Evaluation of Soil Organic Carbon Stock in Coastal Sabkhas under Different Vegetation Covers
Abstract
:1. Introduction
2. Materials and Methods
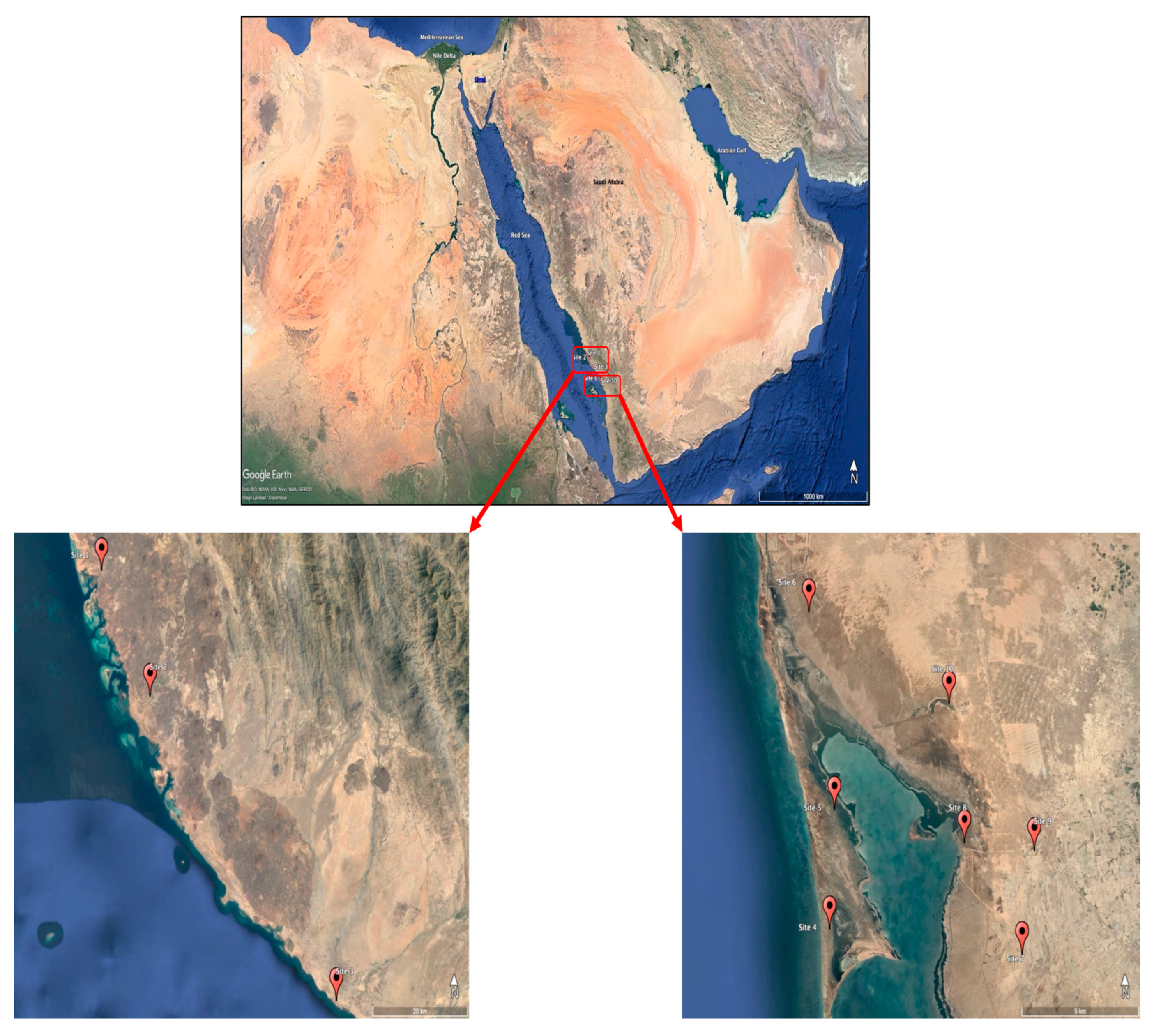
2.1. Study Area
2.2. Soil Sampling
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations and Uncertainties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jaloud, A.A.; Hussain, G. Sabkha ecosystem and halophyte plant communities in Saudi Arabia. In Sabkha Ecosystems; Volume II: West and Central Asia; Ajmal Khan, M., Böer, B., Kust, G.S., Barth, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–7. [Google Scholar]
- Schulz, S.; Horovitz, M.; Rausch, R.; Michelsen, N.; Mallast, U.; Köhne, M.; Siebert, C.; Schüth, C.; Al-Saud, M.; Merz, R. Groundwater evaporation from salt pans: Examples from the eastern Arabian Peninsula. J. Hydrol. 2015, 531, 792–801. [Google Scholar] [CrossRef]
- Barakat, N.; El-Gawad, A.; Laudadio, V.; Kabiel, H.; Tufarelli, V.; Cazzato, E. A contribution to the ecology and floristic markers of plant associations in different habitats of Sinai Peninsula, Egypt. Rend. Lincei 2014, 25, 479–490. [Google Scholar] [CrossRef]
- Sciandrello, S.; Musarella, C.; Puglisi, M.; Spampinato, G.; Tomaselli, V.; Minissale, P. Updated and new insights on the coastal halophilous vegetation of southeastern Sicily (Italy). Plant Sociol. 2019, 56, 81–98. [Google Scholar]
- Kinsman, D.J.J.; Park, R.K. Studies of recent sedimentology and early diagenesis, Trucial Coast, Arabia Gulf. 2nd Regional Technical Symposium Society Ptr. Eng. of AIME, Saudi Arabian Section. 1969. [Google Scholar]
- Batanouny, K.H. Ecology and Flora of Qatar; The Center for Scientific and Applied Research, University of Qatar: Doha, Qatar, 1981. [Google Scholar]
- Dar, B.A.; Assaeed, A.M.; Al-Rowaily, S.L.; Al-Doss, A.A.; Abd-ElGawad, A.M. Vegetation composition of the halophytic grass Aeluropus lagopoides communities within coastal and inland sabkhas of Saudi Arabia. Plants 2022, 11, 666. [Google Scholar] [CrossRef]
- Siikamäki, J.; Sanchirico, J.N.; Jardine, S.; McLaughlin, D.; Morris, D.F. Blue Carbon: Global Options for Reducing Emissions from the Degradation and Development of Coastal Ecosystems; Resources for the Future: Washington, DC, USA, 2012. [Google Scholar]
- Almahasheer, H.; Serrano, O.; Duarte, C.M.; Arias-Ortiz, A.; Masque, P.; Irigoien, X. Low Ccarbon sink capacity of Red Sea mangroves. Sci. Rep. 2017, 7, 9700. [Google Scholar] [CrossRef]
- Chmura, G.L. What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coastal Manag. 2013, 83, 25–31. [Google Scholar] [CrossRef]
- Keshta, A.E.; Yarwood, S.A.; Baldwin, A.H. A new in situ method showed greater persistence of added soil organic matter in natural than restored wetlands. Res. Ecol. 2021, 29, e13437. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Moyer, R.P.; Chappel, A.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Smoak, J.M. Coastal blue carbon assessment of mangroves, salt marshes, and salt barrens in Tempa Bay, Florida, USA. Estuar. Coast. 2018, 41, 1496–1510. [Google Scholar] [CrossRef]
- Ullman, R.; Bilbao-Bastida, V.; Grimsditch, G. Including blue carbon in climate market mechanisms. Ocean Coast. Manag. 2013, 83, 15–18. [Google Scholar] [CrossRef]
- Banerjee, K.; Sappal, S.M.; Ramachandran, P.; Ramesh, R. Salt marsh: Ecologically important, yet least studied blue carbon ecosystems in India. J. Clim. Chang. 2017, 3, 59–72. [Google Scholar] [CrossRef]
- Keshta, A.E.; Riter, J.C.A.; Shaltout, K.H.; Baldwin, A.H.; Kearney, M.; Sharaf El-Din, A.; Eid, E.M. Loss of coastal wetlands in Lake Burullus, Egypt: A GIS and remote-sensing study. Sustainability 2022, 14, 4980. [Google Scholar] [CrossRef]
- Ewers Lewis, C.J.; Carnell, P.E.; Sanderman, J.; Baldock, J.A.; Macreadie, P.I. Variability and vulnerability of coastal ‘blue carbon’ stocks: A case study from southeast Australia. Ecosystems 2018, 21, 263–279. [Google Scholar] [CrossRef]
- Connor, R.F.; Chmura, G.L.; Beecher, B.C. Carbon accumulation in bay of Fundy salt marshes: Implications for restoration of reclaimed marshes. Global Biogeochem. Cycles 2001, 15, 943–954. [Google Scholar] [CrossRef]
- Mahaney, W.M.; Smemo, K.A.; Gross, K.L. Impacts of C4 grass introductions on soil carbon and nitrogen cycling in C3-dominated successional systems. Oecologia 2008, 157, 295–305. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Seliskar, D.; Sommerfield, C.; Gallagher, J. Salt marsh carbon pool distribution in a Mid-Atlantic Lagoon, USA: Sea level rise implications. Wetlands 2011, 31, 87–99. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y. Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 2014, 11, 5057–5071. [Google Scholar] [CrossRef]
- Kelleway, J.J.; Saintilan, N.; Macreadie, P.I.; Baldock, J.A.; Ralph, P.J. Sediment and carbon deposition vary among vegetation assemblages in a coastal salt marsh. Biogeosciences 2017, 14, 3763–3779. [Google Scholar] [CrossRef]
- Gailis, M.; Kohfeld, K.E.; Pellatt, M.G.; Carlson, D. Quantifying blue carbon for the largest salt marsh in southern British Columbia: Implications for regional coastal management. Coast. Eng. J. 2021, 63, 275–309. [Google Scholar] [CrossRef]
- Eid, E.M.; El-Bebany, A.F.; Alrumman, S.A. Distribution of soil organic carbon in the mangrove forests along the southern Saudi Arabian Red Sea coast. Rend. Lincei 2016, 27, 629–637. [Google Scholar] [CrossRef]
- Arshad, M.; Alrumman, S.; Eid, E.M. Evaluation of carbon sequestration in the sediment of polluted and non-polluted locations of mangroves. Fund. Appl. Limnol. 2018, 192, 53–64. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Eid, E.M.; Arshad, M.; Shaltout, K.H.; El-Sheikh, M.A.; Alfarhan, A.H.; Picó, Y.; Barcelo, D. Effect of the conversion of mangroves into shrimp farms on carbon stock in the sediment along the southern Red Sea coast, Saudi Arabia. Environ. Res. 2019, 176, 108536. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Khedher, K.M.; Ayed, H.; Arshad, M.; Moatamed, A.; Mouldi, A. Evaluation of carbon stock in the sediment of two mangrove species, Avicennia marina and Rhizophora mucronata, growing in the Farasan Islands, Saudi Arabia. Oceanologia 2020, 62, 200–213. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Ahmed, M.T.; Alrumman, S.A.; Ahmed, D.A.; Eid, E.M. Evaluation of the carbon sequestration capacity of arid mangroves along nutrient availability and salinity gradients along the Red Sea coastline of Saudi Arabia. Oceanologia 2020, 62, 56–69. [Google Scholar] [CrossRef]
- Negrin, V.L.; Spetter, C.V.; Asteasuain, R.O.; Perillo, G.M.E.; Marcovecchio, J.E. Influence of flooding and vegetation on carbon, nitrogen, and phosphorus dynamics in the pore water of a Spartina alterniflora salt marsh. J. Environ. Sci. 2011, 23, 212–221. [Google Scholar] [CrossRef]
- Bull, I.D.; Bergen, P.F.; Bol, R.; Brown, S.; Gledhill, A.R.; Gray, A.J.; Harkness, D.D.; Woodbury, S.E.; Evershed, R.P. Estimating the contribution of Spartina anglica biomass to salt-marsh sediments using compound specific stable carbon isotope measurements. Org. Geochem. 1999, 30, 477–483. [Google Scholar] [CrossRef]
- Gao, J.H.; Bai, F.L.; Yang, Y.; Gao, S.; Liu, Z.Y.; Li, J. Influence of Spartina colonization on the supply and accumulation of organic carbon in tidal salt marshes of northern Jiangsu Province. Chin. J. Coastal Res. 2012, 28, 486–498. [Google Scholar] [CrossRef]
- Morley, N.J.F. The coastal waters of the Red Sea. Bull. Mar. Res. Cen. 1975, 5, 8–19. [Google Scholar]
- Nabhan, A.I.; Yang, W. Modern sedimentary facies, depositional environments, and major controlling processes on an arid siliciclastic coast, Al qahmah, SE Red Sea, Saudi Arabia. J. Afr. Earth Sci. 2018, 140, 9–28. [Google Scholar] [CrossRef]
- PME (Presidency of Metrology and Environment Protection). Surface Annual Climatological Report; Jizan, Presidency of Metrology and Environment Protection; National Meteorology and Environment Center: Ulaanbaatar, Saudi Arabia, 2012. [Google Scholar]
- Hickey, B.; Goudie, A.S. The Use of TOMS and MODIS to Identify Dust Storm Source Areas: The Tokar Delta (Sudan) and the Seistan Basin (South West) in Geomorphological Variations; Goudie, A.S., Kalvoda, J., Eds.; 2007; Volume 3, pp. 37–57. [Google Scholar]
- Brukner, A.; Rowlands, G.; Riegl, B.; Purkis, S.; William, A.; Renaud, P. Khaled Bin Sultan Living Oceans Foundation Atlas of Saudi Arabian Red Sea Marine Habitats; Panoramic Press: Phoenix, AZ, USA, 2012; p. 262. [Google Scholar]
- Hakami, B.A.; Abu Seif, E.S. Geotechnical aspects and associated problems of Al-Shuaiba Lagoon soil, Red Sea coast, Saudi Arabia. Environ. Earth Sci. 2019, 78, 158. [Google Scholar] [CrossRef]
- Liang, S.; Wang, J. Fractional vegetation cover. In Advanced Remote Sensing: Terrestrial Information Extraction and Applications; Elsevier Inc.: London, UK, 2020; pp. 477–510. [Google Scholar]
- Peet, R.K.; Wentworth, T.R.; White, P.S. A flexible, multipurpose method for recording vegetation composition and structure. Castanea 1998, 63, 262–274. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Arifuzzaman, M.; Habib, M.A.; Al-Turki, M.K.; Khan, M.I.; Ali, M.M. Improvement and characterization of sabkha soil of Saudi Arabia: A review. J. Teknol. 2016, 78, 1–11. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H. Distribution of soil organic carbon in the mangrove Avicennia marina (Forssk.) Vierh. along the Egyptian Red Sea coast. Reg. Stud. Mar. Sci. 2016, 3, 76–82. [Google Scholar] [CrossRef]
- Eid, E.M.; Keshta, A.E.; Shaltout, K.H.; Baldwin, A.H.; El-Din, S.; Ahmed, A. Carbon sequestration potential of the five Mediterranean lakes of Egypt. Fund. Appl. Limnol. 2017, 190, 87–96. [Google Scholar] [CrossRef]
- Wilke, B.M. Determination of chemical and physical soil properties. In Manual for Soil Analysis-Monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 47–95. [Google Scholar]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Craft, C.B.; Seneca, E.D.; Broome, S.W. Loss on ignition and Kjeldahl digestion for estimating organic carbon and total nitrogen in estuarine marsh soils: Calibration with dry combustion. Estuaries 1991, 14, 175–179. [Google Scholar] [CrossRef]
- Han, F.; Hu, W.; Zheng, J.; Du, F.; Zhang, X. Estimating soil organic carbon storage and distribution in a catchment of Loess Plateau, China. Geoderma 2010, 154, 261–266. [Google Scholar] [CrossRef]
- Meersmans, J.; De Ridder, F.; Canters, F.; De Baets, S.; Van Molle, M. A multiple regression approach to assess the spatial distribution of soil organic carbon (SOC) at the regional scale (Flanders, Belgium). Geoderma 2008, 143, 1–13. [Google Scholar] [CrossRef]
- IBM SPSS. IBM SPSS Statistics for Windows, Version 23.0; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Pravin, R.; Dodha, V.; Vidya, D.; Manab, C.; Saroj, M. Soil bulk density as related to soil texture, organic matter content and available total nutrients of coimbatore soil. Int. J. Sci. Res. Pub. 2013, 3, 1–8. [Google Scholar]
- Drewry, J.J.; Cameron, K.C.; Buchan, G.D. Pasture yield and soil physical property responses to soil compaction from treading and grazing-a review. Aust. J. Soil Res. 2008, 46, 237–256. [Google Scholar] [CrossRef]
- Howard, P.J.A.; Loveland, P.J.; Bradley, R.I.; Dry, F.T.; Howard, D.M.; Howard, D.C. The carbon content of soil and its geographical-distribution in Great Britain. Soil Use Manag. 1995, 11, 9–15. [Google Scholar] [CrossRef]
- Drake, K.; Halifax, H.; Adamowicz, S.C.; Craft, C. Carbon sequestration in tidal salt marshes of the northeast United States. Environ. Manag. 2015, 56, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, G.; Zhao, Q.; Lu, Q.; Jia, J.; Cui, B.; Liu, X. Depth-distribution patterns and control of soil organic carbon in coastal salt marshes with different plant covers. Sci. Rep. 2016, 6, 34835. [Google Scholar] [CrossRef] [PubMed]
- Ellison, J.C.; Beasy, K.M. Sediment carbon accumulation in southern latitude saltmarsh communities of Tasmania, Australia. Biology 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Gispert, M.; Phang, C.; Carrasco-Barea, L. The role of soil as a carbon sink in coastal salt-marsh and agropastoral systems at La Pletera, NE Spain. Catena 2020, 185, 104331. [Google Scholar] [CrossRef]
- Sherry, S.; Ramon, A.; Eric, M.; Richard, E.; Barry, W.; Peter, D.; Susan, T. Precambrian shield wetlands: Hydrologic control of the sources and export of dissolved organic matter. Clim. Change 1998, 40, 167–188. [Google Scholar]
- Li, S.B.; Chen, P.H.; Huang, J.S.; Hsueh, M.L.; Hsieh, L.Y.; Lee, C.L.; Lin, H.J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef]
- Osland, M.J.; Gabler, C.A.; Grace, J.B.; Day, R.H.; McCoy, M.L.; McLeod, J.L.; From, A.S.; Enwright, N.M.; Feher, L.C.; Stagg, C.L.; et al. Climate and plant controls on soil organic matter in coastal wetlands. Glob. Chang. Biol. 2018, 24, 5361–5379. [Google Scholar] [CrossRef]
- Bu, N.-S.; Qu, J.-F.; Li, G.; Zhao, B.; Zhang, R.-J. Reclamation of coastal salt marshes promoted carbon loss from previously-sequestered soil carbon pool. Ecol. Eng. 2015, 81, 335–339. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Pethick, J.S. Long-term accretion rates on tidal marshes. J. Sediment. Petrol. 1979, 51, 571–577. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Global Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Núria, M. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Lai, R.; Lagormarsino, A.; Ledda, L.; Roggero, P.P. Variation in soil C and microbial functions across tree canopy projection and open grassland microenvironments. Turk. J. Agric. For. 2014, 38, 62–69. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.; Tang, Y.; Ji, C.; Zheng, C.; He, J.; Zhu, B. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob. Change Biol. 2008, 14, 1592–1599. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, J.; Wu, Y. Recent sediment accumulation and carbon burial in the East China Sea. Global Biogeochem. Cycles 2006, 20, 466–480. [Google Scholar] [CrossRef]
- Gao, J.H.; Feng, Z.X.; Chen, L.; Wang, Y.P.; Bai, F.; Li, J. The effect of biomass variations of Spartina alterniflora on the organic carbon content and composition of a salt marsh in northern Jiangsu Province, China. Ecol. Eng. 2016, 95, 160–170. [Google Scholar] [CrossRef]
- van Ardenne, L.B.; Jolicouer, S.; Bérubé, D.; Burdick, D.; Chmura, G.L. The importance of geomorphic context for estimating the carbon stock of salt marshes. Geoderma 2018, 330, 264–275. [Google Scholar] [CrossRef]
- Harvey, R.J.; Garbutt, A.; Hawkins, S.J.; Skov, M.W. No detectable broad-scale effect of livestock grazing on soil blue-carbon stock in salt marshes. Front. Ecol. Evol. 2019, 7, 151. [Google Scholar] [CrossRef]
- Mueller, P.; Ladiges, N.; Jack, A.; Schmedl, G.; Kutzbach, L.; Jensen, K.; Nolte, S. Assessing the long-term carbon-sequestration potential of the semi-natural salt marshes in the European Wadden Sea. Ecosphere 2019, 10, e02556. [Google Scholar] [CrossRef]
- Adams, C.A.; Andrews, J.E.; Jickells, T. Nitrous oxide and methane fluxes vs. carbon, nitrogen and phosphorous burial in new intertidal and saltmarsh sediments. Sci. Total Environ. 2012, 434, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C.; Borgnis, E.L.; Turner, R.E.; Milan, C.S. Carbon sequestration and sediment accretion in San Francisco Bay Tidal Wetlands. Estuar. Coast. 2012, 35, 1163–1181. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Wang, L.; Guo, J.; Feng, J.; Wu, H.; Lin, G. Distribution patterns and controlling factors for the soil organic carbon in four mangrove forests of China. Global Ecol. Conserv. 2019, 17, e00575. [Google Scholar] [CrossRef]
- Perera, N.; Lokupitiya, E.; Halwatura, D.; Udagedara, S. Quantification of blue carbon in tropical salt marshes and their role in climate change mitigation. Sci. Total Environ. 2022, 820, 153313. [Google Scholar] [CrossRef]
- Neue, H.U.; Gaunt, J.L.; Wang, Z.P.; Becker-Heidmann, P.; Quijano, C. Carbon in tropical wetlands. Geoderma 1997, 79, 163–185. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Amundson, R. The carbon budget in soils. Annu. Rev. Earth Planet. Sci. 2001, 29, 535–562. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Twilley, R.R.; Rovai, A.S.; Riul, P. Coastal morphology explains global blue carbon distributions. Front. Eco. Environ. 2018, 16, 503–508. [Google Scholar] [CrossRef]
- Qiu, D.; Cui, B.; Yan, J.; Ma, X.; Ning, Z.; Wang, F.; Sui, H.; Bai, J. Effect of burrowing crabs on retention and accumulation of soil carbon and nitrogen in an intertidal salt marsh. J. Sea Res. 2019, 154, 101808. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Liu, Q.; Lu, Q.; Gao, Z.; Wang, J. Spatial and seasonal variations of soil carbon and nitrogen content and stock in a tidal salt marsh with Tamarix chinensis, China. Wetlands 2016, 36, S145–S152. [Google Scholar] [CrossRef]
- Kelleway, J.J.; Saintilan, N.; Macreadie, P.I.; Ralph, P.J. Sedimentary factors are key predictors of carbon storage in SE Australian saltmarshes. Ecosystems 2016, 19, 865–880. [Google Scholar] [CrossRef]
- Hansen, K.; Butzeck, C.; Eschenbach, A.; Gröngröft, A.; Jensen, K.; Pfeiffer, E.-M. Factors influencing the organic carbon pools in tidal marsh soils of the Elbe estuary (Germany). J. Soils Sediments 2017, 17, 47–60. [Google Scholar] [CrossRef]
- Howard, J.; Hoyt, S.; Isensee, K.; Pidgeon, E.; Telszewski, M.E. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows; Conservation International, Intergovernmental Oceanographic Commission of UNESCO; International Union for Conservation of Nature: Arlington, VA, USA, 2014. [Google Scholar]
- Alongi, D.M. Carbon balance in salt marsh and mangrove ecosystems: A global synthesis. J. Mar. Sci. Eng. 2020, 8, 767. [Google Scholar] [CrossRef]
- Brown, D.R.; Conrad, S.; Akkerman, K.; Fairfax, S.; Fredericks, J.; Hanrio, E.; Sanders, L.M.; Scott, E.; Skillington, A.; Tucker, J.; et al. Seagrass, mangrove and saltmarsh sedimentary carbon stocks in an urban estuary: Coffs Harbour, Australia. Reg. Stud. Mar. Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Mandura, A.S.; Khafaji, A.K.; Saifullah, S.M. Mangrove ecosystem of southern Red Sea coast of Saudi Arabia. Proc. Saudi Biol. Soc. 1987, 10, 165–193. [Google Scholar]
- Almahasheer, H.; Duarte, C.M.; Irigoien, X. Nutrient limitation in central Red Sea mangroves. Front. Mar. Sci. 2016, 3, 271. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, J.; Xue, L.; Craft, C.B. Distribution of organic carbon storage in different salt-marsh plant communities: A case study at the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2020, 243, 106900. [Google Scholar] [CrossRef]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, J.P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef]
- Liu, J.-E.; Han, R.-M.; Su, H.-R.; Wu, Y.-P.; Zhang, L.-M.; Richardson, C.J.; Wang, G.-X. Effects of exotic Spartina alterniflora on vertical soil organic carbon distribution and storage amount in coastal salt marshes in Jiangsu, China. Ecol. Eng. 2017, 106, 132–139. [Google Scholar]
- Mao, R.; Ye, S.-Y.; Zhang, X.-H. Soil-aggregate-associated organic carbon along vegetation zones in tidal salt marshes in the Liaohe Delta. Clean Soil Air Water 2018, 46, 1800049. [Google Scholar] [CrossRef]
- Kaviarasan, T.; Dahms, H.U.; Gokul, M.S.; Henciya, S.; Muthukumar, K.; Shankar, S.; James, R.A. Seasonal species variation of sediment organic carbon stocks in salt marshes of Tuticorin Area, Southern India. Wetlands 2019, 39, 483–494. [Google Scholar] [CrossRef]
- Byun, C.; Lee, S.-H.; Kang, H. Estimation of carbon storage in coastal wetlands and comparison of different management schemes in South Korea. J. Ecol. Environ. 2019, 43, 8. [Google Scholar] [CrossRef]
- Raw, J.L.; Julie, C.L.; Adams, J.B. A comparison of soil carbon pools across a mangrove-salt marsh ecotone at the southern African warm-temperate range limit. S. Afr. J. Bot. 2019, 127, 301–307. [Google Scholar] [CrossRef]
- Andrews, J.E.; Samways, G.; Shimmield, G.B. Historical storage budgets of organic carbon, nutrient and contaminant elements in saltmarsh sediments: Biogeochemical context for managed realignment, Humber Estuary, UK. Sci. Total Environ. 2008, 405, 1–13. [Google Scholar] [CrossRef]
- González-Alcaraz, M.N.; Egea, C.; Jiménez-Cárceles, F.J.; Párraga, I.; María-Cervantes, A.; Delgado, M.J.; Álvarez-Rogel, J. Storage of organic carbon, nitrogen and phosphorus in the soil–plant system of Phragmites australis stands from a eutrophicated Mediterranean salt marsh. Geoderma 2012, 185–186, 61–72. [Google Scholar] [CrossRef]
- Howe, A.J.; Rodríguez, J.F.; Saco, P.M. Surface evolution and carbon sequestration in disturbed and undisturbed wetland soils of the Hunter estuary, southeast Australia. Estuar. Coast. Shelf Sci. 2009, 84, 75–83. [Google Scholar] [CrossRef]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Santini, N.S.; Lovelock, C.E.; Hua, Q.; Zawadzki, A.; Mazumder, D.; Mercer, T.R.; Muñoz-Rojas, M.; Hardwick, S.A.; Madala, B.S.; Cornwell, W.; et al. Natural and regenerated saltmarshes exhibit similar soil and belowground organic carbon stocks, root production and soil respiration. Ecosystems 2019, 22, 1803–1822. [Google Scholar]
- Unger, V.; Elsey-Quirk, T.; Sommerfield, C.; Velinsky, D. Stability of organic carbon accumulating in Spartina alterniflora-dominated salt marshes of the Mid-Atlantic U.S. Estuar. Coast. Shelf Sci. 2016, 182, 179–189. [Google Scholar]
- Drexler, J.Z.; Woo, I.; Fuller, C.C.; Nakai, G. Carbon accumulation and vertical accretion in a restored versus historic salt marsh in southern Puget Sound, Washington, United States. Restor. Ecol. 2019, 27, 1117–1127. [Google Scholar]
- Ruiz-Fernández, A.C.; Carnero-Bravo, V.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.H.; Amaya-Monterrosa, O.A.; Bojórquez-Sánchez, S.; López-Mendoza, P.G.; Cardoso-Mohedano, J.G.; Dunbar, R.B.; Mucciarone, D.A.; et al. Carbon burial and storage in tropical salt marshes under the influence of sea level rise. Sci. Total Environ. 2018, 630, 1628–1640. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; Gomes, L.E.d.O.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon stocks of mangroves and salt marshes of the Amazon Region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kathiresan, K. The age and species composition of mangroves forest directly influence the net primary productivity and carbon sequestration potential. Biocatal. Agr. Biotechnol. 2019, 20, 101235. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). The Fourth Assessment Report Climate Change 2007; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007.
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using Earth observation satellite data. Global Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar]
- Gedan, K.B.; Silliman, B.; Bertness, M. Centuries of human-driven change in salt marsh ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar]
- Bianchi, T.S.; Allison, M.A.; Zhao, J.; Li, X.; Comeaux, R.S.; Feagin, R.A.; Kulawardhana, R.W. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuar. Coast. Shelf Sci. 2013, 119, 7–16. [Google Scholar]
- Ray, R.; Weigt, M. Seasonal and habitat-wise variations of creek water particulate and dissolved organic carbon in arid mangrove (the Persian Gulf). Cont. Shelf Res. 2018, 165, 60–70. [Google Scholar]




| Vegetation Cover (%) | SBD | SOC Content | SOC Density | SOC Stock |
|---|---|---|---|---|
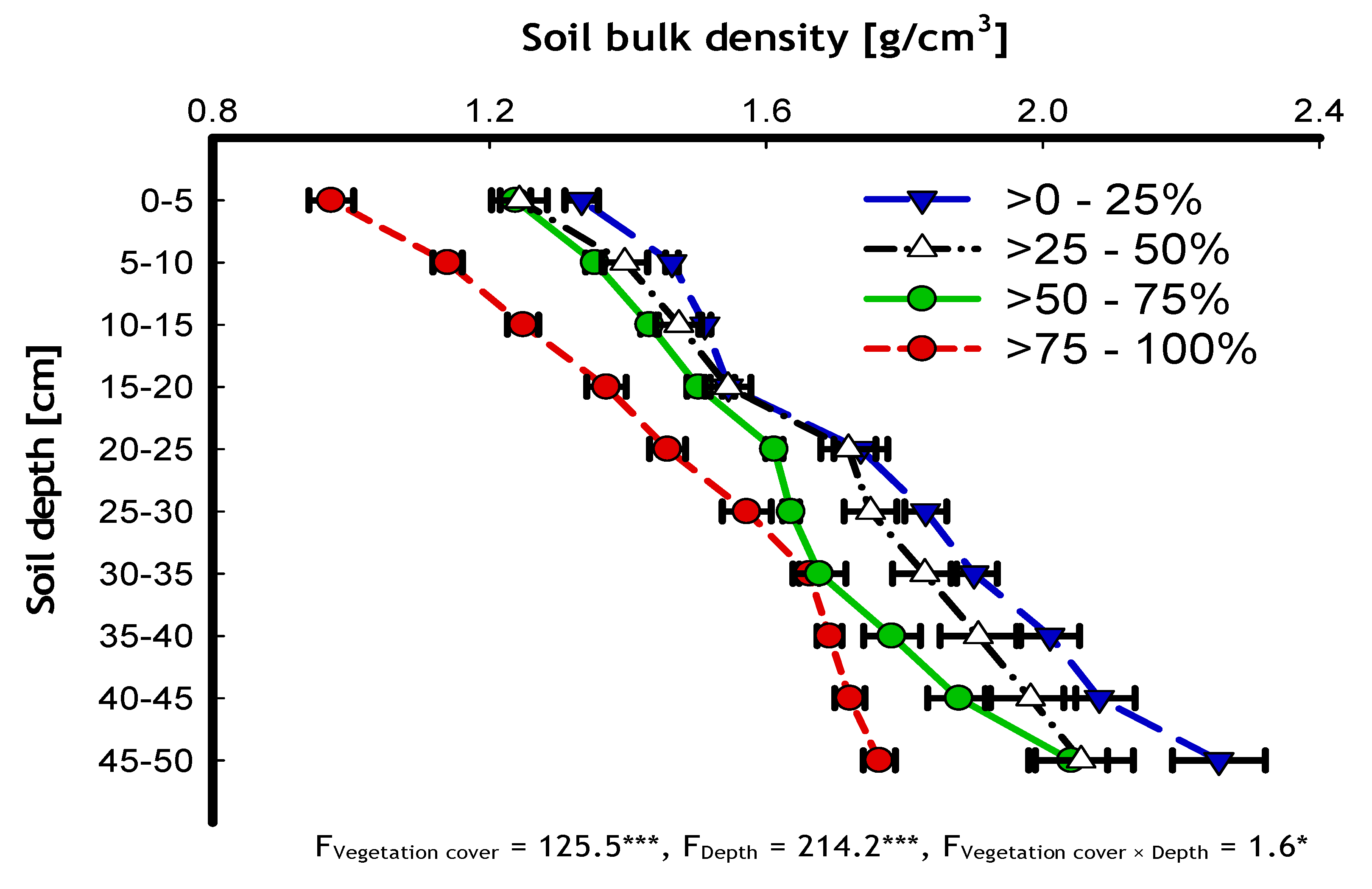
| 0–25 | 1.78 ± 0.02 d [n = 320] | 4.9 ± 0.2 a [n = 320] | 7.3 ± 0.3 a [n = 320] | 3.6 ± 0.2 a [n = 32] |
| >25–50 | 1.69 ± 0.02 c [n = 300] | 11.5 ± 0.4 b [n = 300] | 17.8 ± 0.5 b [n = 300] | 8.9 ± 0.3 b [n = 30] |
| >50–75 | 1.61 ± 0.02 b [n = 330] | 19.0 ± 0.5 c [n = 330] | 30.8 ± 0.6 c [n = 330] | 15.4 ± 0.4 c [n = 33] |
| >75–100 | 1.46 ± 0.02 a [n = 300] | 27.6 ± 0.6 d [n = 300] | 38.9 ± 0.6 d [n = 300] | 19.4 ± 0.7 d [n = 30] |
| F-value | 52.1 *** | 475.6 *** | 756.9 *** | 229.2 *** |
| Location | SBD | SOC Content | SOC Stock | Depth [cm] | Reference |
|---|---|---|---|---|---|
| Southern Red Sea coast, Saudi Arabia | 1.46–1.78 | 4.9–27.6 | 3.6–19.4 | 50 | Present study |
| Coastal sabkha, United Arab Emirates | 1.20–1.25 | 8.2 | 42.8 | Schile et al. [94] | |
| Coastal salt marshes of the Yellow River Delta, China | 1.42–1.76 | 1.7–4.0 | 4.9–5.6 | 100 | Bai et al. [54] |
| Tidal salt marsh of the Yellow River estuary, China | 1.48–1.88 | 2.9–4.1 | 1.6–2.1 | 40 | Zhao et al. [85] |
| Coastal salt marshes in Jiangsu, China | 0.7–5.7 | 7.9 | 300 | Liu et al. [95] | |
| Tidal salt marshes in the Liaohe Delta, China | 1.47–1.55 | 5.3–11.4 | 3.0–4.2 | 30 | Mao et al. [96] |
| Yangtze estuary, China | 1.29–1.36 | 0.7–10.9 | 2.4–4.5 | 100 | Yuan et al. [93] |
| Salt marshes of Tuticorin area, India | 0.13–0.48 | 21.8–38.1 | 0.8–5.4 | 30 | Kaviarasan et al. [97] |
| Eulsukdo salt marsh and Seomjin River estuary, South Korea | 20.9–28.9 | 14.6–25.5 | 100 | Byun et al. [98] | |
| Tropical salt marshes, Sri Lanka | 0.85–1.50 | 7.8–23.2 | 11.0–23.3 | 50 | Perera et al. [77] |
| Salt marshes of Nahoon estuary, South Africa | 11.0 | 50 | Raw et al. [99] | ||
| Humber estuary, UK | 14.5–35.4 | 100 | Andrews et al. [100] | ||
| Elbe estuary, Germany | 0.68–1.22 | 12.5–51.3 | 5.8–35.0 | 100 | Hansen et al. [87] |
| Marina del Carmolí salt marsh, Spain | 1.0–1.3 | 10 | González-Alcaraz et al. [101] | ||
| La Pletera salt marsh, Spain | 1.25–1.54 | 2.0–40.1 | 0.2–2.7 * | 40 | Gispert et al. [56] |
| Hunter estuary, Australia | 65.0 | 100 | Howe et al. [102] | ||
| Salt marsh along the Mornington Peninsula edge, Victoria, Australia | 16.9 | 100 | Livesley and Andrusiak [103] | ||
| Urban estuary, Coffs Harbour, Australia | 0.81 | 56.2 | 33.3 | 100 | Brown et al. [90] |
| Salt marshes in New South Wales, Australia | 16.4 | 100 | Kelleway et al. [86] | ||
| Salt marsh of Tasmania, Australia | 0.50–2.40 | 5.0–70.0 | 30 | Ellison and Beasy [55] | |
| Salt marshes along Haslams Creek, Australia | 1.22–1.57 | 10.9–30.2 | 4.8–6.5 | 30 | Santini et al. [104] |
| Salt marsh along the northern border of Little Assawoman Bay, USA | 0.17–1.63 | 10.0–301.0 | 2.4–8.6 | 22.5 | Elsey-Quirk et al. [19] |
| Tidal salt marshes of the northeast USA | 0.13–0.40 | 82.0–239.0 | 14.4–17.7 | 60 | Drake et al. [53] |
| Three marshes in Barnegat Bay and three marshes in Delaware estuary, USA | 0.14–0.49 | 63.0–302.0 | 50 | Unger et al. [105] | |
| Two marshes on the Gulf of St. Lawrence coast of New Brunswick, Canada and one on the southern coast of Maine, USA | 0.28–0.50 | 21.0–30.0 | 26.1 | 50 | van Ardenne et al. [71] |
| Salt barrens in Tampa Bay, Florida, USA | 1.27–1.44 | 5.0–10.0 | 2.7 | 50 | Radabaugh et al. [12] |
| Salt marshes in Tampa Bay, Florida, USA | 0.95–1.27 | 16.0–66.0 | 6.6 | 50 | Radabaugh et al. [12] |
| Salt marsh in southern Puget Sound, Washington, USA | 0.17–0.65 | 33.0–208.0 | 50 | Drexler et al. [106] | |
| Salt marsh in Boundary Bay, Delta, British Columbia, Canada | 0.53–0.65 | 43.0–113.0 | 3.9–8.3 | 29 | Gailis et al. [22] |
| Tropical salt marshes, Mexico and El Salvador | 2.0–303.0 | 3.0–46.5 | 100 | Ruiz-Fernández et al. [107] | |
| Salt marshes of the Amazon region, Brazil | 0.86–1.57 | 3.4–41.5 | 9.5–12.0 | 100 | Kauffman et al. [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, E.M.; Arshad, M.; Alrumman, S.A.; Al-Bakre, D.A.; Ahmed, M.T.; Almahasheer, H.; Keshta, A.E. Evaluation of Soil Organic Carbon Stock in Coastal Sabkhas under Different Vegetation Covers. J. Mar. Sci. Eng. 2022, 10, 1234. https://doi.org/10.3390/jmse10091234
Eid EM, Arshad M, Alrumman SA, Al-Bakre DA, Ahmed MT, Almahasheer H, Keshta AE. Evaluation of Soil Organic Carbon Stock in Coastal Sabkhas under Different Vegetation Covers. Journal of Marine Science and Engineering. 2022; 10(9):1234. https://doi.org/10.3390/jmse10091234
Chicago/Turabian StyleEid, Ebrahem M., Muhammad Arshad, Sulaiman A. Alrumman, Dhafer A. Al-Bakre, Mohamed T. Ahmed, Hanan Almahasheer, and Amr E. Keshta. 2022. "Evaluation of Soil Organic Carbon Stock in Coastal Sabkhas under Different Vegetation Covers" Journal of Marine Science and Engineering 10, no. 9: 1234. https://doi.org/10.3390/jmse10091234







