Abstract
The removal of oil contaminants in marine intertidal sediments under cold climate is an urgent issue. Although the bioavailability of petroleum hydrocarbons decreases at low temperatures, biosurfactants can promote oil biodegradation. In this study, characteristics of biosurfactants produced by cold-adapted oil-degrading bacteria Planococcus sp. XW-1 were studied. Adding the XW-1 biosurfactant could effectively facilitate the solubility of phenanthrene, pyrene, diesel oil, and crude oil. The solubilization was limited by the number of rings and the molecular weight (WSRphenanthrene = 0.0234; WSRpyrene = 0.0165; WSRdiesel oil = 0.0027; WSRcrude oil = 0.0015). Additional biosurfactants significantly washed out crude oil adsorbed to the sand (reduction from 17.1%, 22.7% to 87.9% and 94.28% in 24 h). With the increase in particle size, the removal efficiency increased from 87.9% to 94.28%. After the addition of biosurfactant, the effect of degradation increased by 20% in 20 days. The results suggest that the biosurfactant-producing bacteria Planococcus sp. XW-1 is a promising candidate used in the in situ bioremediation of petroleum-contaminated intertidal sediment.
1. Introduction
Rapidly increasing demand for petroleum resources has been accompanied by increased potential for pollution of the marine environment [1,2]. Petroleum hydrocarbons may be released into marine environments due to a series of causes, such as the discharge of oily wastewater, oil shipwrecks, leakage from oil pipelines, and offshore oil exploitation [3,4]. Driven by winds and currents, the oil may be transported to the intertidal zones.
Once on shore, cleanup operations become significantly difficult since oil pollutants have higher hydrophobicity, lower volatility, lower solubility, and higher absorption ability [5,6,7]. These properties make the weathered oil thick, sticky, highly absorbed by rocks and sand, and difficult to remove from contaminated intertidal zones [8], particularly when oil spills occur at cold climate. Low temperature changes the physic-chemical properties of petroleum, such as wax content and viscosity, which have a further influence on weathering processes of oil [9]. For instance, the evaporation rate of volatile components is delayed and long-chain hydrocarbon precipitates as waxes in low temperature, decreasing the bioavailability of petroleum.
Oil pollutants in the intertidal sediment could be in situ bioremediated with oil-degrading bacteria. Unfortunately, petroleum bioavailability and microbial activities are usually limited at low temperatures. Although the bioavailability of petroleum hydrocarbons is reduced at low temperature, biosurfactants can promote the oil biodegradation. One effective in situ bioremediation strategy is biosurfactant bioaugmentation which can be implemented by introducing biosurfactant-producing microorganisms or biosurfactant compounds to contaminated sites. The key to degrade petroleum hydrocarbons is to enhance its solubility [10]. The biosurfactant has hydrophilic and hydrophobic groups and it can significantly reduce the surface and interfacial tensions to increase the solubility of petroleum hydrocarbons [11] and it can be served as a solubilizer of oil pollutants. Until now, biosurfactant enhanced bioremediation was regarded as a promising method to promote the removal rate of petroleum [12]. As shown in some research, biosurfactant could promote the biodegradation of PAHs in petrochemical wastewater, especially for the remove of 5–6 ring PAHs [13]. The removal rate of petroleum was enhanced from 40% to 70%, with the addition of biosurfactants (0.2%–0.6% (w/w)) [5]. The removal rate of pyrene was increased from 16% to 67% with the addition of biosurfactant produced by Paenibacillus dendritiformis CN5 strain [14]. Biosurfactants possess some special advantages over chemical surfactants, including sustainable production, better environmental compatibility, better adaptability, higher efficiency, and higher safety [4]. It is just for these reasons it has been used in environmental pollution control to enhance the remove of petroleum [15].
However, there are few reports available on bioremediation for oil polluted intertidal sediments at low temperatures. Moreover, only few cold-adapted bacteria which have the abilities of oil degradation and surfactant production have been reported.
In this paper, characteristics of biosurfactant produced by a Planococcus strain were studied. This strain can produce biosurfactant at a low temperature using petroleum hydrocarbons as the sole carbon source. We aimed to evaluate the feasibility of using the strain and its biosurfactant in removal of oil contamination of the marine intertidal sediment at cold climate.
2. Materials and Methods
2.1. Chemicals and Media
The Zobell Marine 2216E medium used for culture purpose was purchased from Qingdao Haibo Biotechnology Co., Ltd., Qingdao, China. Marine Mineral Culture (MMC) used for culture growth and maintenance had (g/L) NaCl, 24 g; KH2PO4, 2.0 g; Na2HPO4, 3.0 g; NH4NO3, 1.0 g; MgSO4··7H2O, 7.0 g; KCl, 0.7 g and trace elements, including (mg/L) CaCl2 0.02 mg, CuSO4 0.005 mg, FeCl3·6H2O 0.5 mg, ZnSO4·7H2O 0.1 mg, MnCl2·4H2O 0.005 mg, purchased from Damao chemical reagent factory, Tianjin, China. Solvents, such as acetonitrile, n-hexane, and methanol, were high performance liquid chromatography (HPLC) grade.
2.2. Microorganism
Petroleum-degrading strain Planococcus sp. XW-1 was isolated from a petroleum-contaminated area of the Yellow Sea. This strain was selected on the basis of its remarkable capacity to degrade petroleum hydrocarbons and its surface activity. Isolation and identification of XW-1 has been described previously [16].
2.3. Biosurfactant Extraction and Purification
In order to obtain the activated bacterial cultures (OD600 = 1.5), the incubation was performed in 2216E liquid medium at 4 °C, 180 rpm for about 72 h. After incubation, bacteria were separated by centrifuging the medium. Then, bacterial cultures (OD600 = 1.5) were obtained by suspending bacteria with 2216E liquid medium until the optical density reached 1.5. The experiment was carried out in 1-L conical flasks having MMC (400 mL, sterilization at 121 °C for 20 min) supplemented with 1% (v/v) diesel and 1% (v/v) activated bacterial cultures at 4 °C, 180 rpm for about 10 days. After the fermentation broth was centrifuged at 10,000× g for 20 min at 4 °C to obtain the cell-free supernatant, which was adjusted to pH 2 with 6 M HCl and stored at 4 °C overnight. After the acid precipitate, it was extracted with a mixture of chloroform/methanol (2:1 v/v), and the mixture was centrifuged at 8000× g for 20 min at 4 °C. Then, the extracts were obtained by rotary evaporation at 40 °C. The purification of the crude extracts was performed by column chromatography. The crude extracts were dissolved in chloroform and poured in a silica gel (100–120 mesh size). The loaded column was washed with 100 mL chloroform to remove the neutral lipids completely. Then, the mobile phases of different chloroform–methanol ratios were applied to isolate the biosurfactants in sequence: 80 mL:20 mL, 35 mL:65 mL at the speed of 1 mL/min. The purified biosurfactant obtained was combined and dried by rotary evaporation at 40 °C [5,17,18].
2.4. Solubilization Experiments
The solubilization experiments of petroleum hydrocarbons (phenanthrene, pyrene, crude oil, and diesel oil) were carried out in 50-mL glass conical flasks. For the solubilization experiments, overdose phenanthrene, pyrene, crude oil and diesel oil were added into glass conical flasks with 15 mL background solution. The background was composed by biosurfactant solution and distilled water. The concentrations of biosurfactant solutions were 0, 20, 40, 60, 120, 180 and 240 mg/L. After shaking for 48 h, the flasks reached solubilization equilibrium. Undissolved substances were removed by centrifugation at 8000× g for 10 min. A total of 10 mL of aqueous phase was mixed with 10 mL hexane and petroleum ether respectively to extract by vortexing for 2 min. The organic phase was obtained after demulsification by centrifugation at 10,000× g for 20 min and the concentration of different organic matters were measured with UV-Vis and HPLC. The UV wavelengths of crude oil and diesel oil were 258 and 225 nm. The concentrations of phenanthrene and pyrene were quantified by HPLC.
2.5. Elution of Crude Oil
The removal of petroleum was carried out based on the methods described by Luna et al. [19] and Lai et al. [20]. Sand originated from the beach of Dalian, China. In order to study the influence of particle size on elution, the sands used in the experiment were divided into two groups by particle size. The particle size of group one was 0.1 mm–0.45 mm and group two was 2–10 mm. A total of 10 g of the sieved sand was mixed with 2 g crude oil in 50 mL conical flasks, vortexed at high speed until the mixture became muddy. The mixture was aged for 3 days at 45 °C and then weighed.
Next, 25 mL of the biosurfactant solution (60 mg/L ) was added to the flasks which was shaken at 100 rpm for 24 h. After waiting for one hour, the upper water phase was removed, and the sand was dried in an oven for two days at 45 °C before weighing.
2.6. Promoted Biodegradation of Crude Oil
2.6.1. Promoted Biodegradation of Crude Oil in Water
In order to study the effect of biosurfactant on promoting biodegradation of crude oil, biosurfactant and bacteria (OD600 = 1.5) were added into flasks with MMC and crude oil to measure the concentration of residual crude oil after degradation. Three groups of experiments were implemented in 50-mL conical flasks with 1% crude oil as carbon source. Group 1 was designed as the control group with 25 mL MMC. Group 2 was composed of 25 mL MMC and 1% (v/v) isolated bacteria. Group 3 was made of 20 mL MMC, 5 mL biosurfactant solution (60 mg/L) and 1% (v/v) isolated bacteria. Each group was shaken at speeds of 180 rpm at 4 °C. After 21 days, the mixture was extracted with 25 mL of petroleum ether and the organic phase was filtered by anhydrous sodium sulfate to obtain the remaining crude oil. The concentration of crude oil was measured by spectrophotometry at 284 nm after diluting.
2.6.2. Promoted Biodegradation of Crude Oil in the Mixture of Sand and Water
Next, 5 g sand and 0.2 g crude oil was added to the flasks. The mixture was aged for 3 days at 50 °C. The experiment was divided to three groups. The first group was the control group with 25 mL MMC. The second group was made up of 25 mL MMC and 1% isolated bacteria (OD600 = 1.5). The third group was composed of 20 mL MMC, 5 mL biosurfactant (60 mg/L) and 1 % (v/v) isolated bacteria. After 20 days, the mixture was centrifugation at 10,000× g for 20 min to separate aqueous and sand phases. The remaining crude oil was washed out with 25 mL petroleum ether. After diluting, the UV-Vis was used to measure the concentration of crude oil at 284 nm.
3. Results
3.1. The Effect of Biosurfactant on Solubilization of Petroleum Hydrocarbons
Phenanthrene, pyrene, crude oil and diesel oil were selected as the typical pollutants to study the effect on solubilization with the addition of biosurfactant produced by Planococcus sp. XW-1. Figure 1 illustrates the solubility of phenanthrene, pyrene, diesel oil and crude oil in the aqueous phase were under different biosurfactant concentration. As shown in Figure 1, the concentrations of phenanthrene, pyrene, diesel oil and crude oil in the aqueous were 1.12, 0.699, 0.147, and 0.05 mg/L. When the concentration of biosurfactant was 0.24 g/L, the solubility was 5.94, 4.22, 0.89 and 0.35 mg/L, which increased by 430%, 503%, 505% and 600%, respectively. It showed that biosurfactant produced by strain Planococcus sp. XW-1 can significantly promote the solubility of petroleum hydrocarbons. Petroleum hydrocarbons solubilization enhanced with the increase in biosurfactant concentration (as shown in Figure 1). Meanwhile, the solubility increased linearly when the concentration of biosurfactant exceeded the Critical Micelle Concentration (CMC), as shown in Figure 2.

Figure 1.
Effects of biosurfactant on the solubilization of Phen, Pyr, diesel oil and crude oil in aqueous phase.

Figure 2.
The solubility increases linearly of phenanthrene, pyrene, diesel oil and crude oil.
Edwards et al. proposed the concept of weight solubilization ratio to quantitatively describe the solubilization ability of surfactant to organic matters in solution. WSR (weight solubilization ratio) can be calculated by the following formula:
Here, SPAH,mc is the apparent solubility of PAH at a particular total biosurfactant concentration above CMC, mg/L; SPAH,cmc is the apparent solubility of PAHs at CMC, mg/L; Csurf is the concentration of biosurfactant above CMC [21].
The value of WSR is equivalent to the slope of the linear fit between solute concentration and biosurfactant concentration. Table 1 lists the WSR values of phenanthrene, pyrene, diesel oil and crude oil as 0.0234, 0.0165, 0.0027 and 0.0027, respectively. As the results show, WSRphenanthrene > WSRpyrene> WSRdiesel oil >WSRCrude oil, which means the solubilization decreased gradually with the increase in the number of rings and the increase in molecular weight in the presence of biosurfactant [22,23].

Table 1.
Linear regression of solubility enhancement curve.
3.2. Crude Oil Washing Efficiency
In this experiment, the effectiveness of XW-1 biosurfactants for different sand particle size was determined by the kinetic removal experiment of petroleum hydrocarbons adsorbed to sand.
Figure 3 displays the results of the removal experiment of petroleum hydrocarbons. The results show that the biosurfactants could wash out most of the crude oil adsorbed to the sand. The sand showed its natural color with the muddy solution. However, the control groups had no significant changes. Most crude oil was adsorbed to sand and conical flask. The removal rates of crude oil for group one (as shown in Figure 3a) ranged from 17.1% to 87.9%, and in group two (as shown in Figure 3b) the range was from 22.7% to 94.28%.

Figure 3.
(a) Oil washing efficiency of sand with biosurfactant produced by Planococcus sp. XW-1. (1) The end of the sand washing, control group; (2) the end of the sand washing with 50 mL (60 mg/L) biosurfactant. (b) Oil washing efficiency of stone with biosurfactant produced by Planococcus sp. XW-1. (1) The end of the sand washing, control group; (2) the end of the sand washing with 50 mL (60 mg/L) biosurfactant.
3.3. Biosurfactant-Enhanced Bioremediation of Crude Oil
3.3.1. Bioremediation in Sea Water
To evaluate the ability of the biosurfactant produced by Planococcus sp. XW-1 on promotion of the crude oil degradation, experimentation was designed to test the degradation rate of crude oil with and without additional biosurfactant at 4 °C for 20 days. As shown in Figure 4, surface tension of the group one (MMC+bacteria+biosurfactant) decreased to 33.3 mN/m corresponding to the maximum biodegradation rate of crude oil (73%). The surface tension of group two (MMC+bacteria) was 42.3 mN/m, and the biodegradation rate of crude oil was 54%. The surface tension of the control group (MMC) was 54.7 mN/m, and the biodegradation rate was 7% due to the volatilization of low carbon atomic hydrocarbons. The biodegradation rate of crude oil increased from 54% to 73% with the addition of biosurfactant.

Figure 4.
Biodegradation rate of petroleum in water. (1) 25 mL MMC; (2) 25 mL+1% bacteria; (3) 20 mL MMC+ 1% bacteria+ 5 mL biosurfactant solution (60 mg/L).
3.3.2. Bioremediation in Sand Seawater Mixture
Figure 5 illustrated the degradation rate of crude oil after 20 days culturing at 4 °C. The results showed that the biodegradation rates of groups two (MMC+bacteria) and three (MMC+bacteria+biosurfactant) were 65% and 86%, respectively. The biodegradation rate of crude oil increased from 65% to 86% with the addition of the biosurfactant. The results suggested that biosurfactant can promote the degradation of crude oil adsorbed to sand.
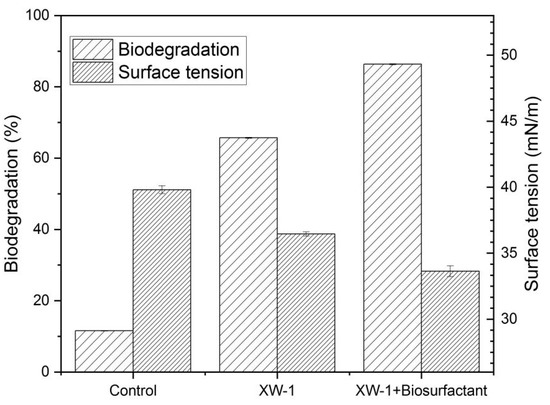
Figure 5.
Biodegradation of petroleum in the mixture of sand and water. (1) 25 mL MMC; (2) 25 mL MMC + 1% bacteria; (3) 20 mL MMC + 1% bacteria + 5 mL biosurfactant solution (60 mg/L).
Comparing the results of the two groups, the crude oil adsorbed on the sand had a higher degradation rate than the crude oil in water. Through two groups of experiments, we found that the biosurfactant produced by Planococcus sp. XW-1 had great degradation effect both in seawater and in the intertidal zone. The biosurfactant produced by this species can effectively promote the degradation of petroleum hydrocarbons. After the addition of biosurfactant, the effect of degradation increased by 20%.
4. Discussion
Bioremediation of petroleum hydrocarbons at a low temperature was limited by high hydrophobicity and poor solubility [23]. The solubility of petroleum hydrocarbons could be increased by biosurfactant [24]. Thus, it was important to explore the influence of XW-1 biosurfactant on the dissolution of petroleum hydrocarbons. The results showed that XW-1 biosurfactant can significantly promote the solubility of phenanthrene, pyrene, crude oil, and diesel oil. Furthermore, the solubilization enhanced with the increase in XW-1 biosurfactant concentration, and the solubility increased linearly when the concentration of biosurfactant exceeded the CMC. The similar results were reported [14,25]. According to Sun et al., the solubility of PAHs increased proportionally after the concentration of biosurfactant produced by P. aeruginosa increased above the CMC [25]. Bezza et al. found that the concentration of biosurfactant under the CMC had a little effect on solubilization of Pyrene but enhanced proportionally if its concentration exceeded the CMC [14]. The micelles are formed when the concentration of surfactant molecules exceed the CMC. Hydrophobic pollutants are wrapped into the surfactant micelle cores, so their solubility increased. With the increase in the biosurfactant concentration, the number of micelles is on the increase to make more insoluble substances be distributed to the micelle cores [25,26].
WSR was used to quantitatively to work out the solubilization ability of the XW-1 biosurfactant to petroleum hydrocarbons in seawater. Our results showed that WSRphenanthrene > WSRpyrene > WSRdiesel oil > WSRCrude oil. When the biosurfactant concentration is constant, the volume of micelles formed by the biosurfactant is constant. Therefore, the solubility of petroleum hydrocarbons would decrease with the increase in their molecular weight. For example, a research study showed that WSR values of phenanthrene, pyrene and diesel oil were 0.0128, 0.0025 and 8 × 10−4, respectively [19]. The bioremediation of oil contaminated areas in a tidal flat area was limited by the low bioavailability of petroleum hydrocarbons, and the high binding force between petroleum hydrocarbons and sand [27]. Therefore, it is difficult to transfer the adsorbed petroleum hydrocarbons from solid phase to aqueous phase [28]. One method to clean oil polluted sediments is the use of biosurfactants to disperse the oil into seawater. The biosurfactant produced by Planococcus sp. XW-1 was used to elute the crude oil which was absorbed by sand. The results showed that the removal rate was up to 94.28%, and the rate was higher when sand particle size increased. Some similar studies also found that the removal efficiency was 84% for 600–850 μm sand, but biosurfactant produced by P. aeruginosa could remove 22% of hexadecane for 300–420 μm sand [29]. Another research study showed that the removal rates were 49 to 54% with biosurfactants produced by P. aeruginosa [30]. The reason is the adsorption capacity of sand is inversely proportional to the particle size. With the decrease in sand particle size, the specific surface area increased, and the absorption capacity increased [30,31].
Bioremediation of oil pollution by adding surfactant-producing strains can improve the remediation effect [32]. Surfactant-producing strain Pseudomonas sp. LP1 can removal 92.34% crude oil and 95.29 diesel oil [33]. Brevibacterium sp. PDM-3 can degrade 93.92% phenanthrene [34]. The cold-adapted strain Planococcus sp. XW-1 can degrade oil and produce surfactant in seawater. The oil degrading rate was up to 54% without additional biosurfactant at 4 °C, and the surface tension decreased to 42.3 mN/m. It showed that strain Planococcus sp. XW-1 is a high-efficiency oil degrader in cold seawater. With additional biosurfactant, the oil degrading rate was increased significantly. After the addition of the XW-1 biosurfactant, the effect of degradation increased by 20% after 20 days. Similar results have been reported by Boqun Liu, through adding surfactant produced by Bacillus licheniformis Y-1 the PAH degradation rate rose up from 40.68% to 45.08 [35]. The biosurfactant produced by Ochrobactrum intermedium CN3 could promote the degradation of petroleum from 40% to 70% [5]. A research study by Pongsak Noparat showed that the oil degradation increased from 32% to 58% with the addition of biosurfactant produced by Sphingobacterium spiritivorum AS43 [36]. The reason for this phenomenon is due to the addition of biosurfactants enhancing the dispersal of petroleum into the aqueous phase to accelerate biodegradation [37].
After an oil spill incident, a small amount of soluble oil and emulsified oil entered the water, and mass of oil pollutant floated on the water surface. Under the effect of winds and tides, oil pollutants tend to persist in intertidal beaches [38]. At present, very little research has been conducted in the field of oil spill clean-up technology in the intertidal zone, especially at low temperatures. Therefore, it is essential to study the effectiveness of the biosurfactant on promotion degradation of crude oil intertidal beaches at low temperatures. Intertidal sediments are periodically covered by seawater. Thus, the experiment of oil degradation was carried in the mixture of sand and water. The results showed that the biodegradation rate of crude oil increased from 65% to 86% with the addition of the biosurfactant. The results suggested that XW-1 biosurfactant can promote the bioremediation of oil contaminated intertidal sediments. Furthermore, the crude oil adsorbed on the sand had a higher degradation rate than the crude oil in seawater. The reason for this phenomenon is due to the application of biosurfactants, which can effectively reduce the adhesion between crude oil and porous media [39].
It has been reported that the strain Planococcus has been isolated from the Polar Regions or marine environments with excellent cold and salt tolerance. It has been widely used in the degradation of industrial wastewater with good industrial application potential in previous research [40,41]. The present study reported Planococcus sp. has the potential to degrade benzene and its derivatives in the environment of high salt and low temperature [42]. Hema et al. [43] found that Planococcus sp. can degrade 80% of different hydrocarbons in crude oil at 30 °C. They also found that biosurfactants produced by Planococcus could promote emulsification and change the hydrophobic property of cells to increase the affinity between the microbial and substrate to enhance their bioavailability. In this study, Planococcus sp. XW-1 could degrade crude oil at low temperatures and its biosurfactant could promote the solubility and degradation of petroleum hydrocarbons in cold environment.
5. Conclusions
The present study reported the potential application of biosurfactant produced by cold-adapted bacteria Planococcus sp. XW-1 in the bioremediation of petroleum contaminated intertidal sediment at low temperatures. The biosurfactant produced by strain XW-1 could effectively facilitate the solubilities of phenanthrene, pyrene, diesel oil, and crude oil. Phenanthrene has the highest WSR value among phenanthrene, pyrene, diesel oil and crude oil, while crude oil has the lowest. Most of the crude oil adsorbed to sand could be washed out by the biosurfactant. Plus, the degradation of crude oil was promoted with the addition of biosurfactant. There was a progressive increase in removal efficiency with the increase in sand particle size. The degradation of crude oil in sea water or intertidal beaches could be promoted by biosurfactants at low temperatures. The strain XW-1 and its extracted biosurfactant could be used to enhance bioremediation of petroleum contaminated marine intertidal sediments at low temperatures.
Author Contributions
Conceptualization, P.G., W.L. and J.-G.L.; methodology, P.G., B.-X.C., M.-X.Z., J.Z. and W.-W.X.; validation, W.-W.X.; formal analysis, W.-W.X.; investigation, W.-W.X., P.G. and D.-N.W.; resources, P.G. and J.-G.L.; data curation, W.-W.X.; writing—original draft preparation, W.-W.X.; writing—review and editing, J.Z., P.G., D.-N.W., S.T.; supervision, P.G.; project administration, P.G.; funding acquisition, P.G., W.L. and J.-G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Fundamental Research Funds for Central Universities of China (3132019304; 3132017097; 3132018177), Liaoning Provincial Natural Science Foundation of China (2019-ZD-0163) and National Natural Science Foundation of China (52171344).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are in agreement with the MDPI Research Data Policies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almeida, D.G.; Da Silva, R.C.S.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.; Mandal, A.B.; Gnanamani, A. Microbial biosurfactant mediated removal and/or solubilization of crude oil contamination from soil and aqueous phase: An approach with Bacillus licheniformis MTCC 5514. Int. Biodeter. Biodegr. 2014, 94, 24–30. [Google Scholar] [CrossRef]
- Margesin, R. Potential of cold-adapted microorganisms for bioremediation of oil-polluted Alpine soils. Int. Biodeter. Biodegr. 2000, 46, 3–10. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, R.; Pandey, L.M. Crude oil degradation and biosurfactant production abilities of isolated Agrobacterium fabrum SLAJ731. Biocatal. Agr. Biotech. 2019, 21, 101322. [Google Scholar] [CrossRef]
- Bezza, F.A.; Beukes, M.; Chirwa, E.M.N. Application of biosurfactant produced by Ochrobactrum intermedium CN3 for enhancing petroleum sludge bioremediation. Process Biochem. 2015, 50, 1911–1922. [Google Scholar] [CrossRef]
- Chandankere, R.; Yao, J.; Cai, M.; Masakorala, K.; Jain, A.K.; Choi, M.M.F. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Chia-Wei, P.; Bakar, N.F.A.; Hamzah, A. A comparative study on biosurfactant activity of crude oil-degrading bacteria and its correlation to total petroleum hydrocarbon degradation. J. Bioremed. 2013, 17, 240–251. [Google Scholar]
- Pereira, M.G.; Mudge, S.M. Cleaning oiled shores: Laboratory experiments testing the potential use of vegetable oil biodiesels. J. Chemosphere 2004, 54, 297–304. [Google Scholar] [CrossRef]
- Walker, J.D.; Colwell, R.R. Microbial degradation of model petroleum at low temperatures. Micro. Ecol. 1974, 1, 63–95. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Bonaunct, K. Biodegradation of petrolcum hydrocarbons in seawater at low temperatures (0–5 °C) and bacterial communities associated with degradation. J. Biodegradation. 2006, 17, 71–82. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, T.A.; Silva, I.A.; Converti, A.; Sarubboa, L.A. Production and formulation of a new low-cost biosurfactant to remediate oil-contaminated seawater. J. Biotech. 2019, 295, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sponza, D.T.; Gok, O. Effect of rhamnolipid on the aerobic removal of polyaromatic hydrocarbons (PAHs) and COD components from petrochemical wastewater. Bioresour. Technol. 2010, 101, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Bezza, F.A.; Chirwa, E.M.N. Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. J. Hazard Mater. 2017, 321, 218–227. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. The role of lipopeptide biosurfactant on microbial remediation of aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil. Chem. Eng. 2017, 309, 563–576. [Google Scholar] [CrossRef]
- Guo, P.; Xu, W.W.; Tang, S.; Wei, D.N.; Zhang, M.X.; Cao, B.X.; Li, W.; Lin, J.G. Isolation and Characterization of a Biosurfactant Producing Strain Planococcus sp. XW-1 from the Cold Marine Environment. Int. J. Environ. Res. Public Health 2022, 19, 782. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, H.; Kwon, B.O.; Khim, J.S.; Yim, U.H.; Kim, B.S.; Kim, J.J. Biosurfactant-assisted bioremediation of crude oil by indigenous bacteria isolated from Taean beach sediment. Environ. Pollut. 2018, 241, 254–264. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Kalita, M.C.; Deka, S. Application of biosurfactant for enhancement of bioremediation process of crude oil contaminated soil. Int. Biodeter. Biodegr. 2018, 129, 50–60. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Albuquerque, C.D.C.; Sarubbo, L.A.; Campos-Takaki, G.M. Economic optimized medium for tensio-ctive agent production by Candida Sphaerica UCP0995 and application in the removal of hydrophobic contaminant from sand. Int. J. Mol. Sci. 2011, 12, 2463–2476. [Google Scholar] [CrossRef]
- Lai, C.C.; Huang, Y.C.; Wei, Y.H.; Chang, J.S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazar. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef]
- Edwards, D.A.; Luithy, R.G.; Liu, Z. Solubilization of polycyclic promatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 1991, 25, 127–133. [Google Scholar] [CrossRef]
- Li, Y.; Mao, H.; Gong, Z.; Sun, Y.; Zhou, C.; Fang, Z. Solubilization of several surfactants on diesel and PAHs. Res. Environ. Sci. 2011, 24, 775–780. [Google Scholar]
- Zenati, B.; Chebbi, A.; Badis, A.; Eddouaouda, K.; Boutoumi, H.; El Hattab, M.; Hentati, D.A.; Chelbi, M.; Sayadi, S.; Chamkha, M.; et al. A non-toxic microbial surfactant from Marinobacter hydrocarbonoclasticus SdK644 for crude oil solubilization enhancement. Ecotoxicol. Environ. Saf. 2018, 154, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, L. Controlling microbiological interfacial behaviors of hydrophobic organic compounds by surfactants in biodegradation process. Front. Env. Sci. Eng. 2014, 8, 305–315. [Google Scholar] [CrossRef]
- Sun, S.L.; Wang, Y.; Zhang, T.; Wei, J.; Wu, H.; Wei, C.; Qiu, G.; Li, F. A biosurfactant-producing Pseudomonas aeruginos S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019, 281, 4281–4428. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, J.; Lou, L. Solubilization properties of polycyclic aromatic hydrocarbons by saponin, a plant-derived biosurfactant. Environ. Pollut. 2011, 159, 1198–1204. [Google Scholar] [CrossRef]
- Li, X.J.; Li, P.J.; Lin, X.; Ver Khonzina, V.A. About the conception of “aging” for organic contaminants in soil. Chin. J. Appl. Ecol. 2007, 18, 1891–1896. [Google Scholar]
- Lee, J.; Han, I.; Kang, B.R.; Kim, S.H.; Sul, W.J.; Lee, T.K. Degradation of crude oil in a contaminated tidal flat area and the resilience of bacterial community. Mar. Pollut. Bull. 2016, 114, 296–301. [Google Scholar] [CrossRef]
- Bai, G.; Brusseau, M.L.; Miller, R.M. Biosurfactant-enhanced removal of residual hydrocarbon from soil. J. Contam. Hydrol. 1997, 25, 157–170. [Google Scholar] [CrossRef]
- Bordoloi, N.K.; Konwar, B.K. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf. B Biointerfaces 2008, 63, 73–82. [Google Scholar] [CrossRef]
- Xiao, Y.; Lu, Q.; Cheng, H.; Zhu, X.; Tang, H. Surface characteristics of sediment and its effect on phosphorus adsorption. Int. J. Sediment Res. 2011, 6, 64–68. [Google Scholar]
- Pacwa-Plociniczak, M.; Plaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Obayori, O.S.; Ilori, M.O.; Adebusoye, S.A.; Oyetibo, G.O.; Omotayo, A.E.; Amund, O.O. Degradation of hydrocarbons and biosurfactant production by Pseudomonas sp. strain LP1. World J. Microbiol. Biotechnol. 2009, 25, 1615–1623. [Google Scholar] [CrossRef]
- Reddy, M.S.; Naresh, B.; Leela, T.; Prashanthi, M.; Madhusudhan, N.C.; Dhanasri, G.; Devi, P. Biodegradation of phenanthrene with biosurfactant production by a new strain of Brevibacillus sp. Bioresour. Technol. 2010, 101, 7980–7983. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Ju, M. Purification and characterization of biosurfactant produced by Bacillus licheniformis Y-1 and its application in remediation of petroleum contaminated soil. Mar. Pollut. Bull. 2016, 107, 46–51. [Google Scholar] [CrossRef]
- Noparat, P.; Maneerat, S.; Saimmai, A. Application of biosurfactant from Sphingobacterium spiritivorum AS43 in the biodegradation of used lubricating Oil. Appl. Biochem. Biotech. 2014, 172, 3949–3963. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, A.H.; Tanveer, S.; Alia, S.; Anees, M.; Sultan, A.; Iqbal, M.; Yousaf, S. Role of nutrients in bacterial biosurfactant production and effect of biosurfactant production on petroleum hydrocarbon biodegradation. Ecol. Engin. 2017, 104, 158–164. [Google Scholar] [CrossRef]
- Pemmaraju, S.C.; Sharma, D.; Singh, N.; Panwar, R.; Cameotra, S.S.; Pruthi, V. Production of microbial surfactants from oily sludge-contaminated soil by Bacillus subtilis DSVP23. Appl. Biochem. Biotech. 2012, 167, 1119–1131. [Google Scholar] [CrossRef]
- Wu, J. Study on Adsorption and Release Process of Petroleum Pollutants on Sandy Beach; Ocean University of China: Qingdao, China, 2006. [Google Scholar]
- Seo, S.; Mastiania, M.; Mosavati, B.; Peters, D.K.; Mandin, P.; Kim, M. Performance evaluation of environmentally benign nonionic biosurfactant for enhanced oil recovery. Fuel 2018, 234, 48–55. [Google Scholar] [CrossRef]
- Choi, J.H.; Im, W.T.; Liu, Q.M.; Yoo, J.S.; Shin, J.H.; Rhee, S.-K.; Roh, D.H. Planococcus donghaensis sp. nov. a starch-degrading bacterium isolated from the East Sea, South Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 2645–2650. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Liu, Y.H.; Luo, N.; Zhang, X.Y.; Luan, T.G.; Hu, J.M.; Wang, P.C.; Chen, M.J.; Lu, J.Q. Biodegradation of benzene and its derivatives by a psychrotolerant and moderately haloalkaliphilic Planococcus sp. strain ZD22. Res. Microbiol. 2006, 157, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Hema, T.; Kiran, G.S.; Sajayyan, A.; Ravendran, A.; Rajb, G.G.; Selvinc, J. Response surface optimization of a glycolipid biosurfactant produced by a sponge associated marine bacterium Planococcus sp. MMD26. Biocat. Agric. Biotec. 2019, 18, 101071. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).