
- American Cancer Society. Cancer Facts & Figures (2019); American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Ferla, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methodsand Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Arti, P. Genetic and environmental factors in cancer pathogenesis. Int. J. Res.-Granthaalayah 2015, 3, 2015. [Google Scholar]
- American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Copeland, G.; Lake, A.; Firth, R. Cancer in North America: 2006–2010. Volume One: Combined Cancer Incidence for the United States, Canada and North America; North American Association of Central Cancer Registries, Inc.: Springfield, IL, USA, 2013. [Google Scholar]
- Stewart, S.L.; Wike, J.M.; Kato, I.; Lewis, D.R.; Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer 2006, 107, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 2009, 16, 3–34. [Google Scholar]
- Baserga, R.; Hongo, A.; Rubini, M.; Prisco, M.; Valentinis, B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim. Biophys. Acta 1997, 1332, F105–F126. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C.; Liu, S.B.; Jung, M.C.; Engelhardt, D.; Baretton, G.B. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer 2002, 95, 2086–2095. [Google Scholar] [CrossRef]
- Guo, Y.; Narayan, S.; Yallampalli, C.; Singh, P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology 2020, 102, 1101–1108. [Google Scholar] [CrossRef]
- Remacle-Bonnet, M.M.; Garrouste, F.L.; Heller, S.; Andre, F.; Marvaldi, J.L.; Pommier, G.J. Insulin-like growth factor-I protects colon cancer cells from death factor-induced apoptosis by potentiating tumor necrosis factor alpha-induced mitogen-activated protein kinase and nuclear factor kappaB signaling pathways. Cancer Res. 2000, 60, 2007–2017. [Google Scholar]
- Zhang, L.; Zhou, W.; Velculescu, V.E.; Kern, S.E.; Hruban, R.H.; Hamilton, S.R.; Vogelstein, B.; Kinzler, K.W. Gene expression profiles in normal and cancer cells. Science 1997, 276, 1268–1272. [Google Scholar] [CrossRef]
- Ravikumar, S.; Kathiresan, K. Marine Pharmacology Published by School of Marine Sciences; Alagappa University: Karaikudi, India, 2010; p. 215. [Google Scholar]
- Kim, S.K. Marine Pharmacognosy: Trends and Applications; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Marchanan, F.L.; Chang, K.J.L.; Nichols, P.D.; Mitchell, W.J.; Polglase, J.L.; Gutierrez, T. Taxonomy, ecology and biotechnological applications of thraustochytrids: A Review. Biotechnol. Adv. 2017, 36, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.K.M.; Marshall, W.; Yokoyama, R.; Honda, D.; Lippmeier, J.C.; Craven, K.D.; Peterson, P.D.; Berbee, M.L. Labyrinthulomycetes phylogeny and its implication for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding. Mol. Phylogenet. Evol. 2009, 50, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kalidasan, K.; Sunil, K.S.; Kayalvizhi, K.; Kathiresan, K. Polyunsaturated fatty acid-producing marine thraustochytrids: A potential source for antimicrobials. J. Coast. Life Med. 2015, 3, 848–851. [Google Scholar]
- Kalidasan, K.; Asmathunisha, N.; Gomathi, V.; Dufossé, L.; Kathiresan, K. Isolation and optimization of culture conditions of Thraustochytrium kinnei for biomass production, nanoparticle synthesis, antioxidant and antimicrobial activities. J. Mar. Sci. Eng. 2021, 9, 678. [Google Scholar] [CrossRef]
- Kalidasan, K.; Vinithkumar, N.V.; Peter, D.M.; Dharani, G.; Dufossé, L. Thraustochytrids of mangrove habitats from Andaman Islands: Species diversity, PUFA profiles and biotechnological potential. Mar. Drugs 2021, 19, 571. [Google Scholar] [CrossRef]
- Visudtiphole, V.; Phromson, M.; Tala, S.; Bunphimpapha, P.; Raweeratanapong, T.; Sittikankaew, K.; Arayamethakorn, S.; Preedanon, S.; Jangsutthivorawat, W.; Chaiyapechara, S.; et al. Aurantiochytrium limacinum BCC52274 improves growth, hyposalinity tolerance and swimming strength of Penaeus vannamei post larvae. Aquaculture 2018, 495, 849–857. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, M.H.; Leong, Y.K.; Chang, J.S.; Lee, D.J. Biodiesel production from heterotrophic oleaginous microalga Thraustochytrium sp. BM2 with enhanced lipid accumulation using crude glycerol as alternative carbon source. Bioresour. Technol. 2020, 306, 123113. [Google Scholar] [CrossRef]
- Bongiorni, L.; Pusceddu, A.; Danovaro, R. Enzymatic activities of epiphytic and benthic thraustochytrids involved inorganic matter degradation. Aquat. Microb. Ecol. 2005, 41, 299–305. [Google Scholar] [CrossRef]
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-production of DHA and squalene by thraustochytrid from forest biomass. Sci. Rep. 2020, 10, 1992. [Google Scholar] [CrossRef]
- Kalidasan, K.; Sunil, K.S.; Narendran, R.; Kathiresan, K. Antioxidant activity of mangrove-derived marine thraustochytrids. Mycosphere 2015, 6, 602–611. [Google Scholar] [CrossRef]
- Xiao, R.; Xi, Y.; Mi, L.; Xiang, L.; Yanzhang, W.; Min, C.; Arthur, R.; Mark, T.; Junhuan, D.; Yi, Z. Investigation of Composition, Structure and Bioactivity of Extracellular Polymeric Substances from Original and Stress-induced Strains of Thraustochytrium striatum. Carbohydr. Polym. 2018, 195, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vega, A.; Rosales-Mendoza, S.; Bañuelos-Hernández, B.; Angulo, C. Prospects on the Use of Schizochytrium sp. to Develop Oral Vaccines. Front. Microbiol. 2018, 9, 2506. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Amoozyan, N.; Fekrat, F.; Maleki, M. Antigastric cancer bioactive Aurantiochytrium oil rich in Docosahexaenoic acid: From media optimization to cancer cells cytotoxicity assessment. J. Food Sci. 2017, 82, 2706–2718. [Google Scholar] [CrossRef]
- Atienza, G.A.M.V.; Arafiles, K.H.V.; Carmona, M.C.M.; Garcia, J.P.C.; Macabago, A.M.B.; Penacerrada, B.J.D.C.; Cordero, P.R.F.; Bennett, R.M.; Dedeles, G.R. Carotenoid analysis of locally isolated Thraustochytrids and their potential as an alternative fish feed for Oreochromis niloticus (Nile tilapia). Mycosphere 2012, 3, 420–428. [Google Scholar] [CrossRef]
- Schmitt, D.; Tran, N.; Peach, J.; Edwards, T.; Greeley, M. Toxicologic evaluations of DHA-rich algal oil in rats: Developmental toxicity study and 3-month dietary toxicity study with an in utero exposure phase. Food Chem. Toxicol. 2012, 50, 4149–4157. [Google Scholar] [CrossRef]
- Mo, C.; Rinkevich, B. A simple reliable and fast protocol for Thraustochytrid DNA extraction. Mar. Biotechnol. 2001, 3, 100–102. [Google Scholar] [CrossRef]
- Honda, D.; Yokochi, T.; Nakahara, T.; Raghukumar, S.; Nakagiri, A.; Schaumann, K.; Higashihara, T. Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J. Eukaryot. Microbiol. 1999, 46, 637–647. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting., position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, R.; Rastogi, S.; Vijayakumar, M.; Rawat, A.K.S.; Shirwaikar, A.; Mehrotra, S.; Pushpangadam, P. Studies on antioxidant activities of Desmodium gangetium. Biol. Pharm. Bull. 2003, 26, 1424–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcin, I.; Sat, I.G.; Beydemi, S.; Kufrevioglu, O.I. Evaluation of the in vitro antioxidant properties of extracts of Broccoli (Brassica oleraceal). Indian J. Food Sci. 2004, 16, 17–30. [Google Scholar]
- Badami, S.; Dongre, S.H.; Suresh, B. In vitro antioxidant properties of Solanum pseudocapsicum leaf extracts. Indian J. Pharmacol. 2005, 37, 251–252. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidant by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of the total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; Technical Note #101; Microbial ID: Newark, DE, USA, 1990; Available online: http://natasha.eng.usf.edu/gilbert/courses/Biotransport%20Phenomena/pdf/bacteria_gc_1.pdf (accessed on 2 January 2022).
- Tamilselvi, T.; Munish, P.; Jitraporn, V.; Colin, J.B. Evaluation of bread crumbs as a potential carbon for the growth of Thraustochytrid species for oil and Omega-3 production. Nutrients 2014, 6, 2104–2114. [Google Scholar]
- Kumaravel, S.; Praveen Kumar, P.; Vasuki, P. GC-MS Study on Microbial degradation of Lindane. Int. J. Appl. Chem. 2010, 6, 363–366. [Google Scholar]
- Lagorce, D.; Sperandio, O.; Baell, J.B.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs3: A web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015, 43, 200–207. [Google Scholar] [CrossRef]
- Singh, R.; Sahu, S.K.; Thangaraj, M. Polychaete fatty acids as potential inhibitor against human. Glioblastoma Multiforme 2013, 4, 1519–1524. [Google Scholar]
- Reisine, T.; Pasternak, G.W. Opioid Analgesics and Antagonists., in Goodman and Gilman’s: The Pharmacological Basis of Therapeutics; Hardman, J.G., Limbird, L.E., Eds.; McGraw-Hill: New York, NY, USA, 1996; pp. 521–556. [Google Scholar]
- Mossman, B.; Light, W.; Wei, E. Asbestos: Mechanisms of Toxicity and Carcinogenicity in the Respiratory Tract. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Alan, G.; Porter Reiner, U.; Jae, N. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar]
- Narayani, S.S.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line HCT-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar]
- Porter, D. Labyrinthulomycota. In Handbook of Protoctista; Margulis, L., Corliss, J.O., Melkonian, M., Chapman, D., Eds.; Jones & Bartlett: Boston, MA, USA, 1990; pp. 388–398. [Google Scholar]
- Dick, M.W. Straminipilous Fungi: Systematics of the Peronosoromycetes including Accounts of the Marine Straminipilous Protists, the Plasmodiophorids and Similar Organisms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Yokoyama, R.; Salleh, B.; Honda, D. Taxonomic rearrangement of the genus Ulkenia sensulato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae., Labyrinthulomycetes): Emendation for Ulkenia and erection of Botryochytrium, Parietichytrium, Sicyoidochytrium gen. nov. Mycoscience 2007, 48, 329–341. [Google Scholar]
- Yokoyama, R.; Honda, D. Taxonomic rearrangement of the genus Schizochytrium sensulato based on morphology, chemotaxonomical characteristics and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes, stramenopiles): Emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 2007, 48, 199–211. [Google Scholar]
- Doi, K.; Honda, D. Proposal of Monorhizochytrium globosum gen. nov., comb. nov. (Stramenopiles., Labyrinthulomycetes) for former Thraustochytrium globosum based on morphological features and phylogenetic relationships. Phycol. Res. 2017, 65, 188–201. [Google Scholar] [CrossRef]
- Leander, C.L.; Porter, D.; Leander, B.S. Comparative morphology and molecular phylogeny of aplanochytrids (Labyrinthulomycota). Eur. J. Protistol. 2004, 40, 317–328. [Google Scholar] [CrossRef]
- Leander, C.; Porter, D. The Labyrinthulomycota is comprised of three distinct lineages. Mycologia 2001, 93, 459–464. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Cote, S.; Carmichael, P.H.; Verreault, R.; Lindsay, J.; Lefebvre, J.; Laurin, D. Non-steroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 219–226. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. Med. Chem. Commun. 2018, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P. n-3 Polyunsaturated fatty acids and cancer. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 121–126. [Google Scholar] [CrossRef]
- Rose, D.P.; Connolly, J.M.; Coleman, M. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin. Cancer Res. 1996, 2, 1751–1756. [Google Scholar]
- Madhavi, N.; Das, U.N. Effect of n-6 and n-3 fatty acids on the survival of vincristine sensitive and resistant human cervical carcinoma cells in vitro. Cancer Lett. 1994, 84, 31–41. [Google Scholar] [CrossRef]
- Farazi, T.; Leichman, J.; Harris, T.; Cahoon, M.; Hedstrom, L. Isolation and characterization of mycophenolic acid-resistant mutants of inosine-50-monophosphate dehydrogenase. J. Biol. Chem. 1997, 272, 961–965. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, O.A.; Wagner, G.K.; Hinkel, P.; Krebs, M.; Stumm, B.; Schmalenberger, S.; Bohm, S.B.; Majewski, F. Fish studies in 45 patients with Rubinstein-Taybisyndrome:deletions associated with polysplenia., hypoplastic left heart and death in infancy. Eur. J. Hum. Genet. 1999, 7, 748–756. [Google Scholar] [CrossRef]
- Shultz, T.D.; Chewa, B.P.; Seamana, W.R.; Luedeckea, L.O. Inhibitory effect of conjugated dienoic derivatives of linoleic acid and β-carotene on the in vitro growth of human cancer cells. Cancer Lett. 1992, 63, 125–133. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [Green Version]
- Tapieroa, H.; Nguyen, B.G.; Couvreura, P.; Tewb, K.D. Polyunsaturated fatty acids (PUFAs) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]















| Compound S. No. | Carbon Atom of Fatty Acid | Name of the Fatty Acid | Content (%) |
|---|---|---|---|
| Comp 1 | 14:00 | Methyl tetradecanoate acid | 1.31 ± 0.18 |
| Comp 2 | 14:01 | Myristoleic acid | 7.04 ± 1.21 |
| Comp 3 | 15:00 | Pentadecanoic acid | 1.15 ± 0.13 |
| Comp 4 | 16:00 | Palmitic acid | 37.32 ± 2.34 |
| Comp 5 | 16:01 | Palmitoleic acid | 0.94 ± 0.11 |
| Comp 6 | 17:00 | Heptadecanoic acid | 0.67 ± 0.14 |
| Comp 7 | 18:00 | Stearic acid | 4.9 ± 0.72 |
| Comp 8 | 18:01 | Oleic acid | 0.58 ± 0.11 |
| Comp 9 | 18:2 n-6 | Linolenic acid | 0.41 ± 0.13 |
| Comp 10 | 18:3 n-3 | α-Linolenic acid | 0.33 ± 0.08 |
| Comp 11 | 18:3 n-6 | γ-Linolenic acid | 0.29 ± 0.10 |
| Comp 12 | 18:04 | Stearidonic acid | 0.27 ± 0.07 |
| Comp 13 | 20:00 | Eicosanoic acid | 0.38 ± 0.12 |
| Comp 14 | 20:3 n-6 | Dihomo-γ-linolenic acid | 0.33 ± 0.14 |
| Comp 15 | 20:4 n-3 | Eicosatetraenoic acid | 1.67 ± 0.25 |
| Comp 16 | 20:4 n-6 | Arachidonic acid | 2.37 ± 0.57 |
| Comp 17 | 20:5 n-3 (EPA) | Eicosapentaenoic acid | 6.76 ± 1.08 |
| Comp 18 | 22:5 n-6 (DPA) | Docosapentaenoic acid | 1.22 ± 0.27 |
| Comp 19 | 22:6 n-3 (DHA) | Docosahexaenoic acid | 33.18 ± 2.16 |
| S. No. | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Hydrogen Donor and Acceptor | Docking Score Kcal/mol |
|---|---|---|---|---|---|
| Comp 10 | Alpha-linolenic acid | C18H30O2 | 278.429 | 1, 2 | −9.2 |
| Comp 11 | Gamma-linolenic acid | C18H30O2 | 278.429 | 1, 2 | −9.0 |
| Comp 16 | Arachidonic acid | C20H32O2 | 304.466 | 1, 2 | −8.0 |
| Comp 17 | Eicosapentaenoic acid | C20H30O2 | 302.451 | 1, 2 | −9.7 |
| Comp 19 | Docosahexaenoic acid | C22H32O2 | 328.488 | 1, 2 | −10.8 |
| S. No. | Compound | MW | logP | logSw | HB Donors | HB Acceptors | Lipinski Violations | Solubility (mg/L) | Solubility | Oral Bio-Availability | Phospo Lipidosis | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp 10 | AA | 304.47 | 6.98 | −5.2 | 1 | 2 | 1 | 1677.77 | Good Solubility | Good | Non-Inducer | Accepted |
| Comp 11 | ALA | 278.43 | 6.46 | −4.78 | 1 | 2 | 1 | 2342.23 | Good Solubility | Good | Non-Inducer | Accepted |
| Comp 16 | GLA | 306.48 | 7.35 | −5.38 | 1 | 2 | 1 | 1411.24 | Good Solubility | Good | Non-Inducer | Accepted |
| Comp 17 | EPA | 302.45 | 6.29 | −4.82 | 1 | 2 | 1 | 2440.06 | Good Solubility | Good | Non-Inducer | Accepted |
| Comp 19 | DHA | 328.49 | 6.19 | −4.85 | 1 | 2 | 1 | 2565.55 | Good Solubility | Good | Non-Inducer | Accepted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalidasan, K.; Dufossé, L.; Manivel, G.; Senthilraja, P.; Kathiresan, K. Antioxidant and Anti-Colorectal Cancer Properties in Methanolic Extract of Mangrove-Derived Schizochytrium sp. J. Mar. Sci. Eng. 2022, 10, 431. https://doi.org/10.3390/jmse10030431
Kalidasan K, Dufossé L, Manivel G, Senthilraja P, Kathiresan K. Antioxidant and Anti-Colorectal Cancer Properties in Methanolic Extract of Mangrove-Derived Schizochytrium sp. Journal of Marine Science and Engineering. 2022; 10(3):431. https://doi.org/10.3390/jmse10030431
Chicago/Turabian StyleKalidasan, Kaliyamoorthy, Laurent Dufossé, Gunasekaran Manivel, Poomalai Senthilraja, and Kandasamy Kathiresan. 2022. "Antioxidant and Anti-Colorectal Cancer Properties in Methanolic Extract of Mangrove-Derived Schizochytrium sp." Journal of Marine Science and Engineering 10, no. 3: 431. https://doi.org/10.3390/jmse10030431







