Abstract
To explore the feasibility of using beak microstructure information to estimate the age of Sthenoteuthis oualaniensis, the microstructures of the upper beaks of individual squid were applied in this work to analyze the ages and growth patterns of squid caught from February–May 2019 and from October–December 2020 in the northwest Indian Ocean. The results indicated that the squid samples in the two years were no older than 9 months, and the samples in 2019 were autumn population and 2020 were spring population. The linear growth model of the autumn population (2019) was the best model for describing the relationship between age and ML, while the power model of the spring population (2020) was the best for describing the relationship between age and ML. The maximum instantaneous growth rate (IGR) and absolute daily growth rate (AGR) values of the spring population were 0.24%/d and 1.09 mm/d, respectively, occurring in squid between 200 and 220 days of age. The maximum IGR and AGR values of the autumn population were 0.69%/d and 1.73 mm/d, respectively, occurring in squid between 200 and 240 days of age. The period from 141–260 days (5–8 months) was considered to correspond to the subadult stage in the whole life history of S. oualaniensis in the Northwest Indian Ocean. The beak microstructure information can be effectively applied to estimate the age of S. oualaniensis individuals.
1. Introduction
Sthenoteuthis oualaniensis, also known as the “Southern squid”, belongs to Cephalopoda, Ommastrephidae, and Sthenoteuthis. This squid is a warm-water, oceanic species that is widely distributed in the equatorial and subtropical waters of the Indian and Pacific Oceans [1]. S. oualaniensis has the characteristics of a rapid swimming speed, strong feeding ability, and a high reproductive capacity. Optimal fishing grounds targeting S. oualaniensis are located in marine areas with high sea surface salinity (SSS > 35) values, high sea surface temperatures (SST 26–28 °C), and unique current conditions [1,2]. The northwest Indian Ocean is a globally famous monsoon region. Under the action of the southwest and northeast monsoons, the northwest Indian Ocean produces a violent upwelling effect [3,4], thus providing superior environmental conditions for the growth of S. oualaniensis. Therefore, S. oualaniensis resources are extremely abundant in the northwest Indian Ocean. S. oualaniensis is an important target of cephalopod fisheries in China, which produce an annual catch of approximately 1.3–2 million tons [5]. Studies have shown that the total biomass of squid in the northwest Indian Ocean is approximately 2–10 million tons [6], corresponding to a high economic development value.
The S. oualaniensis population structure is complex, and the species is widely distributed geographically; according to its various intraspecific morphologies, the species can be divided into different spawning groups and body groups [7,8], in which spawning groups include spring, summer, and autumn groups, and body size groups include large, medium and small groups. As a typical opportunistic species, S. oualaniensis feeds widely in marine ecosystems, eating numerous species, including small fish and crustaceans; the species even exhibits obvious cannibalistic behavior [8,9]. S. oualaniensis is an important part of the oceanic food web and is commonly found in the stomachs of a variety of predators, including seabirds [10], large fishes [11], and marine mammals [12]. This squid species has a fast growth rate and a short life cycle (0.5–1 year) [3], making it highly susceptible to changes in environmental conditions [13].
As it serves as the feeding organ for squid, the squid beak consists of hard, corrosion-resistant tissues with a stable morphological structure. The beak structure exhibits a certain synchronization with the growth of the squid and can store rich life history information [3,7]. Compared to the statoliths of squid, beaks are larger and thus easier to extract and measure [13,14,15]; thus, beaks have gradually become an important material in cephalopod fishery ecology and biology research and have been widely used in cephalopod taxonomy investigations [2]. The results of past studies have revealed that environmental changes could affect the population growth characteristics and genetic changes of this species, which could lead to differential morphologies in S. oualaniensis beaks [16]. The upper beak has been considered a useful tool for estimating age and describing the growth patterns of cephalopods [17]. Consistent with the growth patterns of statoliths, beak growth can be measured as one daily concentric increment representing one day at the larval stage; this makes it easy to determine the ages of squid [18].
Few studies have been conducted in recent years on S. oualaniensis populations in the northwest Indian Ocean near China. In this paper, we estimated the ages of S. oualaniensis specimens captured in the northwest Indian Ocean in 2019 and 2020 using beak information, determined their incubation population size, and analyzed their growth patterns. The squid growth patterns were evaluated by analyzing the relationships among mantle length (ML), body weight (BW), squid age, and ML and BW growth rates. As the basis for exploring fishery biology, ecology, and life history information, it is essential to deeply understand the age and growth patterns of S. oualaniensis in the northwest Indian Ocean. This study provides important information regarding the biology of the S. oualaniensis populations in the northwest Indian Ocean and provides a reference for the actual production and resource management of this species in related fields.
2. Materials and Methods
2.1. Sampling
A total of 1896 squid samples were randomly caught and collected in our survey from Chinese lighting falling-net vessels along the Northwest Indian Ocean (13°05′–19°45′ N, 61°06′–67°50′ E) (Table 1) from February to May 2019 and October 2020 (Figure 1). All squid samples were immediately frozen on the ship and dissected in the laboratory, where the fishery biological experiments and extraction of the complete beaks were also conducted. The ages of the squid specimens were determined by analyzing the beak microstructure, and 677 (Table 1) effective age data were finally obtained in this study.

Table 1.
Basic information of female and male S. oualaniensis specimens collected in different years in the northwest Indian Ocean.
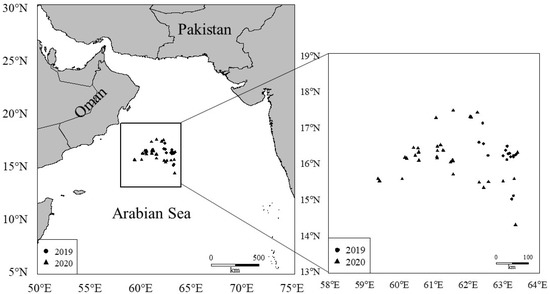
Figure 1.
Sample localities of S. oualaniensis in the northwest Indian Ocean.
2.2. Fishery Biology Measurements
The fishery biological data were measured after thawing the samples; the ML and BW were measured to accuracies of 1 mm and 1 g, respectively, and the sex and maturity stage were identified through visual examinations [19]. According to the original standard gonadal morphological characteristics, the samples of different sexes were divided into five maturity stages (immature: I and II; maturing: III; and mature: IV and V) [15,20]. Stage I. The nidamental gland of female is transparent and filament shaped, the accessory glands were not formed, and the eggs did not appear. The spermatophoric gland of male is white and fibrous, with thin and empty spermatophores. Stage Ⅱ. The nidamental glands of female was granular surface and white, and the accessory glands is not obvious. The spermatophoric gland of male is cream-colored with a few white microplates in the spermatophore. Stage III. the whole accessory gland takes up 1/3 of the mantle cavity of female, the eggs are orange, and the nidamental gland is white. The spermatophore of male is full of spermatozoa, and the size of the spermatophoric gland is increased and white. Stage IV. The accessory gland of female shrinks, there are a few eggs, and the nidamental gland shrinks and becomes light pink. The spermatophoric gland of male becomes soft and semitransparent, there are a few spermatophores. Stage V. The nidamental glands and spermatophoric gland of female and male are obviously undergoing atrophy, and as a rule, die at the end of this stage [15,21]. The upper and lower beaks were removed from the buccal mass of the head and then cleaned and preserved with a 75% alcohol solution.
2.3. Beak Processing and Aging
We chose the upper beak as the subject of study to reduce age estimation errors because the lower beak is more susceptible to erosion during feeding behaviors than the upper beak [22]. Whole-age increments appear on the focal plane of the beak hood. We chose the rostrum sagittal section (RSS) of the beak, as this region exhibits many dark, periodic longitudinal bands that can be easily used to interpret the age of the squid. After cleaning with water, the RSS of the upper beak was cut into two unequal pieces with a cutter bar, and the larger part was immobilized and hardened in a plastic mold with gradually caking epoxy [22]. The focal plane sample was ground on a sander with coarse sandpapers of different sizes (grit: 120, 600, 1200, and 2000) and then polished with an alumina solution on smooth sandpaper (2400) to remove scratches until the age cycle increments were clearly visible on the surface of the sample.
Different parts of the RSS were observed using an Olympus microscope and photographed by a charge-coupled device (CCD) at different magnifications (×10, ×20, ×40, ×100, ×200, ×400). Then, an intact picture was consolidated using Photoshop CS 24.0 for the subsequent age estimation. To reduce bias in the age estimation process, the age information of a sample was considered to reflect the effective squid age when the difference between the average ages identified by two readers was less than 10% [20]. The age and growth patterns of several cephalopods reported in previous studies can be reliably assessed by the daily increments on the beak by comparing statolith increments observed under artificial feeding conditions [15,18,20]. Based on the beak RSS daily increments, we decided to back-calculate the hatching date and explore the growth patterns of the S. oualaniensis specimens living in the Northwest Indian Ocean.
2.4. Population Structure Identification
According to the verified daily deposition of growth increments in Ommastrephidae squid [20,23], the number of increments on an analyzed beak can be said to be equal to the age of that S. oualaniensis individual; thus, the hatching date could be back-calculated from the capture date and age information.
2.5. Data Analysis
Analysis of covariance (ANCOVA) was used to evaluate whether differences existed in the ML and BW growth patterns between females and males and among different hatching groups. We established and selected the following optimal growth models to quantify the relationships among the S. oualaniensis ML, BW, and age data:
Linear function [24]:
L = a x + b
Power function [25]:
L = a x b
Exponential function [26]:
L = a e bx
Logarithmic function [27]:
where L is the ML (in mm) at age, x is the squid age (in days) for the ML growth model establishment, and L is the BW (in g) at age, x is the squid age (in days) for BW growth models establishment, respectively. The least Akaike information criterion (AIC) was used to compare the four types of models and to select the optimal model [28,29]. The absolute daily growth rates (AGRs, in mm/d or g/d) and instantaneous growth rates (IGRs, in %/d) of the ML and BW were estimated at each 30-day interval. The IGRs and AGRs were calculated using the following functions [28]:
where R1 and R2 are the ML or BW means at the start (t1) and end (t2) of the time interval, respectively.
L =
a ln(x) + b
AGR = (R2 − R1)/(t2 − t1)
IGR = [ln(R2) − ln(R1)]/(t2 − t1)
All statistical analyses were conducted in RStudio 4.0.3 and Excel 2019, and the pictures were analyzed in Photoshop 2020.3.
3. Results
3.1. ML and BW Structures
The ANCOVA results showed that in 2019 (F = 16.547, p = 0.000 < 0.05) and 2020 (F = 2579.619, p = 0.000 < 0.05), significant differences were found in the ML between the sexes; thus, the ML composition in S. oualaniensis could be divided into males and females. The ML ranges differed between the S. oualaniensis samples collected in the northwest Indian Ocean in the different years of study, and 50 mm was used as the interval to divide the ML groups. In 2019, the female ML ranged from 129–347 mm (mean ML: 213.9 ± 24.10 mm), with the majority (71.08%) of samples ranging from 121–220 mm (Figure 2a). The male MLs ranged from 138–273 mm (mean ML: 191.7 ± 13.78 mm), with the majority (89.47%) of samples within the 121–220 mm range (Figure 2b). In 2020, the female MLs ranged from 284–570 mm (mean ML: 459.8 ± 26.36 mm), with the majority (90.42%) of samples ranging from 370–530 mm (Figure 2a). The male ML ranged from 94–235 mm (mean ML: 185.9 ± 21.16 mm), with the majority (96.95%) of samples ranging from 121–220 mm (Figure 2b).
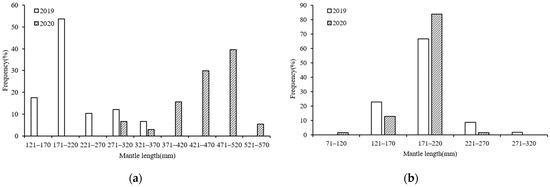
Figure 2.
ML compositions of S. oualaniensis samples in different years: (a) female; (b) male.
The ANCOVA results also showed that in both 2019 (F = 26.271, p = 0.000 < 0.05) and 2020 (F = 814.026, p = 0.000 < 0.05), significant BW differences existed between the sexes; thus, the BW composition of S. oualaniensis could also be divided into male and female groups. The BW ranges differed between the S. oualaniensis samples collected in the northwest Indian Ocean in the different years of study; we have chosen the different but appropriate BW interval for each year (200 g female in 2019, 1000 g for female in 2020, and 60 g for male in 2019 and 2020). In 2019, the female BWs ranged from 76–1224 g (mean BW: 384.1 ± 15.765 g), with the majority (76.51%) of samples ranging from 70–470 g (Figure 3a). The male BWs ranged from 80–632 g (mean BW: 233.1 ± 9.437 g) in 2019, with the majority (78.07%) of samples being within the 160–280 g range (Figure 3c). In 2020, the female BWs ranged from 640–6151 g (mean BW: 3465.2 ± 30.442 g), with the majority (86.83%) of samples ranging from 2271–5270 g (Figure 3b). The 2020 male BWs ranged from 100–375 g (mean BW: 189.7 ± 13.124 g), and the majority (94.65%) of the samples fell within the 100–280 g range (Figure 3c).
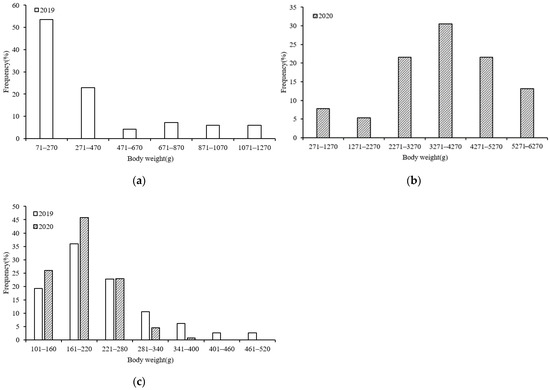
Figure 3.
BW compositions of S. oualaniensis samples in different years: (a,b) female; (c) male.
3.2. Microstructure of the RSS
The microstructural characteristics of the S. oualaniensis RSS are displayed in Figure 4a; the RSS is mainly composed of the hood region and lateral wall region. With dark and light bands, the relatively wide RSS incremental intervals can be clearly seen on the hood region, while narrower intervals are displayed in the lateral wall region (Figure 4b). A clear dividing line can be observed between the hood region and lateral wall region, and the growth rings are bounded by an inner axis with the upper and lower parts forming a “<” shape. The longitudinal increments on the planar view of the hood region can be more clearly observed than those on the lateral wall region, and differences in the incremental width, pattern, and direction can also be seen between the hood region and lateral wall region (Figure 4c,d). Compared to that observed in the 2020 group (Figure 4f), in the 2019 group (Figure 4e), the spacing between each growth ring was relatively narrow, the pigment deposition was relatively shallow, the growth ring bands were relatively dark, and the beak ends were smooth with relatively light wear.
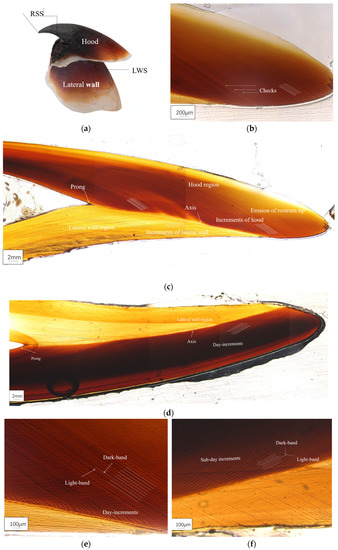
Figure 4.
Upper beak microstructure of S. oualaniensis: (a) upper beak rostrum; (b) growth stripe and marker stripe on the hood; (c) upper beak RSS microstructure in 2019; (d) upper beak RSS microstructure in 2020; (e) light and dark bands and growth stripe in 2019; and (f) light and dark bands and growth whorl stripe in 2020.
3.3. Age Structure and Maturity
The daily age composition of the S. oualaniensis samples collected in the northwest Indian Ocean differed between the two years of study (Figure 5), and 30 days was used as the interval to divide the daily age groups. In 2019, the female ages ranged from 97–263 days, with the majority (78.31%) of samples being 141–230 days old (Figure 5a). The male ages ranged from 131–253 days in 2019, with the majority (95.61%) of samples ranging in age from 141–230 days (Figure 5b). In 2020, the female ages ranged from 148–267 days, with the majority (73.65%) of samples being 201–260 days old (Figure 5a). The male ages in this year ranged from 133–221 days, with the majority (93.12%) being 141–200 days old (Figure 5b).
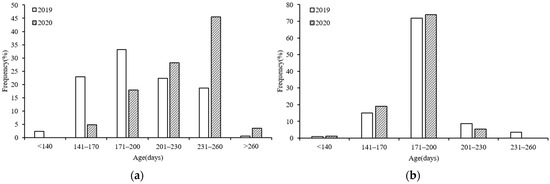
Figure 5.
Age compositions of S. oualaniensis samples in different years: (a) female; (b) male.
Differences were also observed in the gonad maturity compositions between the two years of study. In 2019, the majority (92.17%) of female samples were in stages I and II (Figure 6a), while the majority (81.58%) of male samples were in stages II and III (Figure 6b). In 2020, the majority (80.24%) of female samples were in stages III and IV (Figure 6a), the majority (69.47%) of male samples were in stages III and IV, and the proportions of samples in stages II, III and IV were evenly distributed (Figure 6b).
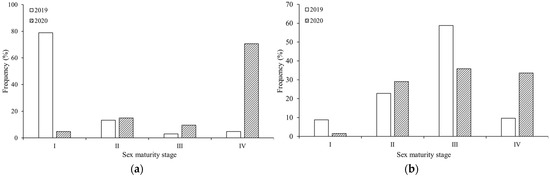
Figure 6.
Maturity stage compositions of the two S. oualaniensis sexes in the northwest Indian Ocean in the two years of study: (a) female; (b) male.
3.4. Hatching Date and Group
Combining the age date and catching date information, the hatching dates of the squid samples were estimated by catching date minus age date. The hatching dates of the S. oualaniensis samples collected in 2019 ranged from May 2018 to January 2019, with the majority (70%) being distributed from September to November 2018. The hatching dates of the S. oualaniensis samples collected in 2020 ranged from March to July, with the majority (85%) being distributed from April to May 2020. According to the calculation results of the hatching dates of the S. oualaniensis samples collected in 2019 and 2020, we concluded that both spring and autumn hatching groups were included in our samples. The 2019 samples were mainly composed of the autumn population, while the 2020 samples were mostly composed of the spring population (Figure 7).
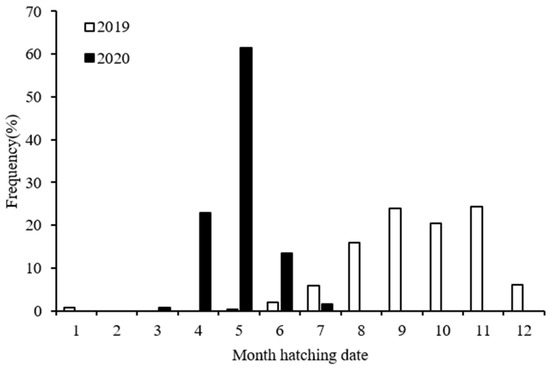
Figure 7.
Hatching date frequency distributions of S. oualaniensis samples in the two years of the study.
3.5. Growth Patterns
The ANCOVA results showed that there was no significant difference between the sexes in the relationship between ML and age (F = 0.316, p = 0.575 > 0.05), but there was a significant difference between the different hatching groups (2019 and 2020) (F = 12.876, p = 0.034 > 0.05); thus, males and females were no longer differentiated in the subsequent growth relationship analyses.
Based on the lowest derived AIC value (Table 2), the age-ML relationship derived for the autumn population (2019) was best described by the linear growth model, while that for the spring population (2020) was best described by the power growth model (Figure 8).

Table 2.
Parameters of the linear, power, exponential, and logarithmic growth models fitted to the ML-age relationships of the S. oualaniensis samples.
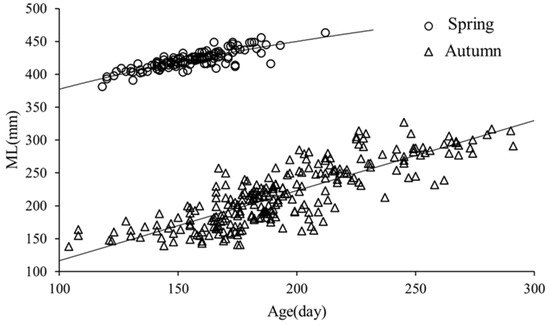
Figure 8.
ML-age relationships of S. oualaniensis samples.
ML-age growth model for the autumn population (2019):
ML = 1.0665 Age + 9.9416 (R2 = 0.6813, n = 258)
ML-age growth model for the spring population (2020):
ML = 117.19 Age 0.2539 (R2 = 0.6960, n = 258)
3.6. Growth Rates
In this study, we found that both the spring (2020) and autumn (2019) S. oualaniensis populations grew rapidly. For the spring population, the lowest IGR and AGR values were 0.13%/d and 0.54 mm/d, respectively, for specimens within the 169–180 days age frame. The maximum IGR and AGR values were 0.24%/d and 1.09 mm/d, respectively, for squid aged within 200–220 days (Figure 9a,b). Regarding the autumn population, the lowest IGR and AGR values were 0.13%/d and 0.35 mm/d, respectively, for samples within 220–260 days old, and the maximum IGR and AGR values were 0.69%/d and 1.73 mm/d, respectively, for squid between 200 and 240 days old (Figure 9a,b).
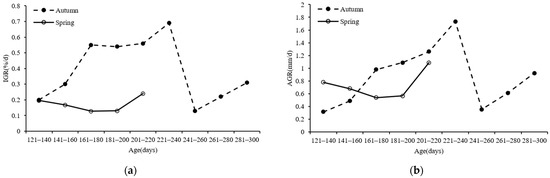
Figure 9.
Relationships between the ML growth rate and age of S. oualaniensis samples: (a) IGR; (b) AGR.
4. Discussion
4.1. ML and BW Distributions
The results showed that compositional differences existed in the ML and BW data between the two years of study, with the largest differences observed among females. Both the ML range and majority ML group of the S. oualaniensis samples collected in 2020 were heavier than the corresponding values of the specimens collected in 2019. According to the ML size and morphological structure at sexual maturity, S. oualaniensis populations can be divided into four groups: large-squid populations (with ML ranging from 400–500 mm), which are mainly distributed in the Indian Ocean and Arabian Sea [30]; medium-squid groups (with ML ranging from 190~250 mm), which are widely distributed [15]; small groups (with ML ranging from 120~160 mm), which are also found in the Indian Ocean [31]; and microgroups (with ML ranging from 90~120 mm), which occur in the eastern Pacific Ocean near the equator [3]. The analysis performed herein showed that the samples collected in 2019 were composed of medium- and small-squid populations, while the individuals sampled in 2020 were composed of large-, medium- and small-squid populations. The BW composition differences observed in the female S. oualaniensis samples between the two years were particularly significant; the samples collected in 2020 were significantly larger than those obtained in 2019, and this finding may have been related to the different populations sampled. The growth of cephalopods is highly affected by temperature. When the SST is too high, the energy consumption rate is higher than the energy intake rate, and both ML and BW growth are inhibited [32,33]. When temperatures are within a suitable range, squid grow larger at lower temperatures and remain smaller at higher temperatures. For example, Loliolus noctiluca [32], Sepioteuthis lessonianas [34], and Loligo opalescens [35], all of which live in regions with cool SSTs, are all relatively large. The Dosidicus gigas individuals [36] affected by El Niño off the coast of California were found to be relatively small. The growth stages of the squid sampled in 2019 in this work were found to be affected by the positive phase of the Indian Ocean dipole, causing the SST in the feeding field to be relatively high; this may have led to the sizes of the squid sampled in 2019 being smaller than those of the squid sampled in 2020. Studies have shown that differences exist in the distributions of S. oualaniensis populations among different sampling areas, and the individual sizes of squid increase with latitude from low latitudes to high latitudes [3,9]. The results of a morphological analysis conducted on an S. oualaniensis population in the northwestern Indian Ocean showed that the large form commonly inhabits north of 18° N, the medium form commonly inhabits between 12° N and 18° N, and the small form commonly inhabits south of 12° N; in addition, the distributions of these three kinds of populations overlapped [3,9]. Additionally, according to our preliminary judgment of the S. oualaniensis population sampled in the South China Sea, small populations exist in the marine area south of 11° N, while medium-sized populations exist in the marine area north of 11° N [31]. In this study, the sampling latitude was 14°–16° N, which further confirmed the above results. These findings further confirmed the latitudinal characteristics of the squid population distribution in the Indian Ocean.
4.2. Beak Microstructure
Similar to statoliths and gladius, beaks are a type of hard tissue in squid, and the growth grain structures of beaks exhibit obvious regularity. The beak grows like statoliths, maintaining a “daily ring” structure [37,38]. Our grinding observations of S. oualaniensis beaks from the northwest Indian Ocean showed that the beak striations grew in a light-dark alternation pattern. These growth rings are displayed in the hood region and lateral wall region, from which the age of the squid is often interpreted; this methodology has also been supported for other cephalopods of the Ommastrephidae family [29,38]. In a past study, researchers observed different degrees of pigment deposition in the beaks of S. oualaniensis individuals [39]. By comparing the growth patterns of the squid beaks between the two years of study, in this work, we found that the pigment deposition in 2019 was lighter than that in 2020, and the dark bands of the 2019 samples were also lighter than those of the 2020 samples. Studies have shown that the pigmentation of squid in the northwest Indian Ocean is positively correlated with gonad development [40].
In addition, the rounded tips of the squid beaks were found to exhibit few notches and wear in the samples; this may have been due to changes in the feeding structure of S. oualaniensis alongside individual growth. During the growth process of S. oualaniensis, its feeding habits change from primarily involving young crustaceans to cephalopods; the beak requires a heightened ability to tear food, and the frequency of use increases; thus, the degree of beak-end wear is higher in large populations than in small ones [15,41,42]. More marks were observed in the RSS of the 2019 samples than in the 2020 samples, and this may reflect events in the life histories of the squid (such as spawning and migration) or changes in their external environment (such as water temperature fluctuations, storms or predator attacks) [18,23,43]. In 2019, an El Niño event occurred, accompanied by frequent fluctuations in hydrological conditions and an increased number of markers on the squid RSS; this finding further confirms that environmental changes can cause growth ring distortions [43] and provides a scientific basis for studying the life histories of squid using squid beak information.
4.3. Age Structure and Hatching Group
By combining the ages of the squid determined from their beak structures with the catch date, the hatching dates of the S. oualaniensis samples could be calculated. We found that the age range of our samples was 97–263 days (entirely less than one year), similar to the life cycles of other cephalopods [22,28]. In 2019, the majority of females were mainly distributed from 141 to 230 days of age (life span: 5–8 months), while males were mainly distributed from 201 to 260 days of age (life span: 7–9 months). In 2020, the majority of females were mainly between 121 and 210 days old (life span: 4–7 months), and males mainly ranged from 141 to 200 days old (life span: 5–7 months). S. oualaniensis usually live in regions with SSTs of 25–28 °C, and their year-round spawning characteristics may be related to their living in regions where SSTs are above 20 °C year-round. Moreover, the superposition of different spawning groups leads to the complex population structure of S. oualaniensis [44].
The results of this work showed that the S. oualaniensis samples distributed in the northwest Indian Ocean can be divided into three spawning populations: spring, summer, and autumn; in addition, the collected samples were from the spring and autumn spawning populations [45,46]. The spring population typically hatches from March to May, although the hatching dates of the spring population sampled in this study occurred approximately one month later; this may have been related to the climate differences between the two years of study. In 2019, the Indian Ocean dipole was in a very highly positive phase. At this time, the SST in the northwest Indian Ocean increased abnormally to values beyond the optimal temperature range for S. oualaniensis, thus inhibiting the individual growth of the squid; thus, the growth rates of the squid were slow, and their hatching dates were late in this year [34,47,48]. As a result, squid growth rates slowed, and hatching dates were delayed. In this study, the hatching dates of the S. oualaniensis specimens collected in 2019 and 2020 spanned the whole year, although the samples collected in 2019 mostly hatched from September to November 2018, and the samples collected in 2020 mostly hatched from April to June 2020. Therefore, we believe that the samples collected in 2019 were members of the autumn population, while the samples collected in 2020 were members of the spring population.
In this study, the ML of the samples collected in 2019 mostly ranged between 120 and 300 mm, and the squid were approximately 200 days old, so we discerned that these samples were mostly members of medium-small groups. However, the MLs of the samples collected in 2020 were quite different, with the female-dominant MLs varying between 370 and 530 mm and the male-dominant MLs ranging from 121~220 mm. The average age of these squid was approximately 230 days, so we judged that there were more individuals from large-medium groups in the 2020 catch. The analyzed samples were collected from the same lighting falling net vessel in the two consecutive years of study and covered all individual squid sizes. Compared to squid jigging, lighting falling net operations have lower sample size selectivity and stronger randomness [29], so the samples collected herein more scientifically and accurately reflect the population structure and composition of S. oualaniensis in the study area.
4.4. Growth Models
We described the ML-age relationships of both males and females together because no significant difference was found in this relationship between the two sexes. The age-ML relationship of the autumn population (2019 catch) was best described by the linear growth model, while that of the spring population (2020 catch) was best described by the power growth model. The two years’ growth models were different, which might be related to the population structure and the different stages of growth and development. Various S. oualaniensis growth models have been obtained in other studies. An exponential growth model was used to fit the ML-age relationships of S. oualaniensis samples collected in the South China Sea [49]. Logistic, von-Bertalanffy, linear and logarithmic growth models have also been fitted to the ML-age relationship for other squid forms in different populations and areas [44]. Similar growth models have also been reported for other cephalopods. Exponential and logarithmic growth models were found to best fit the ML-age relationship in an autumn population of Illex argentinus, while power and linear growth models best described the ML-age relationship in a winter population [50]. The age and growth of squid are both affected by many factors, and optimal growth equations may differ among different squid sexes, populations and habitats [27,51]. No single model can accurately describe the growth relationship of a squid throughout its whole life cycle [52]. Therefore, multiple models should be used to fit the growth characteristics of S. oualaniensis; the fitting effects of the models should be compared, and the most suitable growth model should be selected.
4.5. Growth Rates
The results of this study showed that the growth rates of both the spring and autumn squid populations varied with squid age. The IGR and AGR of the spring population ML (2020) first decreased and then increased, and the maximum values (0.24%/d and 1.09 mm/d, respectively) appeared at 200–220 days of age. Compared to the spring group, the growth pattern of the autumn group was more complex; its IGR and AGR first increased, reached the maxima (0.69%/d and 1.73 mm/d, respectively) at 220–240 days, decreased rapidly to the minima (0.13%/d and 0.35 mm/d, respectively) at 240–260 days, and then gradually increased from 240–260 days. The development and growth stages that cephalopods go through in their early life history are highly influenced by SST [51]. The S. oualaniensis samples collected in 2020 lived in a sea area with abnormally high SST. At this time, the northwest Indian Ocean was affected by an extremely strong positive dipole phenomenon, so the SSTs recorded in this period exceeded the optimal temperature range for squid, thus resulting in their slow growth rates. When the S. oualaniensis samples were 200–220 days of age, the spring population grew until December 2020, which is the peak period of cold SST in the Arabian Sea [53]. Therefore, during this period, the S. oualaniensis growth rates accelerated as the SST decreased to the appropriate level. The change in the ML-age growth rate of the S. oualaniensis samples collected in 2019 was more similar to the changes reported by other scholars. The IGR and AGR increased during the larval development stage but decreased when the squid reached sexual maturity; this may have been due to the requirement of the squid to put more energy into gonad development [44]. Therefore, many factors affect the growth rates of cephalopods, such as water temperature changes [54], food abundance [55], gonad development, and hatching date [56]. The age and growth of cephalopods are complex biological issues. The study of the ML-age relationship of S. oualaniensis conducted in this paper provides a reference for the subsequent scientific analysis and conservation of S. oualaniensis resources in the northwest Indian Ocean.
4.6. Maturity
We found differences in the gonad maturity composition of the collected S. oualaniensis samples between the two years of study and between male and female individuals collected in the same year. Males reached the mature stage faster than females [8], which was consistent with the finding that the females in the 2019 samples were mostly immature, while the males were mostly mature. Most of the S. oualaniensis samples collected in 2020 had reached maturity, which may have been due to the Indian Ocean dipole conditions affecting the samples collected in 2019, while 2020 was a normal year, thus resulting in the more obvious differences observed in gonad development. In addition, studies have shown that the S. oualaniensis populations in the northwest Indian Ocean mainly comprise immature individuals [30,31]; this is consistent with the sampling results obtained in 2019 in this study [8], possibly because the gonad maturity composition is greatly affected by marine environmental conditions, such as water temperature, and because certain differences exist with regard to the latitudinal distribution of squid. In this paper, we summarized all past studies on the age and growth patterns of S. oualaniensis. After combining the age ranges and hatching dates of our samples collected from the northwest Indian Ocean, we found that our study samples mostly consisted of large- and medium-sized squid from the spring-autumn hatching groups.
5. Conclusions
In this study, we have summarized all the results of studies on the age and growth patterns of S. oualaniensis. The age and ML ranges in this study indicate that our S. oualaniensis samples belong to the spring/autumn hatching group in the Northwestern Indian Ocean. The period from 141–260 days (5–8 months) was considered to correspond to the subadult stage in the whole life history of S. oualaniensis in the Northwest Indian Ocean. The relationship between the beak growth and age of S. oualaniensis is complicated, and the optimum growth model may be different in different populations and environments. Therefore, in the follow-up study, we will extend the sampling time and expand the sampling area, so as to provide a scientific basis for carrying out the beak microstructure study on its migration and rationally developing squid resources in the northwest Indian Ocean.
Author Contributions
Conceptualization, H.-J.L. and X.-J.C.; methodology, H.-J.L.; software, M.-L.Z.; validation, H.-J.L., Y.-Z.O. and J.-R.H.; formal analysis, Y.-Z.O.; investigation, J.-R.H.; resources, H.-J.L.; data curation, H.-J.L.; writing—original draft preparation, Y.-Z.O.; visualization, M.-L.Z.; supervision, Z.-Y.C.; project administration, H.-J.L.; funding acquisition, H.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (NSFC 41506184) and National Key Research and Development Program of China 489 (2019YFD0901402).
Institutional Review Board Statement
The study was conducted according to the guidelines of the code of Ethics of the University Department of Marine Studies; we only used specimens obtained from the surveys that were already dead.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
We would like to express our thanks to support for two scientific surveys made by “Xinhai 1223” is gratefully acknowledged. Thanks for the partial support of the Natural Science Foundation of China (NSFC 41506184) and National Key Research and Development Program of China 489 (2019YFD0901402). Finally, we thank the editor and the anonymous reviewers whose comments greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.G.; Chen, X.J. Marine Economic Ommastrephids Resources and Fishery in the World; China Ocean Press: Beijing, China, 2005; pp. 78–93. [Google Scholar]
- Zhou, J.G.; Chen, X.J.; Liu, B.L. Notes on the present status of exploitation and potential of cephalopod resources on the world. Mar. Fish. 2008, 30, 268–275. [Google Scholar]
- Trotsenko, B.G.; Pinchukov, M.A. Mesoscale distribution features of the purpleblack squid Sthenoteuthis oualaniensis with reference to the structure of the upper quasi-homogeneous layer in the West India Ocean. Oceanology 1994, 34, 380–385. [Google Scholar]
- Qian, W.G.; Chen, X.J.; Liu, B.L.; Tian, S.Q.; Ye, X.C. The relationship between fishing ground distribution of Sthenoteuthis oualaniensisand zooplankton in the northwestern Indian Ocean in autumn. Mar. Fish. 2006, 28, 265–271. [Google Scholar]
- Fan, J.T.; Feng, X.; Qiu, Y.S.; Huang, Z.; Chen, G.B. Review on the biology of purple back flying squid in South China Sea. Guangdong Agric. Sci. 2013, 40, 122–128. [Google Scholar]
- Chen, X.J.; Qian, W.G. Study on the resource density distribution of Sthenoteuthis oualaniensis in the northwestern Indian Ocean. J. Shanghai Fish. Univ. 2004, 13, 218–223, (In Chinese with English Abstract). [Google Scholar]
- Perales, R.C.; Almansa, E.; Aurora, B.; Beatriz, C.F.; José, I.; Francisco, J.S.; José, F.C.; Carmen, R. Age validation in octopus vulgaris beaks across the full ontogenetic range: Beaks as recorders of life events in octopuses. J. Shellfish Res. 1943, 33, 481–493. [Google Scholar] [CrossRef]
- Jereb, P.; Roper, C.F.E. Cephalopods of the World. An Annotated and Illustrated Catalog of Cephalopod Species Known to Date; FAO: Rome, Italy, 2010; Volume 2, pp. 315–318. [Google Scholar]
- Chen, X.J.; Liu, J.L. Morphological analysis on population structure of Sthenoteuthis oualaniensis in the northwestern Indian Ocean. J. Shanghai Fish. Univ. 2007, 16, 174–179. [Google Scholar]
- Corre, M.L.; Cherel, Y.; Lagarde, F.; Lormee, H.; Jouventin, P. Seasonal and interannual variation in the feeding ecology of a tropical oceanic seabird, the red-tailed tropicbird Phaethon rubricauda. Mar. Ecol. Prog. Ser. 2003, 255, 289–301. [Google Scholar] [CrossRef][Green Version]
- Parry, M. Feeding behavior of two ommastrephid squids Ommastrephes bartramiiand Sthenoteuthis oualaniensis off Hawaii. Mar. Ecol. Prog. Ser. 2006, 318, 229–235. [Google Scholar] [CrossRef]
- Colombo, E.M.; Luis, C.F.; José, F.E.; Felipe, G.M.; Alejandro, S.L.; David, C.S.; Carlos, J.P. Feeding habits and trophic level of the smooth hammerhead shark, Sphyrna zygaena (Carcharhiniformes: Sphyrnidae), off Ecuador. J. Mar. Biol. Assoc. 2018, 99, 673–680. [Google Scholar]
- Beal, L.M.; Hormann, V.; Lumpkin, R.; Foltz, G.R. The Response of the Surface Circulation of the Arabian Sea to Monsoonal Forcing. J. Phys. Oceanogr. 2013, 43, 2008–2022. [Google Scholar] [CrossRef]
- Clarke, M.R. A Review of the systematics and ecology of oceanic squids. Adv. Mar. Biol. 1966, 4, 91–300. [Google Scholar]
- Dong, Z.Z. Cephalopod Biology of the World’s Oceanic Economy; Shandong Science and Technology Press: Jinan, China, 1991; pp. 27–105. [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Liu, W.; Liu, K.; Chen, X.J. Beak Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Xisha Islands Waters of the South China Sea. Fishes 2022, 7, 187. [Google Scholar] [CrossRef]
- Canali, E.; Ponte, G.; Belcari, P.; Rocha, F.; Fiorito, G. Evaluating age in Octopus vulgaris: Estimation, validation and seasonal differences. Mar. Ecol. Prog. Ser. 2011, 441, 141–149. [Google Scholar] [CrossRef]
- Perales, R.C.; Bartolomé, A.; García-Santamaría, M.T.; Teresa, G.S.; Pedro, P.A.; Eduardo, A. Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: Improvements and comparisons. Fish. Res. 2010, 106, 171–176. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, C.J.; Chen, X.J. Preliminary study on the biological characteristics of Sthenoteuthis oualaniensis in the high seas nearby the equator of eastern Pacific during April to June. J. Shanghai Ocean. Univ. 2014, 23, 441–447. [Google Scholar]
- Fang, Z.; Xu, L.L.; Chen, X.J.; Liu, B.L.; Li, J.H.; Chen, Y. Beak growth pattern of purpleback flying squid Sthenoteuthis oualaniensis in the eastern tropical Pacific equatorial waters. Fish Sci. 2015, 81, 443–452. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Reproductive System Structure, Development and Function in Cephalopods with a New General Scale for Maturity Stages. J. Northwest Atl. Fish. Sci. 1985, 12, 63–74. [Google Scholar] [CrossRef]
- Fang, Z.; Li, J.H.; Thompson, K. Age, growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak microstructure. Fish. Bull. 2016, 114, 34–44. [Google Scholar] [CrossRef]
- Hu, G.Y.; Jin, Y.; Chen, X.J. Beak morphological characteristics of Dosidicus gigas off the Peruvian Exclusive Economic Zone (EEZ) and their relationship with body size and daily age. Mar. Fish. 2017, 39, 361–371, (In Chinese with English Abstract). [Google Scholar]
- Rodhouse, P.G.; Hatfield, E.M.C. Dynamics of Growth and Maturation in the Cephalopod Illex argentinus de Castellanos, 1960 (Teuthoidea: Ommastrephidae). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 329, 229–241. [Google Scholar]
- Jackson, G.D. Application and future potential of statolith increment analysis in squids and sepioids. Can. J. Fish. Aquat. 1994, 51, 2612–2625. [Google Scholar] [CrossRef]
- Froese, R.; Thorson, J.T.; Reyes, R.B. A Bayesian approach for estimating length-weight relationships in fishes. J. Appl. Ichthyol. 2014, 30, 78–85. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Age and growth of the mesopelagic squid Ancistrocheirus lesueurii (Oegopsida: Ancistrocheiridae) from the central-east Atlantic based on statolith microstructure. Mar. Biol. 1997, 129, 103–111. [Google Scholar] [CrossRef]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Chen, Y. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. J. Mar. Biol. Assoc. 2011, 91, 229–235. [Google Scholar] [CrossRef]
- Lu, H.J.; Chen, X.J.; Fang, Z. Comparison of the beak morphologic growth characteristics between two spawning populations of Illex argentinus in Southwest Atlantic Ocean. J. Ocean Univ. China 2012, 42, 33–40. [Google Scholar]
- Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G.; Zhao, X.H. Fishery biology of purpleback squid, Sthenoteuthis oualaniensis, in the northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar]
- Ye, X.C.; Chen, X.J. Study of biological characteristics of Sthenoteuthis oualaniensis in the northwestern Indian Ocean. J. Shanghai Ocean. Univ. 2004, 13, 316–322. [Google Scholar]
- Jackson, G.D.; Moltschaniwskyj, N.A. Temporal variation in growth rates and reproductive parameters in the small near-shore tropical squid Loliolus noctiluca; is cooler better. Mar. Ecol. Prog. Ser. 2001, 218, 167–177. [Google Scholar] [CrossRef]
- Ceriola, L.; Jackson, G.D. Growth, hatch size and maturation in a southern population of the loliginid squid Loliolus noctiluca. J. Mar. Biol. Assoc. 2010, 90, 755–767. [Google Scholar] [CrossRef]
- Jackson, G.D.; Moltschaniwskyj, N.A. Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae). Mar. Biol. 2002, 140, 747–754. [Google Scholar]
- Jackson, G.D.; Domeier, M.L. The effects of an extraordinary El Niño/La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Mar. Biol. 2003, 142, 925–935. [Google Scholar] [CrossRef]
- Hoving, H.J.T.; Gilly, W.F.; Markaida, U.; Kelly, B.B.J.; Brown, Z.W.; Daniel, P.; Field, J.C.; Parassenti, L.; Liu, B.L.; Campos, B. Extreme plasticity in life-history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Glob. Chang. Biol. 2013, 19, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Doubleday, Z.A.; White, J.; Pecl, G.T.; Semmens, J.M. Age determination in merobenthic octopuses using stylet increment analysis: Assessing future challenges using Macroctopus maorum as a model. ICES J. Mar. Sci. 2011, 68, 2059–2063. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, X.C.; Lu, H.J.; Liu, K.; Ren, P.; Ning, X.; Chen, X.J. Beak microstructure and growth characteristics of Sthenoteuthis oualaniensis in the Xinsha Islands waters of the South China Sea. Oceanol. Limnol. Sin. 2021, 52, 1293–1302. [Google Scholar]
- Lu, H.J.; Chen, Z.Y.; Ning, X.; Chen, X.J. Pigmentation change on beak for Sthenoteuthis oualaniensis in the Xisha Islands waters of the South China Sea. Chin. J. Ecol. 2020, 39, 1600–1608. [Google Scholar]
- Chen, X.Y.; Lu, H.J.; Wang, H.H.; He, J.R.; Liu, K.; Chen, X.J. Analysis of pigmentation character on beak for Sthenoteuthis oualaniensis in Northwest Indian Ocean. J. Zoo 2020, 55, 468–476, (In Chinese with English Abstract). [Google Scholar]
- Shchetinnikov, A.S. Feeding spectrum of squid Sthenoteuthis oualaniensis (Oegopsida) in the eastern Pacific. J. Mar. Biol. Assoc. 1992, 72, 849–860. [Google Scholar] [CrossRef]
- Castro, J.J.; Hernández-García, V. Ontogenetic changes in mouth structures, foraging behaviour and habitat use of Scomber japonicus and Illex coindetii. Sci. Mar. 1995, 59, 347–355. [Google Scholar]
- Perales, R.C.; Jurado, R.A.; Bartolom, A.; Verónica, D.; María, N.C.; Eugenio, F.N. Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydrobiologia. 2014, 725, 105–114. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Zhong, J.S. Age, growth and population structure of squid Sthenoteuthis oualaniensis in northwest Indian Ocean by statolith microstructure. J. Dalian Fish. Univ. 2009, 24, 206–212. [Google Scholar]
- Fang, Z.; Chen, X.J.; Lu, H.J.; Li, J.H.; Liu, B.L. Morphology and growth of beaks in two cohorts for neon flying squid (Ommastrephesbartramii) in the North Pacific Ocean. Sheng Tai Xue Bao 2014, 34, 5405–5415. [Google Scholar]
- Clarke, M. Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. S. Afr. J. Mar. Sci. 1980, 20, 41–44. [Google Scholar] [CrossRef]
- Pecl, G.T.; Jackson, G.D. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish Biol. Fish 2008, 18, 373–385. [Google Scholar] [CrossRef]
- Keyl, F.; Argüelles, J.; Mariátegui, L.; Tafur, R.; Yamashiro, C. A hypothesis on range expansion and spatiotemporal shifts in size at maturity of jumbo squid (Dosidicus gigas) in the Eastern Pacific Ocean. CalCOFI Rep. 2014, 49, 119–128. [Google Scholar]
- Zhao, C.X.; Chen, Z.P.; He, X.B.; Deng, Y.S.; Feng, B.; Yan, Y.R. Age, growth and population structure of purple beak flying squid, sthenoteuthis oualaniensis the south China Sea in spring based on statolith microstructure. Acta Hydrobiol. Sin. 2017, 41, 884–890. [Google Scholar]
- Lu, H.J.; Chen, X.J. Age, growth and population structure of Illex argentinus based on statolith microstructure in Southwest Atlantic Ocean. Shuichan Xuebao 2012, 36, 1049–1056. [Google Scholar] [CrossRef]
- Jackson, G.D.; Wadley, V.A. Age, growth, and reproduction of the tropical squid Nototodarus hawaiiensis (Cephalopoda: Ommastrephidae) off the North West Slope of Australia. Fish. Bull. 1998, 96, 779–787. [Google Scholar]
- Liu, B.L.; Chen, X.J.; Yi, Q. A comparison of fishery biology of jumbo flying squid, Dosidicus gigas outside three Exclusive Economic Zones in the Eastern Pacific Ocean. Chin. J. Oceanol. Limnol. 2013, 31, 523–533. [Google Scholar] [CrossRef]
- Gao, L.; Xu, J.D.; Qiu, F.; Lin, X.Y. Bimmodal character of upper ocean temperature in the Arabian Sea. J. Appl. Oceanogr. 2018, 37, 161–172. [Google Scholar]
- Forsythe, J.W.; Hanlon, R.T. Growth of the eastern Atlantic squid, Loligo forbesi Steenstrup (Molluska: Cephalopoda). Aquac. Res. 1989, 20, 1–14. [Google Scholar]
- Forsythe, J. A Working Hypothesis of How Seasonal Temperature Change May Impact the Field Growth of Young Cephalopods. Recent Adv. Cephalop. Fish. Biol. 1993, 133–143. [Google Scholar]
- Gonzlez, A.F.; Bernardino, G.C.; Guerra, A. Age and growth of short-finned squid Illex coindetii in Galicianwaters (NW Spain) based on statolith analysis. ICES J. Mar. Sci. 1996, 53, 802–810. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).