Effects of Seawater Acidification on Echinoid Adult Stage: A Review
Abstract
:1. Introduction
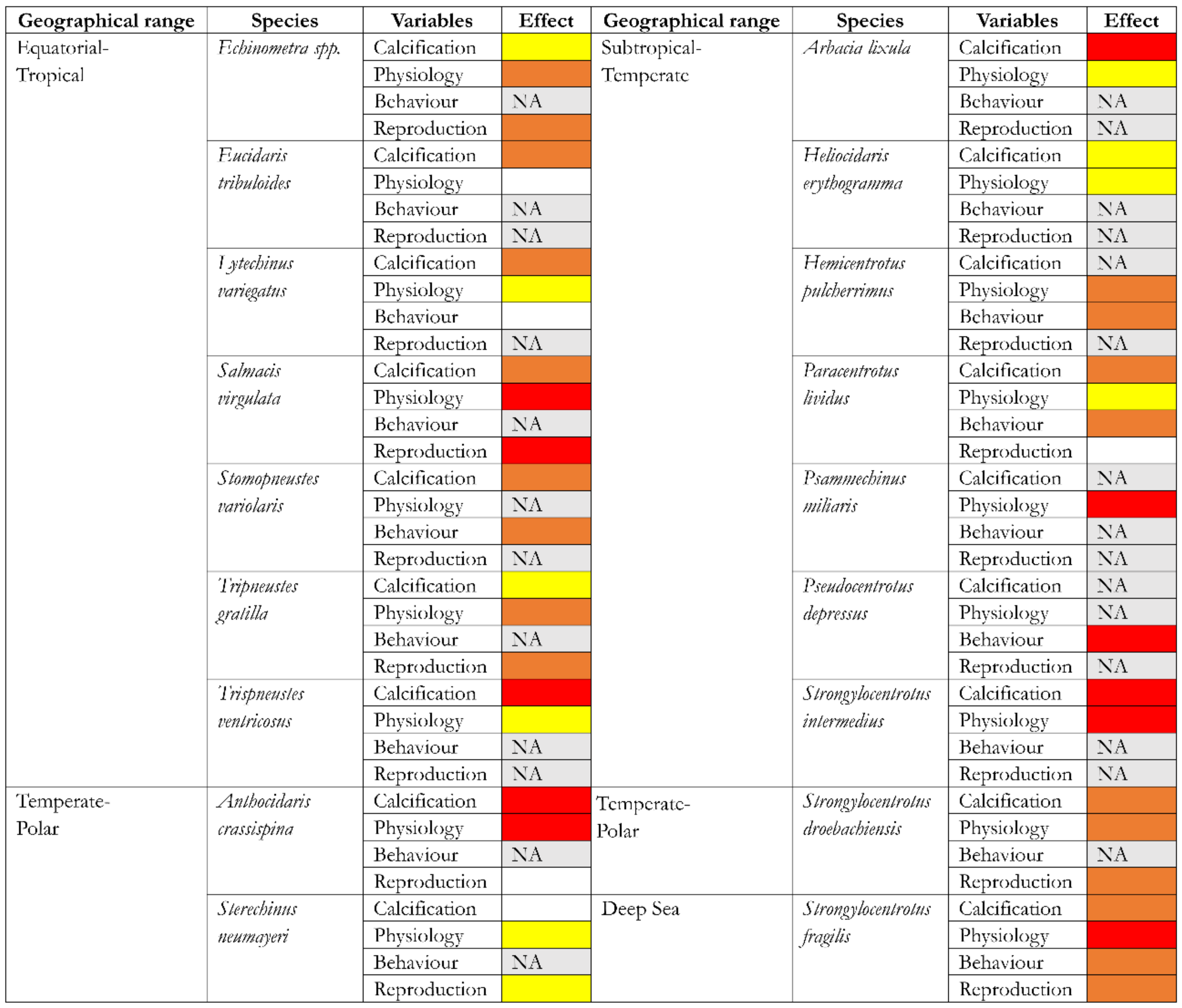
2. SWA Effects on Echinoid Calcification
| N | Response Detail | Species | Latitudinal Range | pH Level | ΔpH | Exposure Time (Days) | ΔpH Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| 1 | Growth | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.8 | 0.3 | 14 | = | [34] |
| 2 | Growth | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.6–7.4 | 0.5–0.7 | 14 | = | [34] |
| 3 | Growth | Echinometra mathaei | Eq.-Trop. | 8.04–7.69 | 0.35 | 49 | = | [35] |
| 4 | Growth | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.82 | 0.28 | 60 | Altered | [36] |
| 5 | Growth | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.68–7.55 | 0.42–0.55 | 60 | Altered | [36] |
| 6 | Growth | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | = | [37] |
| 7 | Growth | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | Slower | [37] |
| 8 | Growth | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | = | [38] |
| 9 | Growth | Strongylocentrotus fragilis | Deep Sea | 7.92–7.64 | 0.28 | 140 | = | [39] |
| 10 | Growth | Strongylocentrotus fragilis | Deep Sea | 7.92–7.23–6.61 | 0.69–1.31 | 140 | Altered | [39] |
| 11 | Growth | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | = | [40] |
| 12 | Growth | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | altered | [40] |
| 13 | Growth | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [41] |
| 14 | Growth | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | Decreased | [41] |
| 15 | Growth | Echinometra mathaei | Eq.-Trop. | 8.09–7.63 | 0.46 | 360 | = | [42] |
| 16 | Growth | Echinometra sp. | Eq.-Trop. | 8.06–7.89–7.79 | 0.17–0.27 | 600 | = | [43] |
| 17 | Growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 18 | Growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | = | [44] |
| 19 | Growth | Echinometra sp. | Eq.-Trop. | 8.0–7.48 | 0.52 | Resident | = | [45] |
| 20 | Growth | Echinometra sp. | Eq.-Trop. | 8.0–7.48 | 0.52 | Resident | Increase | [45] |
| 21 | Growth (jaw size) | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | Decreased | [37] |
| 22 | Growth (jaw size) | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | Decreased | [37] |
| 23 | Growth (ossicles) | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.66 | 0.34 | 42 | Reduced | [46] |
| 24 | Growth (ossicles) | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.19 | 0.81 | 42 | Reduced | [46] |
| 25 | Mineral ionic content | Paracentrotus lividus | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | Altered | [47] |
| 26 | Mineral ionic content | Arbacia lixula | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | Altered | [47] |
| 27 | Mineral ionic content | Paracentrotus lividus | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | Altered | [47] |
| 28 | Mineral ionic content | Arbacia lixula | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | = | [47] |
| 29 | Porosity | Heliocidaris erythogramma | Sub.-Temp. | 8.01–7.6 | 0.39 | 210 | = | [48] |
| 30 | Skeletal degradation | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.68 | 0.37 | 45 | Higher (Spines) | [49] |
| 31 | Skeletal degradation | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.39 | 0.66 | 45 | Higher (Spines) | [49] |
| 32 | Skeletal degradation | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.65 | 0.39 | 45 | Higher (Spines) | [49] |
| 33 | Skeletal degradation | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.41 | 0.63 | 45 | Higher (Spines) | [49] |
| 34 | Skeletal degradation | Paracentrotus lividus | Sub.-Temp. | 8.11–7.75–7.50–7.48 | 0.36–0.61–0.63 | Resident | Higher | [50] |
| 35 | Skeletal degradation | Arbacia lixula | Sub.-Temp. | 8.11–7.75–7.50–7.48 | 0.36–0.61–0.63 | Resident | Higher | [50] |
| 36 | Skeletal integrity | Echinometra sp. | Eq.-Trop. | 8.1–7.7 | 0.4 | 330 | = | [51] |
| 37 | Skeletal integrity | Paracentrotus lividus | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 38 | Skeletal integrity | Arbacia lixula | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | Lowered | [52] |
| 39 | Skeletal mechanical properties | Paracentrotus lividus | Sub.-Temp. | 8.0–7.8–7.7 | 0.2–0.3 | 30 | = | [53] |
| 40 | Skeletal mechanical properties | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.66 | 0.34 | 42 | = | [46] |
| 41 | Skeletal mechanical properties | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.19 | 0.81 | 42 | Lower force and high dissolution | [46] |
| 42 | Skeletal mechanical properties | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [54] |
| 43 | Skeletal mechanical properties | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | Decreased | [54] |
| 44 | Skeletal mechanical properties | Heliocidaris erythogramma | Sub.-Temp. | 8.01–7.6 | 0.39 | 210 | = | [48] |
| 45 | Skeletal mechanical properties | Echinometra mathaei | Eq.-Trop. | 8.09–7.63 | 0.46 | 360 | = | [42] |
| 46 | Skeletal mechanical properties | Paracentrotus lividus | Sub.-Temp. | 8.0–7.9–7.8 | 0.1–0.2 | 360 | = | [55] |
| 47 | Skeletal mechanical properties | Paracentrotus lividus | Sub.-Temp. | 8.02–7.865–7.65 | 0.155–0.37 | Resident | = | [55] |
| 48 | Skeletal mechanical properties | Paracentrotus lividus | Sub.-Temp. | 8.1–7.8 | 0.3 | Resident | = | [55] |
| 49 | Skeletal mechanical properties | Paracentrotus lividus | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 50 | Skeletal mechanical properties | Arbacia lixula | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | Altered | [52] |
| 51 | Skeletal mechanical properties | Sterechinus neumayeri | Temp.-Pol. | 8.1–7.8 | 0.3 | Resident | = | [56] |
| 52 | Skeletal mineralogy | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.66 | 0.34 | 42 | = | [46] |
| 53 | Skeletal mineralogy | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.00–7.19 | 0.81 | 42 | Dissolution | [46] |
| 54 | Skeletal mineralogy | Echinometra sp. | Eq.-Trop. | 8.1–7.9 | 0.2 | 70 | = | [57] |
| 55 | Skeletal mineralogy | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [54] |
| 56 | Skeletal mineralogy | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | = | [54] |
| 57 | Skeletal mineralogy | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | = | [58] |
| 58 | Skeletal mineralogy (Aristotle’s lantern) | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | Thinner | [40] |
| 59 | Skeletal mineralogy (Aristotle’s lantern) | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | Thinner | [40] |
| 60 | Skeletal mineralogy (FTIR-TGA) | Salmacis virgulata | Eq.-Trop. | 8.26–8.00 | 0.26 | 14 | = | [59] |
| 61 | Skeletal mineralogy (FTIR-TGA) | Salmacis virgulata | Eq.-Trop. | 8.26–7.81–7.63 | 0.45–0.63 | 14 | Loss of weight, less calcite | [59] |
| 62 | Skeletal mineralogy (gene expr.) | Lytechinus variegatus | Eq.-Trop. | 7.93–7.7 | 0.23 | 56 | = | [60] |
| 63 | Skeletal mineralogy (gene expr.) | Lytechinus variegatus | Eq.-Trop. | 7.93–7.47 | 0.46 | 56 | Upregulated | [60] |
| 64 | Skeletal mineralogy (gene expr.) | Paracentrotus lividus | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 65 | Skeletal mineralogy (gene expr.) | Arbacia lixula | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | Altered | [52] |
| 66 | Skeletal mineralogy (Mg, Sr, Ca) | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.76 | 0.2 | 210 | = | [61] |
| 67 | Skeletal mineralogy (Mg, Sr, Ca) | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.46 | 0.5 | 210 | Altered | [61] |
| 68 | Skeletal mineralogy (Mn, Sr, Zn) | Paracentrotus lividus | Sub.-Temp. | 8.11–7.75–7.50–7.48 | 0.36–0.61–0.63 | Resident | Altered | [50] |
| 69 | Skeletal mineralogy (Mn, Sr, Zn) | Arbacia lixula | Sub.-Temp. | 8.11–7.75–7.50–7.48 | 0.36–0.61–0.63 | Resident | Altered | [50] |
| 70 | Spine development | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.8 | 0.3 | 14 | = | [34] |
| 71 | Spine development | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.6–7.4 | 0.5–0.7 | 14 | Impaired | [34] |
| 72 | Spine development | Lytechinus variegatus | Eq.-Trop. | 7.93–7.7 | 0.23 | 56 | = | [60] |
| 73 | Spine development | Lytechinus variegatus | Eq.-Trop. | 7.93–7.47 | 0.46 | 56 | Impaired | [60] |
| 74 | Spine length | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | Decreased | [38] |
| 75 | Spine mechanical strength | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.68 | 0.37 | 45 | = | [49] |
| 76 | Spine mechanical strength | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.39 | 0.66 | 45 | = | [49] |
| 77 | Spine mechanical strength | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.65 | 0.39 | 45 | = | [49] |
| 78 | Spine mechanical strength | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.41 | 0.63 | 45 | Decreased | [49] |
| 79 | Spine tips integrity | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.8 | 0.3 | 14 | = | [34] |
| 80 | Spine tips integrity | Heliocidaris erythrogramma | Sub.-Temp. | 8.1–7.6–7.4 | 0.5–0.7 | 14 | Dissolved (7,4) | [34] |
| 81 | Spines integrity | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.76 | 0.2 | 210 | = | [61] |
| 82 | Spines integrity | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.46 | 0.5 | 210 | Reduced | [61] |
| 83 | Test robustness | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | = | [38] |
| 84 | Test thickness | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | Thinner | [38] |
| 85 | Test thickness | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [54] |
| 86 | Test thickness | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | Decreased | [54] |
3. SWA Effects on Echinoid Physiology
| N | Response Detail | Species | Latitudinal Range | pH Level | ΔpH | Exposure Time (Days) | Specific Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| 1 | Absorption efficiency | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 2 | Absorption efficiency | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | = | [44] |
| 3 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | Altered | [47] |
| 4 | Acid–base regulation | Arbacia lixula | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | = | [47] |
| 5 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | Altered | [47] |
| 6 | Acid–base regulation | Arbacia lixula | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | Altered | [47] |
| 7 | Acid–base regulation | Arbacia lixula | Sub.-Temp. | 8.04–7.72 | 0.32 | 4 | = | [76] |
| 8 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 8.04–7.72 | 0.32 | 4 | Altered | [76] |
| 9 | Acid–base regulation | Psammechinus miliaris | Sub.-Temp. | 7.96–7.44–6.63–6.16 | 0.52–1.33–1.8 | 7 | Altered | [29] |
| 10 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 8.14–7.68 | 0.46 | 14 | Altered | [77] |
| 11 | Acid–base regulation | Strongylocentrotus fragilis | Deep Sea | 7.98–7.51–7.11–6.65 | 0.47–0.87–1.33 | 31 | Altered | [39] |
| 12 | Acid–base regulation | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.68 | 0.37 | 35 | = | [78] |
| 13 | Acid–base regulation | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.39 | 0.66 | 35 | = | [78] |
| 14 | Acid–base regulation | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.65 | 0.39 | 35 | = | [78] |
| 15 | Acid–base regulation | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.41 | 0.63 | 35 | Altered | [78] |
| 16 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 7.98–7.66 | 0.22 | 35 | Altered | [78] |
| 17 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 7.98–7.47 | 0.51 | 35 | Altered | [78] |
| 18 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | = | [37] |
| 19 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | = | [37] |
| 20 | Acid–base regulation | Echinometra mathaei | Eq.-Trop. | 8.09–7.63 | 0.46 | 360 | Higher | [42] |
| 21 | Acid–base regulation | Paracentrotus lividus | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 22 | Acid–base regulation | Arbacia lixula | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 23 | Ammonia excretion rate | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.01–7.60–7.16 | 0.41–0.85 | 45 | Higher | [75] |
| 24 | Ammonia excretion rate | Paracentrotus lividus | Sub.-Temp. | 8.0–7.7 | 0.3 | 60 | = | [79] |
| 25 | Ammonia excretion rate | Paracentrotus lividus | Sub.-Temp. | 8.0–7.4 | 0.6 | 60 | = | [79] |
| 26 | Ammonia excretion rate | Echinometra sp. | Eq.-Trop. | 8.1–7.9 | 0.2 | 70 | = | [57] |
| 27 | Ammonia excretion rate | Heliocidaris erythogramma | Sub.-Temp. | 8.0–7.6 | 0.4 | 84 | = | [80] |
| 28 | Ammonia excretion rate | Paracentrotus lividus site 1 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 29 | Ammonia excretion rate | Paracentrotus lividus site 2 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | Altered | [25] |
| 30 | Ammonia excretion rate | Echinometra sp. | Eq.-Trop. | 8.06–7.89–7.79 | 0.17–0.27 | 600 | = | [43] |
| 31 | Ammonia excretion rate | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 32 | Ammonia excretion rate | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | = | [44] |
| 33 | Ammonia excretion rate | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | = | [58] |
| 34 | Antioxidant capacity | Paracentrotus lividus | Sub.-Temp. | 8.14–7.68 | 0.46 | 14 | = | [77] |
| 35 | Antioxidant capacity | Salmacis virgulata | Eq.-Trop. | 8.26–8.00 | 0.26 | 14 | Oxidative stress induction | [59] |
| 36 | Antioxidant capacity | Salmacis virgulata | Eq.-Trop. | 8.26–7.81–7.63 | 0.45–0.63 | 14 | Oxidative stress induction | [59] |
| 37 | Antioxidant capacity | Paracentrotus lividus | Sub.-Temp. | 8.0–7.7 | 0.3 | 60 | = | [79] |
| 38 | Antioxidant capacity | Paracentrotus lividus | Sub.-Temp. | 8.0–7.4 | 0.6 | 60 | Slight induction | [79] |
| 39 | Antioxidant capacity | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | Higher | [58] |
| 40 | Assimilation efficiency | Paracentrotus lividus | Sub.-Temp. | 8.0–7.7 | 0.3 | 60 | = | [79] |
| 41 | Assimilation efficiency | Paracentrotus lividus | Sub.-Temp. | 8.0–7.4 | 0.6 | 60 | = | [79] |
| 42 | Coelomic fluid ions concentration | Strongylocentrotus droebachiensis | Temp.-Pol. | 7.89–7.44–7.16–6.78 | 0.45–0.73–1.11 | 5 | Mostly = | [81] |
| 43 | Coelomic fluid ions concentration | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | Altered | [40] |
| 44 | Coelomic fluid ions concentration | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | Altered | [40] |
| 45 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | Altered | [47] |
| 46 | Coelomic fluid pH | Arbacia lixula | Sub.-Temp. | 8.06–7.69 | 0.37 | 1 | = | [47] |
| 47 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | = | [47] |
| 48 | Coelomic fluid pH | Arbacia lixula | Sub.-Temp. | 8.05–7.73 | 0.32 | 4 | = | [47] |
| 49 | Coelomic fluid pH | Arbacia lixula | Sub.-Temp. | 8.04–7.72 | 0.32 | 4 | = | [76] |
| 50 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.04–7.72 | 0.32 | 4 | Altered | [76] |
| 51 | Coelomic fluid pH | Strongylocentrotus droebachiensis | Temp.-Pol. | 7.89–7.44–7.16–6.78 | 0.45–0.73–1.11 | 5 | Decreased | [81] |
| 52 | Coelomic fluid pH | Lytechinus variegatus | Eq.-Trop. | 8.0–7.6 | 0.4 | 5 | Decreased | [82] |
| 53 | Coelomic fluid pH | Lytechinus variegatus | Eq.-Trop. | 8.0–7.3 | 0.7 | 5 | Decreased | [82] |
| 54 | Coelomic fluid pH | Echinometra lucunter | Eq.-Trop. | 8.0–7.6 | 0.4 | 5 | Decreased | [82] |
| 55 | Coelomic fluid pH | Echinometra lucunter | Eq.-Trop. | 8.0–7.3 | 0.7 | 5 | Decreased | [82] |
| 56 | Coelomic fluid pH | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.2–7.7 | 0.5 | 7 | = | [83] |
| 57 | Coelomic fluid pH | Psammechinus miliaris | Sub.-Temp. | 7.96–7.44–6.63–6.16 | 0.52–1.33–1.8 | 7 | Altered | [29] |
| 58 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.14–7.68 | 0.46 | 14 | = | [77] |
| 59 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.1–7.7 | 0.4 | 19 | Decreased | [74] |
| 60 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.1–7.4 | 0.7 | 19 | Decreased | [74] |
| 61 | Coelomic fluid pH | Strongylocentrotus fragilis | Deep Sea | 7.98–7.51–7.11–6.65 | 0.47–0.87–1.33 | 31 | Altered | [39] |
| 62 | Coelomic fluid pH | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.68 | 0.37 | 35 | = | [78] |
| 63 | Coelomic fluid pH | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.39 | 0.66 | 35 | = | [78] |
| 64 | Coelomic fluid pH | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.65 | 0.39 | 35 | = | [78] |
| 65 | Coelomic fluid pH | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.41 | 0.63 | 35 | = | [78] |
| 66 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 7.98–7.66 | 0.22 | 35 | = | [78] |
| 67 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 7.98–7.47 | 0.51 | 35 | = | [78] |
| 68 | Coelomic fluid pH | Echinometra mathaei | Eq.-Trop. | 8.04–7.69 | 0.35 | 49 | = | [35] |
| 69 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | = | [37] |
| 70 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | = | [37] |
| 71 | Coelomic fluid pH | Echinometra sp. | Eq.-Trop. | 8.1–7.9 | 0.2 | 70 | = | [57] |
| 72 | Coelomic fluid pH | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [41] |
| 73 | Coelomic fluid pH | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | Decreased | [41] |
| 74 | Coelomic fluid pH | Hemicentrotus pulcherrimus | Sub.-Temp. | 8.1–7.83 | 0.27 | 270 | Decreased | [84] |
| 75 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 76 | Coelomic fluid pH | Arbacia lixula | Sub.-Temp. | 7.93–7.63 | 0.3 | Resident | = | [52] |
| 77 | Coelomic fluid pH | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | = | [58] |
| 78 | Energy consumed | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | Decreased | [44] |
| 79 | Energy consumed | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | Decreased | [44] |
| 80 | Faecal production | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | Decreased | [40] |
| 81 | Faecal production | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | Decreased | [40] |
| 82 | Food intake | Echinometra lucunter | Eq.-Trop. | 8.23–7.75 | 0.48 | 21 | Increased | [85] |
| 83 | Food intake | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.68 | 0.37 | 35 | = | [78] |
| 84 | Food intake | Eucidaris tribuloides | Eq.-Trop. | 8.05–7.39 | 0.66 | 35 | = | [78] |
| 85 | Food intake | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.65 | 0.39 | 35 | = | [78] |
| 86 | Food intake | Tripneustes ventricosus | Eq.-Trop. | 8.04–7.41 | 0.63 | 35 | = | [78] |
| 87 | Food intake | Paracentrotus lividus | Sub.-Temp. | 7.98–7.66 | 0.22 | 35 | = | [78] |
| 88 | Food intake | Paracentrotus lividus | Sub.-Temp. | 7.98–7.47 | 0.51 | 35 | = | [78] |
| 89 | Food intake | Lytechinus variegatus | Eq.-Trop. | 7.87–7.57 | 0.3 | 42 | = | [86] |
| 90 | Food intake | Lytechinus variegatus | Eq.-Trop. | 7.97–7.60 | 0.37 | 42 | = | [86] |
| 91 | Food intake | Heliocidaris erythrogramma | Sub.-Temp. | 8.0–7.5 | 0.5 | 60 | = | [72] |
| 92 | Food intake | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.82 | 0.28 | 60 | = | [36] |
| 93 | Food intake | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.68–7.55 | 0.42–0.55 | 60 | Decreased | [36] |
| 94 | Food intake | Heliocidaris erythogramma | Sub.-Temp. | 8.0–7.6 | 0.4 | 84 | = | [80] |
| 95 | Food intake | Strongylocentrotus fragilis | Deep Sea | 7.92–7.64 | 0.28 | 140 | = | [39] |
| 96 | Food intake | Strongylocentrotus fragilis | Deep Sea | 7.92–7.23–6.61 | 0.69–1.31 | 140 | Decreased | [39] |
| 97 | Food intake | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | Decreased | [40] |
| 98 | Food intake | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | Decreased | [40] |
| 99 | Food intake | Hemicentrotus pulcherrimus | Sub.-Temp. | 8.1–7.83 | 0.27 | 270 | Rate reduced | [84] |
| 100 | Immune system | Lytechinus variegatus | Eq.-Trop. | 8.0–7.6 | 0.4 | 5 | Altered | [82] |
| 101 | Immune system | Lytechinus variegatus | Eq.-Trop. | 8.0–7.3 | 0.7 | 5 | Altered | [82] |
| 102 | Immune system | Echinometra lucunter | Eq.-Trop. | 8.0–7.6 | 0.4 | 5 | Altered | [82] |
| 103 | Immune system | Echinometra lucunter | Eq.-Trop. | 8.0–7.3 | 0.7 | 5 | Altered | [82] |
| 104 | Immune system | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.2–7.7 | 0.5 | 7 | = | [83] |
| 105 | Immune system | Lytechinus variegatus | Eq.-Trop. | 7.93–7.7 | 0.23 | 56 | = | [60] |
| 106 | Immune system | Lytechinus variegatus | Eq.-Trop. | 7.93–7.47 | 0.46 | 56 | = | [60] |
| 107 | Immune system | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | Upregulated | [58] |
| 108 | Lactate concentration | Strongylocentrotus droebachiensis | Temp.-Pol. | 7.89–7.44–7.16–6.78 | 0.45–0.73–1.11 | 5 | Increased | [81] |
| 109 | Metabolism enzyme activity | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.82 | 0.28 | 60 | Altered | [36] |
| 110 | Metabolism enzyme activity | Strongylocentrotus intermedius | Sub.-Temp. | 8.10–7.68–7.55 | 0.42–0.55 | 60 | Altered | [36] |
| 111 | Metabolism enzyme activity | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | Upregulated | [58] |
| 112 | Oxidative damage | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | = | [58] |
| 113 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 8.1–7.7 | 0.4 | 19 | = | [74] |
| 114 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 8.1–7.4 | 0.7 | 19 | Lower | [74] |
| 115 | Respiration rate | Echinometra lucunter | Eq.-Trop. | 8.23–7.75 | 0.48 | 21 | Higher | [85] |
| 116 | Respiration rate | Lytechinus variegatus | Eq.-Trop. | 7.87–7.57 | 0.3 | 42 | = | [86] |
| 117 | Respiration rate | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.01–7.60–7.16 | 0.41–0.85 | 45 | = | [75] |
| 118 | Respiration rate | Echinometra mathaei | Eq.-Trop. | 8.04–7.69 | 0.35 | 49 | = | [35] |
| 119 | Respiration rate | Heliocidaris erythrogramma | Sub.-Temp. | 8.0–7.5 | 0.5 | 60 | Increased | [72] |
| 120 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | = | [37] |
| 121 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | = | [37] |
| 122 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 8.0–7.7 | 0.3 | 60 | = | [79] |
| 123 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 8.0–7.4 | 0.6 | 60 | = | [79] |
| 124 | Respiration rate | Echinometra sp. | Eq.-Trop. | 8.1–7.9 | 0.2 | 70 | = | [57] |
| 125 | Respiration rate | Heliocidaris erythogramma | Sub.-Temp. | 8.0–7.6 | 0.4 | 84 | Increased | [80] |
| 126 | Respiration rate | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | = | [40] |
| 127 | Respiration rate | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | Decreased | [40] |
| 128 | Respiration rate | Paracentrotus lividus site 1 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 129 | Respiration rate | Paracentrotus lividus site 2 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 130 | Respiration rate | Hemicentrotus pulcherrimus | Sub.-Temp. | 8.1–7.83 | 0.27 | 270 | = | [84] |
| 131 | Respiration rate | Echinometra mathaei | Eq.-Trop. | 8.09–7.63 | 0.46 | 360 | = | [42] |
| 132 | Respiration rate | Echinometra sp. | Eq.-Trop. | 8.06–7.89–7.79 | 0.17–0.27 | 600 | = | [43] |
| 133 | Respiration rate | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 134 | Respiration rate | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | Increased | [44] |
| 135 | Respiration rate | Paracentrotus lividus | Sub.-Temp. | 8.02–7.8 | 0.18 | Resident | = | [58] |
| 136 | Respiration rate | Echinometra sp. | Eq.-Trop. | 8.0–7.48 | 0.52 | Resident | = | [45] |
| 137 | Scope for growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 138 | Scope for growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | = | [44] |
| 139 | Weight gain | Lytechinus variegatus | Eq.-Trop. | 7.97–7.60 | 0.37 | 42 | = | [86] |
| 140 | Weight gain | Lytechinus variegatus | Eq.-Trop. | 7.93–7.7 | 0.23 | 56 | = | [60] |
| 141 | Weight gain | Lytechinus variegatus | Eq.-Trop. | 7.93–7.47 | 0.46 | 56 | = | [60] |
4. SWA Effects on Echinoid Behaviour
| N | Response Detail | Species | Latitudinal Range | Ph Level | ΔpH | Exposure Time (Days) | Specific Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| 1 | Attachment | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | = | [38] |
| 2 | Feeding | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.76 | 0.2 | 210 | = | [61] |
| 3 | Feeding | Stomopneustes variolaris | Eq.-Trop. | 7.96–7.46 | 0.5 | 210 | Altered | [61] |
| 4 | Foraging time | Strongylocentrotus fragilis | Deep Sea | 7.6–7.1 | 0.5 | 27 | Longer | [102] |
| 5 | Predator-avoidance | Paracentrotus lividus | Sub.-Temp. | 8.04–7.78 | 0.26 | 90 | Lowered | [38] |
| 6 | Resistance to flow | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | Decreased | [37] |
| 7 | Resistance to flow | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | Decreased | [37] |
| 8 | Righting response | Lytechinus variegatus | Eq.-Trop. | 7.97–7.60 | 0.37 | 42 | = | [86] |
| 9 | Righting response | Lytechinus variegatus | Eq.-Trop. | 7.93–7.7 | 0.23 | 56 | = | [60] |
| 10 | Righting response | Lytechinus variegatus | Eq.-Trop. | 7.93–7.47 | 0.46 | 56 | = | [60] |
| 11 | Righting response | Paracentrotus lividus | Sub.-Temp. | 8.0–7.7 | 0.3 | 60 | = | [79] |
| 12 | Righting response | Paracentrotus lividus | Sub.-Temp. | 8.0–7.4 | 0.6 | 60 | = | [79] |
| 13 | Righting response | Strongylocentrotus fragilis | Deep Sea | 7.92–7.64 | 0.28 | 140 | = | [39] |
| 14 | Righting response | Strongylocentrotus fragilis | Deep Sea | 7.92–7.23–6.61 | 0.69–1.31 | 140 | Increased | [39] |
| 15 | Righting response | Paracentrotus lividus site 1 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 16 | Righting response | Paracentrotus lividus site 2 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 17 | Shelter seeking | Paracentrotus lividus site 1 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | Impaired | [25] |
| 18 | Shelter seeking | Paracentrotus lividus site 2 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | Impaired | [25] |
| 19 | Speed | Strongylocentrotus fragilis | Deep Sea | 7.6–7.1 | 0.5 | 27 | = | [102] |
| 20 | Speed | Paracentrotus lividus site 1 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | = | [25] |
| 21 | Speed | Paracentrotus lividus site 2 | Sub.-Temp. | 8.0–7.6 | 0.4 | 180 | Impaired | [25] |
| 22 | Tube feet characteristics | Pseudocentrotus depressus | Sub.-Temp. | 8.2–7.56-(6.90) | 0.64–1.3 | 48 | Impaired | [28] |
| 23 | Tube feet characteristics | Paracentrotus lividus | Sub.-Temp. | 7.9–7.7 | 0.2 | 60 | = | [37] |
| 24 | Tube feet characteristics | Paracentrotus lividus | Sub.-Temp. | 7.9–7.4 | 0.5 | 60 | = | [37] |
| N | Response Detail | Species | Latitudinal Range | pH Level | ΔpH | Exposure Time (Days) | Specific Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| 1 | Female fecundity | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.1–7.7 | 0.4 | 120 | Decreased | [122] |
| 2 | Female fecundity | Hemicentrotus pulcherrimus | Sub.-Temp. | 8.1–7.83 | 0.27 | 270 | = | [84] |
| 3 | Female fecundity | Strongylocentrotus droebachiensis | Temp.-Pol. | 8.1–7.7 | 0.4 | 480 | = | [122] |
| 4 | Gonad histology | Salmacis virgulata | Eq.-Trop. | 8.26–8.00 | 0.26 | 14 | Oocytes lesions | [59] |
| 5 | Gonad histology | Salmacis virgulata | Eq.-Trop. | 8.26–7.81–7.63 | 0.45–0.63 | 14 | Oocytes lesions | [59] |
| 6 | Gonad histology | Sterechinus neumayeri | Temp.-Pol. | 8.12–7.8 | 0.32 | 30 | Anomalies | [123] |
| 7 | Gonad histology | Sterechinus neumayeri | Temp.-Pol. | 8.12–7.6 | 0.42 | 30 | Anomalies | [123] |
| 8 | Gonad histology | Echinometra sp. | Eq.-Trop. | 8.1–7.9 | 0.2 | 70 | Male development delayed | [57] |
| 9 | Gonad histology | Hemicentrotus pulcherrimus | Sub.-Temp. | 8.1–7.83 | 0.27 | 270 | Delayed | [84] |
| 10 | Gonad histology | Echinometra sp. | Eq.-Trop. | 8.1–7.7 | 0.4 | 330 | = | [51] |
| 11 | Gonadal growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.72 | 0.26 | 1200 | = | [44] |
| 12 | Gonadal growth | Sterechinus neumayeri | Temp.-Pol. | 7.98–7.52 | 0.46 | 1200 | = | [44] |
| 13 | Gonadal index | Sterechinus neumayeri | Temp.-Pol. | 8.12–7.8 | 0.32 | 30 | = | [123] |
| 14 | Gonadal index | Sterechinus neumayeri | Temp.-Pol. | 8.12–7.6 | 0.42 | 30 | = | [123] |
| 15 | Gonadal index | Strongylocentrotus fragilis | Deep Sea | 7.92–7.64 | 0.28 | 140 | = | [39] |
| 16 | Gonadal index | Strongylocentrotus fragilis | Deep Sea | 7.92–7.23–6.61 | 0.69–1.31 | 140 | Decreased | [39] |
| 17 | Gonadal index | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.83 | 0.32 | 140 | = | [40] |
| 18 | Gonadal index | Anthocidaris crassispina | Temp.-Pol. | 8.15–7.33 | 0.82 | 140 | = | [40] |
| 19 | Gonadal index | Tripneustes gratilla | Eq.-Trop. | 8.1–7.8 | 0.3 | 146 | = | [41] |
| 20 | Gonadal index | Tripneustes gratilla | Eq.-Trop. | 8.1–7.6 | 0.5 | 146 | Reduced | [41] |
| 21 | Gonadal index | Paracentrotus lividus site 1 | Sub.-Temp. | 8.1–7.7 | 0.4 | 180 | = | [25] |
| 22 | Gonadal index | Paracentrotus lividus site 2 | Sub.-Temp. | 8.1–7.7 | 0.4 | 180 | = | [25] |
| 23 | Gonadal index | Echinometra sp. | Eq.-Trop. | 8.1–7.7 | 0.4 | 330 | = | [51] |
| 24 | Gonadal weight | Echinometra sp. | Eq.-Trop. | 8.0–7.48 | 0.52 | resident | Reduced | [45] |
5. SWA Effects on Echinoid Reproduction
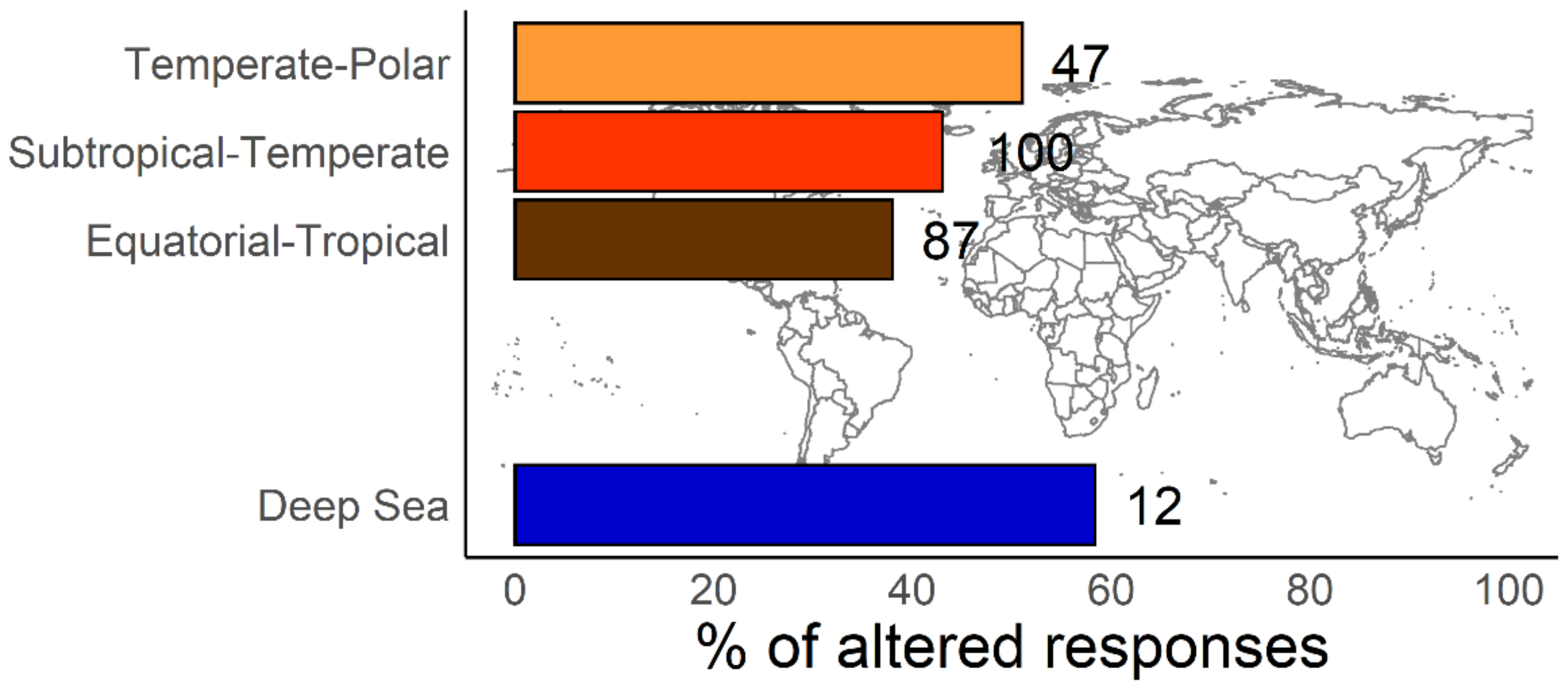
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Masson-Delmotte, V.; Zhai, P.; Tignor, M.; Poloczanska, E.; Mintenbeck, K.; Alegría, A.; Nicolai, M.; Okem, A.; et al. (Eds.) IPCC 2019 Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; 755p. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Zachos, J.C.; Caldeira, K.; Tyrrell, T. OCEANS: Carbon emissions and acidification. Science 2008, 321, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. (Eds.) IPCC 2018 Summary for Policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty; World Meteorological Organization: Geneva, Switzerland, 2018; 32p., Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2019/05/SR15_SPM_version_report_LR.pdf (accessed on 23 March 2022).
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide; The Royal Society: London, UK, 2005; ISBN 0-85403-617-2. [Google Scholar]
- Hu, M.; Tseng, Y.-C.; Su, Y.-H.; Lein, E.; Lee, H.-G.; Lee, J.-R.; Dupont, S.; Stumpp, M. Variability in larval gut pH regulation defines sensitivity to ocean acidification in six species of the Ambulacraria superphylum. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171066. [Google Scholar] [CrossRef]
- Chan, K.Y.K.; Grunbaum, D.; O’Donnell, M.J. Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J. Exp. Biol. 2011, 214, 3857–3867. [Google Scholar] [CrossRef] [Green Version]
- Couturier, C.S.; Stecyk, J.A.W.; Rummer, J.L.; Munday, P.L.; Nilsson, G.E. Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2013, 166, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Spady, B.L.; Nay, T.J.; Rummer, J.L.; Munday, P.L.; Watson, S.-A. Aerobic performance of two tropical cephalopod species unaltered by prolonged exposure to projected future carbon dioxide levels. Conserv. Physiol. 2019, 7, coz024. [Google Scholar] [CrossRef]
- Range, P.; Chícharo, M.A.; Ben-Hamadou, R.; Piló, D.; Fernandez-Reiriz, M.J.; Labarta, U.; Marin, M.G.; Bressan, M.; Matozzo, V.; Chinellato, A.; et al. Impacts of CO2-induced seawater acidification on coastal Mediterranean bivalves and interactions with other climatic stressors. Reg. Environ. Chang. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Wang, M.; Jeong, C.B.; Lee, Y.H.; Lee, J.S. Effects of ocean acidification on copepods. Aquat. Toxicol. 2018, 196, 17–24. [Google Scholar] [CrossRef]
- Yamada, Y.; Ikeda, T. Acute toxicity of lowered pH to some oceanic zooplankton. Plankt. Biol. Ecol. 1999, 46, 62–67. [Google Scholar]
- McClintock, J.B.; Amsler, M.O.; Angus, R.A.; Challener, R.C.; Schram, J.B.; Amsler, C.D.; Mah, C.L.; Cuce, J.; Baker, B.J. The Mg-calcite composition of Antarctic echinoderms: Important implications for predicting the impacts of ocean acidification. J. Geol. 2011, 119, 457–466. [Google Scholar] [CrossRef]
- Dupont, S.; Dorey, N.; Thorndyke, M. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast. Shelf Sci. 2010, 89, 182–185. [Google Scholar] [CrossRef]
- Wootton, J.T.; Pfister, C.A.; Forester, J.D. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl. Acad. Sci. USA 2008, 105, 18848–18853. [Google Scholar] [CrossRef] [Green Version]
- Moschino, V.; Marin, M.G. Spermiotoxicity and embryotoxicity of triphenyltin in the sea urchin Paracentrotus lividus Lmk. Appl. Organomet. Chem. 2002, 16, 175–181. [Google Scholar] [CrossRef]
- Bellas, J.; Granmo, Å.; Beiras, R. Embryotoxicity of the antifouling biocide zinc pyrithione to sea urchin (Paracentrotus lividus) and mussel (Mytilus edulis). Mar. Pollut. Bull. 2005, 50, 1382–1385. [Google Scholar] [CrossRef]
- Bellas, J.; Fernández, N.; Lorenzo, I.; Beiras, R. Integrative assessment of coastal pollution in a Ría coastal system (Galicia, NW Spain): Correspondence between sediment chemistry and toxicity. Chemosphere 2008, 72, 826–835. [Google Scholar] [CrossRef]
- Dupont, S.; Ortega-Martínez, O.; Thorndyke, M. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 2010, 19, 449–462. [Google Scholar] [CrossRef]
- Vargas, C.A.; Lagos, N.A.; Lardies, M.A.; Duarte, C.; Manríquez, P.H.; Aguilera, V.M.; Broitman, B.; Widdicombe, S.; Dupont, S. Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 2017, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asnicar, D.; Novoa-Abelleira, A.; Minichino, R.; Badocco, D.; Pastore, P.; Finos, L.; Munari, M.; Marin, M.G. When site matters: Metabolic and behavioural responses of adult sea urchins from different environments during long-term exposure to seawater acidification. Mar. Environ. Res. 2021, 169, 105372. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, V.M.; Vargas, C.A.; Lardies, M.A.; Poupin, M.J. Adaptive variability to low-pH river discharges in Acartia tonsa and stress responses to high pCO2 conditions. Mar. Ecol. 2016, 37, 215–226. [Google Scholar] [CrossRef]
- Vargas, C.A.; Cuevas, L.A.; Broitman, B.R.; San Martin, V.A.; Lagos, N.A.; Gaitán-Espitia, J.D.; Dupont, S. Upper environmental pCO2 drives sensitivity to ocean acidification in marine invertebrates. Nat. Clim. Chang. 2022, 12, 200–207. [Google Scholar] [CrossRef]
- Nasuchon, N.; Hirasaka, K.; Yamaguchi, K.; Okada, J.; Ishimatsu, A. Effects of elevated carbon dioxide on contraction force and proteome composition of sea urchin tube feet. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 21, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Miles, H.; Widdicombe, S.; Spicer, J.I.; Hall-Spencer, J. Effects of anthropogenic seawater acidification on acid-base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 2007, 54, 89–96. [Google Scholar] [CrossRef]
- Smith, A.M.; Clark, D.E.; Lamare, M.D.; Winter, D.J.; Byrne, M. Risk and resilience: Variations in magnesium in echinoid skeletal calcite. Mar. Ecol. Prog. Ser. 2016, 561, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chave, K.E. Aspects of the Biogeochemistry of Magnesium 1. Calcareous Marine Organisms. J. Geol. 1954, 62, 266–283. [Google Scholar] [CrossRef]
- Andersson, A.J.; Mackenzie, F.T.; Bates, N.R. Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 2008, 373, 265–273. [Google Scholar] [CrossRef]
- Lebrato, M.; McClintock, J.B.; Amsler, M.O.; Ries, J.B.; Egilsdottir, H.; Lamare, M.; Amsler, C.D.; Challener, R.C.; Schram, J.B.; Mah, C.L.; et al. From the Arctic to the Antarctic: The major, minor, and trace elemental composition of echinoderm skeletons. Ecology 2013, 94, 1434. [Google Scholar] [CrossRef]
- Wolfe, K.; Dworjanyn, S.A.; Byrne, M. Effects of ocean warming and acidification on survival, growth and skeletal development in the early benthic juvenile sea urchin (Heliocidaris erythrogramma). Glob. Chang. Biol. 2013, 19, 2698–2707. [Google Scholar] [CrossRef]
- Moulin, L.; Grosjean, P.; Leblud, J.; Batigny, A.; Dubois, P. Impact of elevated pCO2 on acid–base regulation of the sea urchin Echinometra mathaei and its relation to resistance to ocean acidification: A study in mesocosms. J. Exp. Mar. Bio. Ecol. 2014, 457, 97–104. [Google Scholar] [CrossRef]
- Zhan, Y.; Cui, D.; Xing, D.; Zhang, J.; Zhang, W.; Li, Y.; Li, C.; Chang, Y. CO2-driven ocean acidification repressed the growth of adult sea urchin Strongylocentrotus intermedius by impairing intestine function. Mar. Pollut. Bull. 2020, 153, 110944. [Google Scholar] [CrossRef]
- Cohen-Rengifo, M.; Agüera, A.; Bouma, T.; M’Zoudi, S.; Flammang, P.; Dubois, P. Ocean warming and acidification alter the behavioral response to flow of the sea urchin Paracentrotus lividus. Ecol. Evol. 2019, 9, 12128–12143. [Google Scholar] [CrossRef] [Green Version]
- Asnaghi, V.; Chindris, A.; Leggieri, F.; Scolamacchia, M.; Brundu, G.; Guala, I.; Loi, B.; Chiantore, M.; Farina, S. Decreased pH impairs sea urchin resistance to predatory fish: A combined laboratory-field study to understand the fate of top-down processes in future oceans. Mar. Environ. Res. 2020, 162, 105194. [Google Scholar] [CrossRef]
- Taylor, J.R.; Lovera, C.; Whaling, P.J.; Buck, K.R.; Pane, E.F.; Barry, J.P. Physiological effects of environmental acidification in the deep-sea urchin Strongylocentrotus fragilis. Biogeosciences 2014, 11, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yagi, M.; Yin, R.; Lu, W.; Ishimatsu, A. Effects of elevated seawater CO2 on feed intake, oxygen consumption and morphology of Aristotle’s lantern in the sea urchin Anthocidaris crassispina. J. Mar. Sci. Technol. 2013, 21, 192–200. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Byrne, M. Impacts of ocean acidification on sea urchin growth across the juvenile to mature adult life-stage transition is mitigated by warming. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172684. [Google Scholar] [CrossRef] [Green Version]
- Moulin, L.; Grosjean, P.; Leblud, J.; Batigny, A.; Collard, M.; Dubois, P. Long-term mesocosms study of the effects of ocean acidification on growth and physiology of the sea urchin Echinometra mathaei. Mar. Environ. Res. 2015, 103, 103–114. [Google Scholar] [CrossRef]
- Uthicke, S.; Patel, F.; Karelitz, S.; Luter, H.; Webster, N.; Lamare, M. Key biological responses over two generations of the sea urchin Echinometra sp. A under future ocean conditions. Mar. Ecol. Prog. Ser. 2020, 637, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Morley, S.A.; Suckling, C.C.; Clark, M.S.; Cross, E.L.; Peck, L.S. Long-term effects of altered pH and temperature on the feeding energetics of the Antarctic sea urchin, Sterechinus neumayeri. Biodiversity 2016, 17, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Uthicke, S.; Ebert, T.; Liddy, M.; Johansson, C.; Fabricius, K.E.; Lamare, M. Echinometra sea urchins acclimatized to elevated pCO2 at volcanic vents outperform those under present-day pCO2 conditions. Glob. Chang. Biol. 2016, 22, 2451–2461. [Google Scholar] [CrossRef]
- Holtmann, W.C.; Stumpp, M.; Gutowska, M.A.; Syré, S.; Himmerkus, N.; Melzner, F.; Bleich, M. Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: The role of body cavity epithelia and stereom dissolution. Mar. Biol. 2013, 160, 2631–2645. [Google Scholar] [CrossRef]
- Calosi, P.; Rastrick, S.P.S.; Graziano, M.; Thomas, S.C.; Baggini, C.; Carter, H.A.; Hall-Spencer, J.M.; Milazzo, M.; Spicer, J.I. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar. Pollut. Bull. 2013, 73, 470–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.; Harianto, J.; Thomson, M.; Byrne, M. The effects of long-term exposure to low pH on the skeletal microstructure of the sea urchin Heliocidaris erythrogramma. J. Exp. Mar. Bio. Ecol. 2020, 523, 151250. [Google Scholar] [CrossRef]
- Dery, A.; Collard, M.; Dubois, P. Ocean Acidification Reduces Spine Mechanical Strength in Euechinoid but Not in Cidaroid Sea Urchins. Environ. Sci. Technol. 2017, 51, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.; Pancucci-Papadopulou, M.A.; Hall-Spencer, J.M. Sea urchin response to rising pCO2 shows ocean acidification may fundamentally alter the chemistry of marine skeletons. Mediterr. Mar. Sci. 2014, 15, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Hazan, Y.; Wangensteen, O.S.; Fine, M. Tough as a rock-boring urchin: Adult Echinometra sp. EE from the Red Sea show high resistance to ocean acidification over long-term exposures. Mar. Biol. 2014, 161, 2531–2545. [Google Scholar] [CrossRef]
- Di Giglio, S.; Spatafora, D.; Milazzo, M.; M’Zoudi, S.; Zito, F.; Dubois, P.; Costa, C. Are control of extracellular acid-base balance and regulation of skeleton genes linked to resistance to ocean acidification in adult sea urchins? Sci. Total Environ. 2020, 720, 137443. [Google Scholar] [CrossRef]
- Asnaghi, V.; Collard, M.; Mangialajo, L.; Gattuso, J.P.; Dubois, P. Bottom-up effects on biomechanical properties of the skeletal plates of the sea urchin Paracentrotus lividus (Lamarck, 1816) in an acidified ocean scenario. Mar. Environ. Res. 2019, 144, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.; Smith, A.M.; West, S.; Collard, M.; Dubois, P.; Graba-landry, A.; Dworjanyn, S.A. Warming Influences Mg2+ Content, While Warming and Acidification Influence Calcification and Test Strength of a Sea Urchin. Environ. Sci. Technol. 2014, 48, 12620–12627. [Google Scholar] [CrossRef]
- Collard, M.; Rastrick, S.P.S.; Calosi, P.; Demolder, Y.; Dille, J.; Findlay, H.S.; Hall-Spencer, J.M.; Milazzo, M.; Moulin, L.; Widdicombe, S.; et al. The impact of ocean acidification and warming on the skeletal mechanical properties of the sea urchin Paracentrotus lividus from laboratory and field observations. ICES J. Mar. Sci. 2016, 73, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Di Giglio, S.; Agüera, A.; Pernet, P.; M’Zoudi, S.; Angulo-Preckler, C.; Avila, C.; Dubois, P. Effects of ocean acidification on acid-base physiology, skeleton properties, and metal contamination in two echinoderms from vent sites in Deception Island, Antarctica. Sci. Total Environ. 2021, 765, 142669. [Google Scholar] [CrossRef]
- Uthicke, S.; Liddy, M.; Nguyen, H.D.; Byrne, M. Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin, Echinometra sp. A. Coral Reefs 2014, 33, 831–845. [Google Scholar] [CrossRef] [Green Version]
- Migliaccio, O.; Pinsino, A.; Maffioli, E.; Smith, A.M.; Agnisola, C.; Matranga, V.; Nonnis, S.; Tedeschi, G.; Byrne, M.; Gambi, M.C.; et al. Living in future ocean acidification, physiological adaptive responses of the immune system of sea urchins resident at a CO2 vent system. Sci. Total Environ. 2019, 672, 938–950. [Google Scholar] [CrossRef]
- Anand, M.; Rangesh, K.; Maruthupandy, M.; Jayanthi, G.; Rajeswari, B.; Priya, R.J. Effect of CO2 driven ocean acidification on calcification, physiology and ovarian cells of tropical sea urchin Salmacis virgulata—A microcosm approach. Heliyon 2021, 7, e05970. [Google Scholar] [CrossRef]
- Emerson, C.E.; Reinardy, H.C.; Bates, N.R.; Bodnar, A.G. Ocean acidification impacts spine integrity but not regenerative capacity of spines and tube feet in adult sea urchins. R. Soc. Open Sci. 2017, 4, 170140. [Google Scholar] [CrossRef] [Green Version]
- Shetye, S.S.; Naik, H.; Kurian, S.; Shenoy, D.; Kuniyil, N.; Fernandes, M.; Hussain, A. pH variability off Goa (eastern Arabian Sea) and the response of sea urchin to ocean acidification scenarios. Mar. Ecol. 2020, 41, 1–11. [Google Scholar] [CrossRef]
- Mos, B.; Byrne, M.; Dworjanyn, S.A. Biogenic acidification reduces sea urchin gonad growth and increases susceptibility of aquaculture to ocean acidification. Mar. Environ. Res. 2016, 113, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Wood, H.L.; Spicer, J.I.; Widdicombe, S. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B Biol. Sci. 2008, 275, 1767–1773. [Google Scholar] [CrossRef] [Green Version]
- Long, W.C.; Swiney, K.M.; Foy, R.J. Effects of ocean acidification on the embryos and larvae of red king crab, Paralithodes camtschaticus. Mar. Pollut. Bull. 2013, 69, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Melzner, F.; Gutowska, M.A.; Langenbuch, M.; Dupont, S.; Lucassen, M.; Thorndyke, M.C.; Bleich, M.; Pörtner, H.O. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences 2009, 6, 2313–2331. [Google Scholar] [CrossRef] [Green Version]
- Kaniewska, P.; Campbell, P.R.; Kline, D.I.; Rodriguez-Lanetty, M.; Miller, D.J.; Dove, S.; Hoegh-Guldberg, O. Major cellular and physiological impacts of ocean acidification on a reef building coral. PLoS ONE 2012, 7, e34659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pörtner, H. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.O.; Bock, C.; Reipschläger, A. Modulation of the cost of pHi regulation during metabolic depression: A 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol. 2000, 203, 2417–2428. [Google Scholar] [CrossRef]
- Reipschläger, A.; Pörtner, H.O. Metabolic depression during environmental stress: The role of extracellular versus intracellular pH in Sipunculus nudus. J. Exp. Biol. 1996, 199, 1801–1807. [Google Scholar] [CrossRef]
- Langenbuch, M.; Bock, C.; Leibfritz, D.; Pörtner, H.O. Effects of environmental hypercapnia on animal physiology: A 13C NMR study of protein synthesis rates in the marine invertebrate Sipunculus nudus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Carey, N.; Harianto, J.; Byrne, M. Sea urchins in a high-CO2 world: Partitioned effects of body size, ocean warming and acidification on metabolic rate. J. Exp. Biol. 2016, 219, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Brockington, S.; Peck, L. Seasonality of respiration and ammonium excretion in the Antarctic echinoid Sterechinus neumayeri. Mar. Ecol. Prog. Ser. 2001, 219, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Catarino, A.I.; Bauwens, M.; Dubois, P. Acid-base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ. Sci. Pollut. Res. 2012, 19, 2344–2353. [Google Scholar] [CrossRef]
- Stumpp, M.; Trübenbach, K.; Brennecke, D.; Hu, M.Y.; Melzner, F. Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat. Toxicol. 2012, 110–111, 194–207. [Google Scholar] [CrossRef]
- Small, D.P.; Milazzo, M.; Bertolini, C.; Graham, H.; Hauton, C.; Hall-Spencer, J.M.; Rastrick, S.P.S. Temporal fluctuations in seawater pCO2 may be as important as mean differences when determining physiological sensitivity in natural systems. ICES J. Mar. Sci. 2016, 73, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; Ellis, R.P.; Vernon, E.; Elliot, K.; Newbatt, S.; Wilson, R.W. Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Collard, M.; Dery, A.; Dehairs, F.; Dubois, P. Euechinoidea and Cidaroidea respond differently to ocean acidification. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2014, 174, 45–55. [Google Scholar] [CrossRef]
- Marčeta, T.; Matozzo, V.; Alban, S.; Badocco, D.; Pastore, P.; Marin, M.G. Do males and females respond differently to ocean acidification? An experimental study with the sea urchin Paracentrotus lividus. Environ. Sci. Pollut. Res. 2020, 27, 39516–39530. [Google Scholar] [CrossRef]
- Harianto, J.; Aldridge, J.; Torres Gabarda, S.A.; Grainger, R.J.; Byrne, M. Impacts of Acclimation in Warm-Low pH Conditions on the Physiology of the Sea Urchin Heliocidaris erythrogramma and Carryover Effects for Juvenile Offspring. Front. Mar. Sci. 2021, 7, 1–18. [Google Scholar] [CrossRef]
- Spicer, J.I.; Widdicombe, S.; Needham, H.R.; Berge, J.A. Impact of CO2-acidified seawater on the extracellular acid-base balance of the northern sea urchin Strongylocentrotus dröebachiensis. J. Exp. Mar. Bio. Ecol. 2011, 407, 19–25. [Google Scholar] [CrossRef]
- Leite Figueiredo, D.A.; Branco, P.C.; dos Santos, D.A.; Emerenciano, A.K.; Iunes, R.S.; Shimada Borges, J.C.; Machado Cunha da Silva, J.R. Ocean acidification affects parameters of immune response and extracellular pH in tropical sea urchins Lytechinus variegatus and Echinometra luccunter. Aquat. Toxicol. 2016, 180, 84–94. [Google Scholar] [CrossRef]
- Dupont, S.; Thorndyke, M. Relationship between CO2-driven changes in extracellular acid-base balance and cellular immune response in two polar echinoderm species. J. Exp. Mar. Bio. Ecol. 2012, 424–425, 32–37. [Google Scholar] [CrossRef]
- Kurihara, H.; Yin, R.; Nishihara, G.N.G.; Soyano, K.; Ishimatsu, A. Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus. Aquat. Biol. 2013, 18, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Rich, W.A.; Schubert, N.; Schläpfer, N.; Carvalho, V.F.; Horta, A.C.L.; Horta, P.A. Physiological and biochemical responses of a coralline alga and a sea urchin to climate change: Implications for herbivory. Mar. Environ. Res. 2018, 142, 100–107. [Google Scholar] [CrossRef]
- Burnham, K.A.; Nowicki, R.J.; Hall, E.R.; Pi, J.; Page, H.N. Effects of ocean acidification on the performance and interaction of fleshy macroalgae and a grazing sea urchin. J. Exp. Mar. Bio. Ecol. 2022, 547, 151662. [Google Scholar] [CrossRef]
- Sun, T.; Tang, X.; Jiang, Y.; Wang, Y. Seawater acidification induced immune function changes of haemocytes in Mytilus edulis: A comparative study of CO2 and HCl enrichment. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Lane, J.M. The utilization of nutrients by post-metamorphic echinoderms. In Echinoderm Nutrition; Jangoux, M., Lawrence, J.M., Eds.; CRC Press: London, UK, 2020; pp. 331–371. [Google Scholar]
- Shick, J.M. Respiratory Gas Exchange in Echinoderms. In Echinoderm Studies; Jangoux, M., Lawrence, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 67–110. [Google Scholar]
- Cattano, C.; Claudet, J.; Domenici, P.; Milazzo, M. Living in a high CO2 world: A global meta-analysis shows multiple trait-mediated fish responses to ocean acidification. Ecol. Monogr. 2018, 88, 320–335. [Google Scholar] [CrossRef]
- Havenhand, J.N.; Buttler, F.-R.; Thorndyke, M.C.; Williamson, J.E. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008, 18, R651–R652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniash, E.; Ivanina, A.; Lieb, N.S.; Kurochkin, I.; Sokolova, I.M. Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar. Ecol. Prog. Ser. 2010, 419, 95–108. [Google Scholar] [CrossRef]
- Mayzaud, P. Respiration and nitrogen excretion of zooplankton. II. Studies of the metabolic characteristics of starved animals. Mar. Biol. 1973, 21, 19–28. [Google Scholar] [CrossRef]
- Bayne, B.L.; Widdows, J.; Thompson, T.J. Physiological integrations. In Marine Mussels: Their Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1976; pp. 121–206. [Google Scholar]
- Bayne, B.L.; Brown, D.A.; Burns, K.; Dixon, D.R.; Ivanovici, A.; Livingstone, D.R.; Lowe, D.M.; Moore, M.N.; Stebbing, A.R.D.; Widdows, J. The Effects of Stress and Pollution on Marine Animals; Praeger: Westport, CT, USA, 1985; ISBN 0030570190. [Google Scholar]
- Clements, J.C.; Hunt, H.L. Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 2015, 536, 259–279. [Google Scholar] [CrossRef]
- Clements, J.C.; Poirier, L.A.; Pérez, F.F.; Comeau, L.A.; Babarro, J.M.F. Behavioural responses to predators in Mediterranean mussels (Mytilus galloprovincialis) are unaffected by elevated pCO2. Mar. Environ. Res. 2020, 161, 105148. [Google Scholar] [CrossRef]
- Clements, J.C.; Darrow, E.S. Eating in an acidifying ocean: A quantitative review of elevated CO2 effects on the feeding rates of calcifying marine invertebrates. Hydrobiologia 2018, 820, 1–21. [Google Scholar] [CrossRef]
- Watson, S.A.; Lefevre, S.; McCormick, M.I.; Domenici, P.; Nilsson, G.E.; Munday, P.L. Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc. R. Soc. B Biol. Sci. 2013, 281. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dixson, D.L.; Domenici, P.; McCormick, M.I.; Sørensen, C.; Watson, S.-A.; Munday, P.L. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012, 2, 201–204. [Google Scholar] [CrossRef]
- Clements, J.C.; Sundin, J.; Clark, T.D.; Jutfelt, F. Meta-analysis reveals an extreme “decline effect” in the impacts of ocean acidification on fish behavior. PLoS Biol. 2022, 20, e3001511. [Google Scholar] [CrossRef]
- Barry, J.P.; Lovera, C.; Buck, K.R.; Peltzer, E.T.; Taylor, J.R.; Walz, P.; Whaling, P.J.; Brewer, P.G. Use of a free ocean CO2 enrichment (FOCE) system to evaluate the effects of ocean acidification on the foraging behavior of a deep-sea urchin. Environ. Sci. Technol. 2014, 48, 9890–9897. [Google Scholar] [CrossRef] [Green Version]
- Persons, M.H.; Walker, S.E.; Rypstra, A.L.; Marshall, S.D. Wolf spider predator avoidance tactics and survival in the presence of diet-associated predator cues (Araneae: Lycosidae). Anim. Behav. 2001, 61, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Weis, J.S.; Smith, G.; Zhou, T.; Santiago-Bass, C.; Weis, P. Effects of contaminants on behavior: Biochemical mechanisms and ecological consequences. Bioscience 2001, 51, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Bao, Z.; Chang, Y. Fitness-related consequences shed light on the mechanisms of covering and sheltering behaviors in the sea urchin Glyptocidaris crenularis. Mar. Ecol. 2016, 37, 998–1007. [Google Scholar] [CrossRef]
- Borell, E.M.; Steinke, M.; Fine, M. Direct and indirect effects of high pCO2 on algal grazing by coral reef herbivores from the Gulf of Aqaba (Red Sea). Coral Reefs 2013, 32, 937–947. [Google Scholar] [CrossRef]
- Campbell, J.E.; Craft, J.D.; Muehllehner, N.; Langdon, C.; Paul, V.J. Responses of calcifying algae (Halimeda spp.) to ocean acidification: Implications for herbivores. Mar. Ecol. Prog. Ser. 2014, 514, 43–56. [Google Scholar] [CrossRef]
- Percy, J.A. Thermal adaptation in the boreo-arctic echinoid Strongylocentrotus droebachiensis (Müller, 1776). II. Seasonal acclimatization and urchin activity. Physiol. Zool. 1973, 46, 129–138. [Google Scholar] [CrossRef]
- Bayed, A.; Quiniou, F.; Benrha, A.; Guillou, M. The Paracentrotus lividus populations from the northern Moroccan Atlantic coast: Growth, reproduction and health condition. J. Mar. Biol. Assoc. UK 2005, 85, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.M.; Cowell, B.C. The righting response as an indication of stress in Stichaster striatus (Echinodermata, asteroidea). Mar. Freshw. Behav. Physiol. 1996, 27, 239–248. [Google Scholar] [CrossRef]
- Verling, E.; Crook, A.C.; Barnes, D.K.A. Covering behaviour in Paracentrotus lividus: Is light important? Mar. Biol. 2002, 140, 391–396. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. In Sea Urchins: Biology and Ecology; Lawrence, J.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 447–485. [Google Scholar]
- Brothers, C.J.; McClintock, J.B. The effects of climate-induced elevated seawater temperature on the covering behavior, righting response, and Aristotle’s lantern reflex of the sea urchin Lytechinus variegatus. J. Exp. Mar. Bio. Ecol. 2015, 467, 33–38. [Google Scholar] [CrossRef]
- Pinna, S.; Pais, A.; Campus, P.; Sechi, N.; Ceccherelli, G. Habitat preferences of the sea urchin Paracentrotus lividus. Mar. Ecol. Prog. Ser. 2012, 445, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Dumont, C.P.; Drolet, D.; Deschênes, I.; Himmelman, J.H. Multiple factors explain the covering behaviour in the green sea urchin, Strongylocentrotus droebachiensis. Anim. Behav. 2007, 73, 979–986. [Google Scholar] [CrossRef]
- Farina, S.; Tomas, F.; Prado, P.; Romero, J.; Alcoverro, T. Seagrass meadow structure alters interactions between the sea urchin Paracentrotus lividus and its predators. Mar. Ecol. Prog. Ser. 2009, 377, 131–137. [Google Scholar] [CrossRef]
- Ziegenhorn, M.A. Sea urchin covering behavior: A comparative review. In Sea Urchin—From Environment to Aquaculture and Biomedicine; InTech: London, UK, 2017. [Google Scholar]
- Richner, H.; Milinski, M. On the functional significance of masking behaviour in sea urchins—An experiment with Paracentrotus lividus. Mar. Ecol. Prog. Ser. 2000, 205, 307–308. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, J.; Yang, M.; Shi, D.; Yin, D.; Hu, F.; Sun, J.; Chi, X.; Zhang, L.; Chang, Y. Transcriptomes reveal genes involved in covering and sheltering behaviors of the sea urchin Strongylocentrotus intermedius exposed to UV-B radiation. Ecotoxicol. Environ. Saf. 2019, 167, 236–241. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Shi, D.; Wei, J.; Chang, Y.; Zhao, C. Effects of long-term elevated temperature on covering, sheltering and righting behaviors of the sea urchin Strongylocentrotus intermedius. PeerJ 2017, 5, e3122. [Google Scholar] [CrossRef] [Green Version]
- Chi, X.; Sun, J.; Yu, Y.; Luo, J.; Zhao, B.; Han, F.; Chang, Y.; Zhao, C. Fitness benefits and costs of shelters to the sea urchin Glyptocidaris crenularis. PeerJ 2020, 8, e8886. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.; Dorey, N.; Stumpp, M.; Melzner, F.; Thorndyke, M. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2013, 160, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Dell’Acqua, O.; Ferrando, S.; Chiantore, M.; Asnaghi, V. The impact of ocean acidification on the gonads of three key Antarctic benthic macroinvertebrates. Aquat. Toxicol. 2019, 210, 19–29. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef]
- Birkhead, T.; Møller, A. Sperm Competition and Sexual Selection; Academic Press Inc.: New York, NY, USA, 1998. [Google Scholar]
- Parker, G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef]
- Bednaršek, N.; Calosi, P.; Feely, R.A.; Ambrose, R.; Byrne, M.; Chan, K.Y.K.; Dupont, S.; Padilla-Gamiño, J.L.; Spicer, J.I.; Kessouri, F.; et al. Synthesis of Thresholds of Ocean Acidification Impacts on Echinoderms. Front. Mar. Sci. 2021, 8, 261. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Foo, S.A.; Byrne, M. Marine gametes in a changing ocean: Impacts of climate change stressors on fecundity and the egg. Mar. Environ. Res. 2017, 128, 12–24. [Google Scholar] [CrossRef]
- Dijkstra, J.A.; Harris, L.G.; Mello, K.; Litterer, A.; Wells, C.; Ware, C. Invasive seaweeds transform habitat structure and increase biodiversity of associated species. J. Ecol. 2017, 105, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- Fagerli, C.; Norderhaug, K.; Christie, H. Lack of sea urchin settlement may explain kelp forest recovery in overgrazed areas in Norway. Mar. Ecol. Prog. Ser. 2013, 488, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Cardona, L.; Moranta, J.; Reñones, O.; Hereu, B. Pulses of phytoplanktonic productivity may enhance sea urchin abundance and induce state shifts in Mediterranean rocky reefs. Estuar. Coast. Shelf Sci. 2013, 133, 88–96. [Google Scholar] [CrossRef]
- Hereu, B.; Zabala, M.; Sala, E. Multiple controls of community structure and dynamics in a sublittoral marine environment. Ecology 2008, 89, 3423–3435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef] [Green Version]
- Gianguzza, P.; Visconti, G.; Gianguzza, F.; Vizzini, S.; Sarà, G.; Dupont, S. Temperature modulates the response of the thermophilous sea urchin Arbacia lixula early life stages to CO2-driven acidification. Mar. Environ. Res. 2014, 93, 70–77. [Google Scholar] [CrossRef]
- Shaw, E.C.; Carpenter, R.C.; Lantz, C.A.; Edmunds, P.J. Intraspecific variability in the response to ocean warming and acidification in the scleractinian coral Acropora pulchra. Mar. Biol. 2016, 163, 210. [Google Scholar] [CrossRef]
- Kurihara, H.; Takahashi, A.; Reyes-Bermudez, A.; Hidaka, M. Intraspecific variation in the response of the scleractinian coral Acropora digitifera to ocean acidification. Mar. Biol. 2018, 165, 38. [Google Scholar] [CrossRef]
- Dupont, S.; Lundve, B.; Thorndyke, M. Near future ocean acidification increases growth rate of the lecithotrophic larvae and juveniles of the sea star Crossaster papposus. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314B, 382–389. [Google Scholar] [CrossRef]
- Schlegel, P.; Havenhand, J.N.; Gillings, M.R.; Williamson, J.E. Individual variability in reproductive success determines winners and losers under ocean acidification: A case study with sea urchins. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.; Navarro, J.M.; Acuña, K.; Torres, R.; Manríquez, P.H.; Lardies, M.A.; Vargas, C.A.; Lagos, N.A.; Aguilera, V. Intraspecific variability in the response of the edible mussel Mytilus chilensis (Hupe) to ocean acidification. Estuaries Coasts 2015, 38, 590–598. [Google Scholar] [CrossRef]
- Ellis, R.P.; Davison, W.; Queirós, A.M.; Kroeker, K.J.; Calosi, P.; Dupont, S.; Spicer, J.I.; Wilson, R.W.; Widdicombe, S.; Urbina, M.A. Does sex really matter? Explaining intraspecies variation in ocean acidification responses. Biol. Lett. 2017, 13. [Google Scholar] [CrossRef]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar. Biol. 2011, 158, 689–697. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.A.; Bange, H.W.; Hansen, H.P.; Körtzinger, A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- Salisbury, J.; Green, M.; Hunt, C.; Campbell, J. Coastal acidification by rivers: A threat to shellfish? Eos Trans. Am. Geophys. Union 2008, 89, 513. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, J.; Gutowska, M.A.; Saphörster, J.; Heinemann, A.; Trübenbach, K.; Fietzke, J.; Hiebenthal, C.; Eisenhauer, A.; Körtzinger, A.; Wahl, M.; et al. Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 2010, 7, 3879–3891. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, G.E.; Evans, T.G.; Kelly, M.W.; Padilla-Gamiño, J.L.; Blanchette, C.A.; Washburn, L.; Chan, F.; McManus, M.A.; Menge, B.A.; Gaylord, B.; et al. Exploring local adaptation and the ocean acidification seascape—Studies in the California Current Large Marine Ecosystem. Biogeosciences 2014, 11, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.; Peck, L.S.; Tyler, P.A.; Southgate, P.C.; Tan, K.S.; Day, R.W.; Morley, S.A. Marine invertebrate skeleton size varies with latitude, temperature and carbonate saturation: Implications for global change and ocean acidification. Glob. Chang. Biol. 2012, 18, 3026–3038. [Google Scholar] [CrossRef]
- Foo, S.A.; Koweek, D.A.; Munari, M.; Gambi, M.C.; Byrne, M.; Caldeira, K. Responses of sea urchin larvae to field and laboratory acidification. Sci. Total Environ. 2020, 723, 138003. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asnicar, D.; Marin, M.G. Effects of Seawater Acidification on Echinoid Adult Stage: A Review. J. Mar. Sci. Eng. 2022, 10, 477. https://doi.org/10.3390/jmse10040477
Asnicar D, Marin MG. Effects of Seawater Acidification on Echinoid Adult Stage: A Review. Journal of Marine Science and Engineering. 2022; 10(4):477. https://doi.org/10.3390/jmse10040477
Chicago/Turabian StyleAsnicar, Davide, and Maria Gabriella Marin. 2022. "Effects of Seawater Acidification on Echinoid Adult Stage: A Review" Journal of Marine Science and Engineering 10, no. 4: 477. https://doi.org/10.3390/jmse10040477
APA StyleAsnicar, D., & Marin, M. G. (2022). Effects of Seawater Acidification on Echinoid Adult Stage: A Review. Journal of Marine Science and Engineering, 10(4), 477. https://doi.org/10.3390/jmse10040477







