Nitrous Oxide from Abiotic Processes of Hydroxylamine and Nitrite in Estuarine and Coastal Ecosystems: A Review
Abstract
:1. Introduction
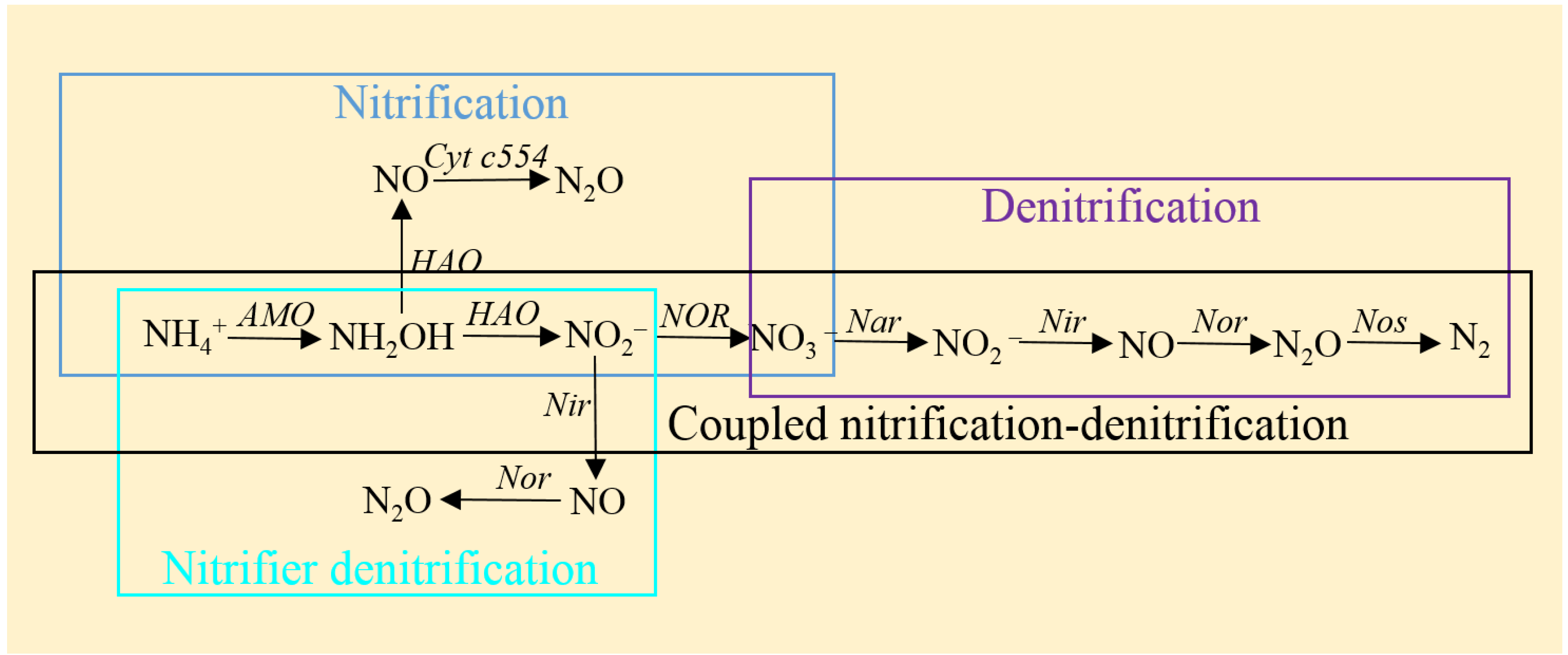
2. N2O Production from Abiotic Processes of Hydroxylamine and Nitrite
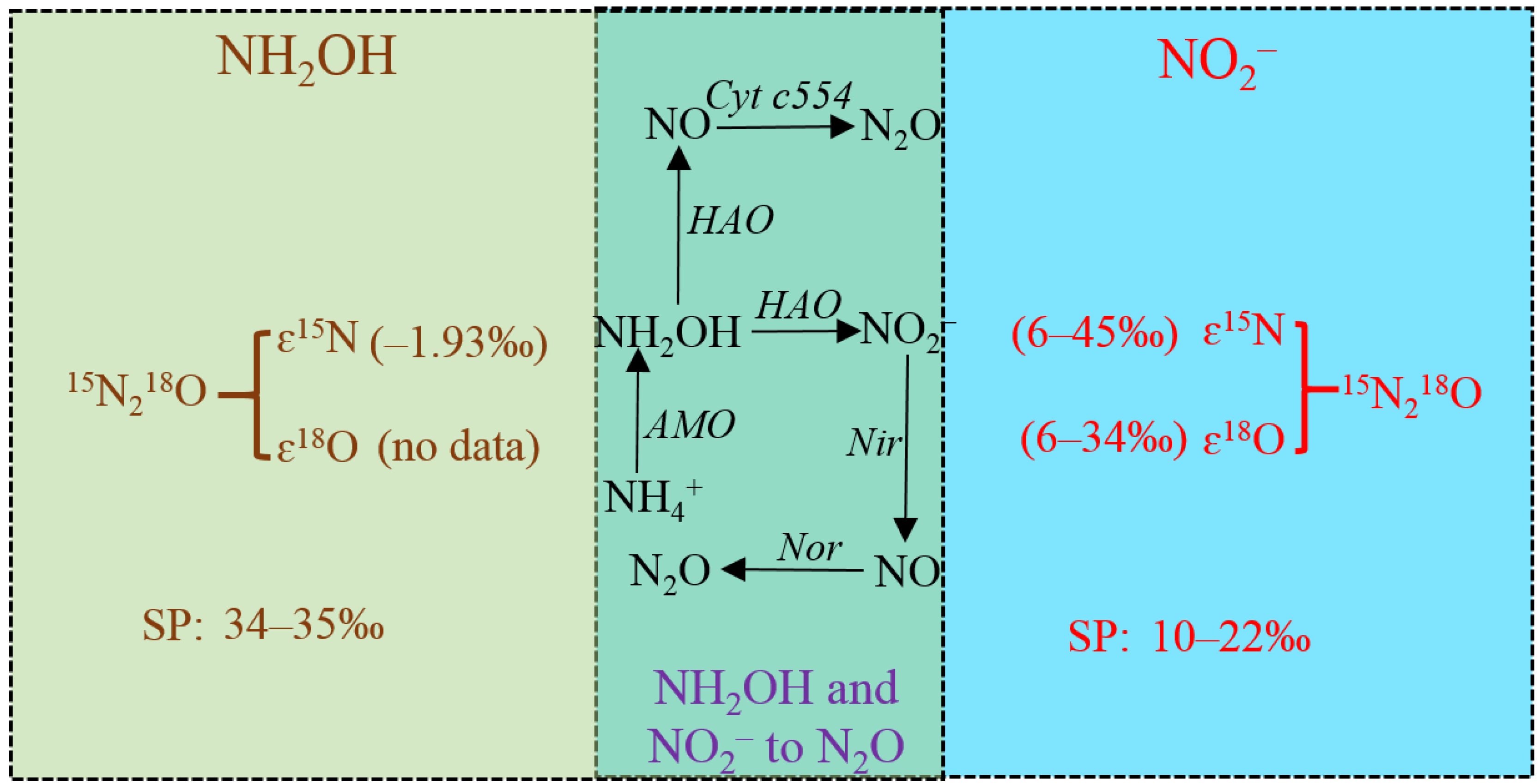
3. Isotopic Fractionation Effects of N2O Production from Abiotic Processes of Hydroxylamine and Nitrite
4. Key Factors of N2O Production from Abiotic Processes of Hydroxylamine and Nitrite
5. Study Limitation and Future Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Chen, G.; Lu, C.; Xu, X.; Ren, W.; Zhang, B.; Banger, K.; Tao, B.; Pan, S.; Liu, M.; et al. Global methane and nitrous oxide emissions from terrestrial ecosystems due to multiple environmental changes. Ecosyst. Health Sustain. 2015, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Wells, N.S.; Maher, D.T.; Erler, D.V.; Hipsey, M.; Rosentreter, J.A.; Eyre, B.D. Estuaries as Sources and Sinks of N2O Across a Land Use Gradient in Subtropical Australia. Glob. Biogeochem. Cycles 2018, 32, 877–894. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, J.; Tang, Q.; Wei, Z.; Schwenke, G.; Liu, D.L.; Yan, X. Indirect N2O emissions from groundwater under high nitrogen-load farmland in eastern China. Environ. Pollut. 2019, 248, 238–246. [Google Scholar] [CrossRef]
- Wei, H.; Gao, D.; Liu, Y.; Lin, X. Sediment nitrate reduction processes in response to environmental gradients along an urban river-estuary-sea continuum. Sci. Total Environ. 2020, 718, 137185. [Google Scholar] [CrossRef]
- WMO. The state of greenhouse gases in the atmosphere based on global observations through 2012. WMO Greenh. Gas Bull. 2013, 9, 1–4. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Lin, X.; Li, X.; Gao, D.; Liu, M.; Cheng, L. Ammonium Production and Removal in the Sediments of Shanghai River Networks: Spatiotemporal Variations, Controlling Factors, and Environmental Implications. J. Geophys. Res. Biogeosci. 2017, 122, 2461–2478. [Google Scholar] [CrossRef]
- Wells, N.S.; Eyre, B.D. δ15N patterns in three subtropical estuaries show switch from nitrogen “reactors” to “pipes” with increasing degradation. Limnol. Oceanogr. 2019, 64, 860–876. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.; Hu, W.F.; Zeng, F.; He, L.; Yin, K.D. Nitrogen cycling processes in sediments of the Pearl River Estuary: Spatial variations, controlling factors, and environmental implications. Catena 2021, 206, 105545. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liu, M.; Hou, L.; Gao, D.; Li, X.; Lu, K.; Gao, J. Nitrogen Losses in Sediments of the East China Sea: Spatiotemporal Variations, Controlling Factors, and Environmental Implications. J. Geophys. Res. Biogeosci. 2017, 122, 2699–2715. [Google Scholar] [CrossRef]
- Zhu-Barker, X.; Cavazos, A.R.; Ostrom, N.E.; Horwath, W.R.; Glass, J.B. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 2015, 126, 251–267. [Google Scholar] [CrossRef]
- Kristie, R.; Klara, R.; Caleb, L.; Biles, S.; Huling, G. Abiotic hydroxylamine nitrification involving manganese- and iron-bearing minerals. Sci. Total Environ. 2018, 644, 567–575. [Google Scholar]
- Butler, J.H.; Gordon, L.I. Rates of nitrous oxide production in the oxidation of hydroxylamine by iron(III). Inorg. Chem. 1986, 25, 4573–4577. [Google Scholar] [CrossRef]
- Bremner, J.; Blackmer, A.; Waring, S. Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biol. Biochem. 1980, 12, 263–269. [Google Scholar] [CrossRef]
- Picardal, F. Abiotic and Microbial Interactions during Anaerobic Transformations of Fe(II) and NOX−. Front. Microbiol. 2012, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Herbst, M.; Bol, R.; Gottselig, N.; Pütz, T.; Weymann, D.; Wiekenkamp, I.; Vereecken, H.; Brüggemann, N. The contribution of hydroxylamine content to spatial variability of N2O formation in soil of a Norway spruce forest. Geochim. Cosmochim. Acta 2016, 178, 76–86. [Google Scholar] [CrossRef]
- Grabb, K.C.; Buchwald, C.; Hansel, C.M.; Wankel, S.D. A dual nitrite isotopic investigation of chemodenitrification by mineral-associated Fe(II) and its production of nitrous oxide. Geochim. Cosmochim. Acta 2017, 196, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Otte, J.M.; Blackwell, N.; Ruser, R.; Kappler, A.; Kleindienst, S.; Schmidt, C. N2O formation by nitrite-induced (chemo)denitrification in coastal marine sediment. Sci. Rep. 2019, 9, 10691. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.H.; Erler, D.; Eyre, B. Nitrous oxide fluxes in estuarine environments: Response to global change. Glob. Chang. Biol. 2015, 21, 3219–3245. [Google Scholar] [CrossRef]
- Yang, W.H.; Silver, W.L. Gross nitrous oxide production drives net nitrous oxide fluxes across a salt marsh landscape. Glob. Chang. Biol. 2016, 22, 2228–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, L.; Tian, H.; Jiang, H.; Mou, X.; Sun, W. Fluxes of nitrous oxide and methane in different coastal Suaeda salsa marshes of the Yellow River estuary, China. Chemosphere 2013, 90, 856–865. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Ren, H.; Li, J.; Tong, C.; Musenze, R.S. Seasonal variations of nitrous oxide fluxes and soil denitrification rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci. Total Environ. 2018, 616−617, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Khanal, S.K. Nitrous Oxide (N2O) Emission from Aquaculture: A Review. Environ. Sci. Technol. 2012, 46, 6470–6480. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.G.; Sun, Z.G.; Gan, Z.T.; Sun, W.L.; Wang, W. Contribution of defferentprocesses in wetland soil N2O production in different restoration phases of the Yellow River Estuary, China. Environ. Sci. 2014, 35, 3110–3119. [Google Scholar]
- Gao, D.; Hou, L.; Li, X.; Liu, M.; Zheng, Y.; Yin, G.; Yang, Y.; Liu, C.; Han, P. Exotic Spartina alterniflora invasion alters soil nitrous oxide emission dynamics in a coastal wetland of China. Plant Soil 2019, 442, 233–246. [Google Scholar] [CrossRef]
- Wang, F.; Chen, N.; Yan, J.; Lin, J.; Guo, W.; Cheng, P.; Liu, Q.; Huang, B.; Tian, Y. Major Processes Shaping Mangroves as Inorganic Nitrogen Sources or Sinks: Insights From a Multidisciplinary Study. J. Geophys. Res. Biogeosci. 2019, 124, 1194–1208. [Google Scholar] [CrossRef]
- Yan, X.; Wan, X.S.; Liu, L.; Xu, M.N.; Tan, E.; Zheng, Z.; Zou, W.; Tian, L.; Li, D.; Trull, T.W.; et al. Biogeochemical Dynamics in a Eutrophic Tidal Estuary Revealed by Isotopic Compositions of Multiple Nitrogen Species. J. Geophys. Res. Biogeosci. 2019, 124, 1849–1864. [Google Scholar] [CrossRef]
- Heil, J.; Wolf, B.; Brüggemann, N.; Emmenegger, L.; Tuzson, B.; Vereecken, H.; Mohn, J. Site-specific 15N isotopic signatures of abiotically produced N2O. Geochim. Cosmochim. Acta 2014, 139, 72–82. [Google Scholar] [CrossRef]
- Jones, L.C.; Peters, B.; Pacheco, J.S.L.; Casciotti, K.L.; Fendorf, S. Stable Isotopes and Iron Oxide Mineral Products as Markers of Chemodenitrification. Environ. Sci. Technol. 2015, 49, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.R.; Han, P.; Hink, L.; Prosser, J.I.; Wagner, M.; Bruggemann, N. Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation. Environ. Sci. Technol. 2017, 51, 13122–13132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Shao, B.; Fu, M.; Luo, C.; Liu, Z. Denitrification of nitrite by ferrous hydroxy complex: Effects on nitrous oxide and ammonium formation. Chem. Eng. J. 2015, 279, 149–155. [Google Scholar] [CrossRef]
- Buchwald, C.; Grabb, K.C.; Hansel, C.M.; Wankel, S.D. Constraining the role of iron in environmental nitrogen transformations: Dual stable isotope systematics of abiotic NO2− reduction by Fe(II) and its production of N2O. Geochim. Cosmochim. Acta 2016, 186, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; De Beer, D.; Dentinger, J.; Xu, Z.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Cavazos, A.R.; Taillefert, M.; Tang, Y.; Glass, J.B. Kinetics of nitrous oxide production from hydroxylamine oxidation by birnessite in seawater. Mar. Chem. 2018, 202, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Jensen, M.M.; Smets, B.F.; Thamdrup, B. Pathways and controls of N2O production in nitritation–anammox biomass. Environ. Sci. Technol. 2017, 51, 8981–8991. [Google Scholar] [CrossRef]
- Peters, B.; Casciotti, K.L.; Samarkin, V.A.; Madigan, M.T.; Schutte, C.A.; Joye, S.B. Stable isotope analyses of NO2−, NO3−, and N2O in the hypersaline ponds and soils of the McMurdo Dry Valleys, Antarctica. Geochim. Cosmochim. Acta 2014, 135, 87–101. [Google Scholar] [CrossRef]
- Ostrom, N.E.; Gandhi, H.; Trubl, G.; Murray, A.E. Chemodenitrification in the cryoecosystem of Lake Vida, Victoria Valley, Antarctica. Geobiology 2016, 14, 575–587. [Google Scholar] [CrossRef]
- Wei, J.; Ibraim, E.; Brüggemann, N.; Vereecken, H.; Mohn, J. First real-time isotopic characterisation of N2O from chemodenitrification. Geochim. Cosmochim. Acta 2019, 267, 17–32. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heil, J.; Liu, S.; Vereecken, H.; Brüggemann, N. Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol. Biochem. 2015, 84, 107–115. [Google Scholar] [CrossRef]
- Su, Q.; Domingo-Félez, C.; Jensen, M.M.; Smets, B.F. Abiotic Nitrous Oxide (N2O) Production Is Strongly pH Dependent, but Contributes Little to Overall N2O Emissions in Biological Nitrogen Removal Systems. Environ. Sci. Technol. 2019, 53, 3508–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Schloter, M.; Hu, R.; Vereecken, H.; Brüggemann, N. Hydroxylamine Contributes More to Abiotic N2O Production in Soils Than Nitrite. Front. Environ. Sci. 2019, 7, 47. [Google Scholar] [CrossRef]
- Ma, W.; Bedard-Haughn, A.; Siciliano, S.; Farrell, R. Relationship between nitrifier and denitrifier community composition and abundance in predicting nitrous oxide emissions from ephemeral wetland soils. Soil Biol. Biochem. 2008, 40, 1114–1123. [Google Scholar] [CrossRef]
- Thorn, K.A.; Mikita, M.A. Nitrite Fixation by Humic Substances Nitrogen-15 Nuclear Magnetic Resonance Evidence for Potential Intermediates in Chemodenitrification. Soil Sci. Soc. Am. J. 2000, 64, 568–582. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Qi, M.; Lin, W.; Li, X. Nitrous Oxide from Abiotic Processes of Hydroxylamine and Nitrite in Estuarine and Coastal Ecosystems: A Review. J. Mar. Sci. Eng. 2022, 10, 623. https://doi.org/10.3390/jmse10050623
Xu C, Qi M, Lin W, Li X. Nitrous Oxide from Abiotic Processes of Hydroxylamine and Nitrite in Estuarine and Coastal Ecosystems: A Review. Journal of Marine Science and Engineering. 2022; 10(5):623. https://doi.org/10.3390/jmse10050623
Chicago/Turabian StyleXu, Chaobin, Mengting Qi, Weisheng Lin, and Xiaofei Li. 2022. "Nitrous Oxide from Abiotic Processes of Hydroxylamine and Nitrite in Estuarine and Coastal Ecosystems: A Review" Journal of Marine Science and Engineering 10, no. 5: 623. https://doi.org/10.3390/jmse10050623




