The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean
Abstract
:1. Introduction
2. Geological Setting and Oceanography
3. Samples and Methods
3.1. Mineralogy
3.2. Geochemistry
4. Results
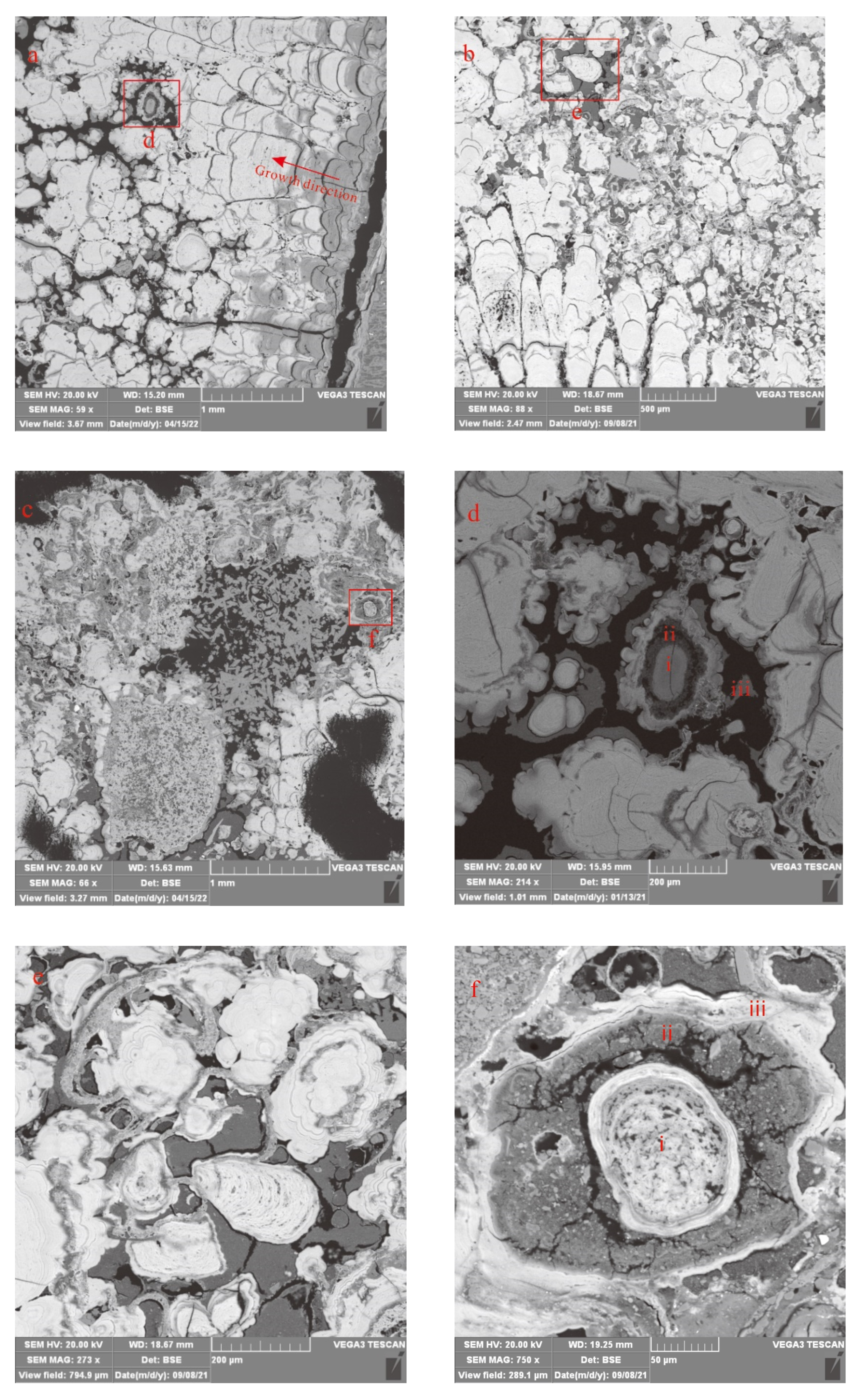
4.1. Texture and Microstructure
4.1.1. Textural Morphology
4.1.2. Microstructure
4.2. Mineralogy
4.3. Geochemistry
4.3.1. Geochemistry Compositions of the Ferromanganese Crusts
4.3.2. Geochemistry Compositions of the Ferromanganese Nodule
4.4. Growth Rates and Age Model
4.5. Element Correlations and Factor Analysis
5. Discussion
5.1. Texture and Surface
5.2. Genesis Type of Ferromanganese Crusts and the Nodule
5.3. Genesis of Ferromanganese Deposits
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry, 2nd ed.; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 273–291. [Google Scholar]
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.; Kang, J.-K.; Roberts, L. Cobalt-Rich Ferromanganese Crusts in the Pacific. In Handbook of Marine Mineral Deposits; Cronan, D., Ed.; CRC Press: London, UK, 2000. [Google Scholar]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Heller, C.; Kuhn, T.; Versteegh, G.J.M. The geochemical behavior of metals during early diagenetic alteration of buried manganese nodules. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 2018, 142, 16–33. [Google Scholar] [CrossRef]
- Fitzgerald, C.E.; Gillis, K.M. Hydrothermal manganese oxide deposits from Baby Bare seamount in the Northeast Pacific Ocean. Mar. Geol. 2006, 225, 145–156. [Google Scholar] [CrossRef]
- Pelleter, E.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Labanieh, S.; Josso, P.; Langlade, J. Ni-Cu-Co-rich hydrothermal manganese mineralization in the Wallis and Futuna back-arc environment (SW Pacific). Ore Geol. Rev. 2017, 87, 126–146. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dobretsova, I.G.; Dubinchuk, V.T. Hydrothermal manganese mineralization in the Peterbourgskoye ore field (North Atlantic). Oceanology 2014, 54, 222–230. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Hein, J.R.; Medialdea, T.; León, R.; Urgorri, V.; Reyes, J.; Martín-Rubí, J.A. Phosphorites, Co-rich Mn nodules, and Fe-Mn crusts from Galicia Bank, NE Atlantic: Reflections of Cenozoic tectonics and paleoceanography. Geochem. Geophys. Geosyst. 2016, 17, 346–374. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.-K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Special Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Halbach, P. Processes controlling the heavy metal distribution inPacific f erromanganese nodules and crusts. Geol. Rundsch. 1986, 75, 235–247. [Google Scholar] [CrossRef]
- Hein, J.R.; Schwab, W.C.; Davis, A. Cobalt- and platinum-rich ferromanganese crusts and associated substrate rocks from the Marshall Islands. Mar. Geol. 1988, 78, 255–283. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- McMurtry, G.M.; VonderHaar, D.L.; Eisenhauer, A.; Mahoney, J.J.; Yeh, H.W. Cenozoic accumulation history of a Pacific ferromanganese crust. Earth Planet. Sci. Lett. 1994, 125, 105–118. [Google Scholar] [CrossRef]
- Jiang, X.D.; Sun, X.M.; Guan, Y.; Gong, J.L.; Lu, Y.; Lu, R.F.; Wang, C. Biomineralisation of the ferromanganese crusts in the Western Pacific Ocean. J. Asian Earth Sci. 2017, 136, 58–67. [Google Scholar] [CrossRef]
- Jiang, X.D.; Gong, J.L.; Ren, J.B.; Liu, Q.S.; Zhang, J.; Chou, Y.M. An interdependent relationship between microbial ecosystems and ferromanganese nodules from the Western Pacific Ocean. Sediment. Geol. 2020, 398, 105588. [Google Scholar] [CrossRef]
- Sujith, P.P.; Gonsalves, M.J.B.D. Ferromanganese oxide deposits: Geochemical and microbiological perspectives of interactions of cobalt and nickel. Ore Geol. Rev. 2021, 139, 104458. [Google Scholar] [CrossRef]
- Benites, M.; Hein, J.R.; Mizell, K.; Blackburn, T.; Jovane, L. Genesis and Evolution of Ferromanganese Crusts from the Summit of Rio Grande Rise, Southwest Atlantic Ocean. Minerals 2020, 10, 349. [Google Scholar] [CrossRef]
- Chen, S.; Yin, X.; Wang, X.; Huang, X.; Ma, Y.; Guo, K.; Zeng, Z. The geochemistry and formation of ferromanganese oxides on the eastern flank of the Gagua Ridge. Ore Geol. Rev. 2018, 95, 118–130. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on ferromanganese crust composition and reconnaissance resource potential, Ninetyeast Ridge, Indian Ocean. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Hein, J.R.; Konstantinova, N.; Mikesell, M.; Mizell, K.; Fitzsimmons, J.N.; Lam, P.J.; Till, C.P. Arctic deep water ferromanganese-oxide deposits reflect the unique characteristics of the Arctic Ocean. Geochem. Geophys. Geosyst. 2017, 18, 3771–3800. [Google Scholar] [CrossRef]
- Epp, D. Possible perturbations to hotspot traces and implications for the origin and structure of the Line Islands. J. Geophys. Res.-Solid Earth 1984, 89, 11273–11286. [Google Scholar] [CrossRef]
- Lonsdale, P. Geography and history of the Louisville Hotspot Chain in the southwest Pacific. J. Geophys. Res. 1988, 93, 3078. [Google Scholar] [CrossRef]
- Wessel, P.; Kroenke, L. A geometric technique for relocating hotspots and refining absolute plate motions. Nature 1997, 387, 365–369. [Google Scholar] [CrossRef]
- He, G.; Zhao, Z.; Zhu, K. Cobalt-Rich Crust Resources in the Western Pacific; Geological Publishing House: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Koppers, A.P.; Staudigel, H.; Pringle, M.S.; Wijbrans, J.R. Short-lived and discontinuous intraplate volcanism in the South Pacific: Hot spots or extensional volcanism? Geochem. Geophys. Geosyst. 2003, 4, 1089. [Google Scholar] [CrossRef]
- Ren, X.W. The Metallogenic System of Co-rich Manganese Crusts in Western Pacific. Master’s Thesis, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China, 2005. (In Chinese). [Google Scholar]
- Zhao, B.; Wei, Z.; Yang, Y.; He, G.; Zhang, H.; Ma, W. Sedimentary characteristics and the implications of cobalt-rich crusts resources at Caiwei Guyot in the Western Pacific Ocean. Mar. Georesour. Geotechnol. 2019, 38, 1037–1045. [Google Scholar] [CrossRef]
- Yang, Y.; He, G.; Ma, J.; Yu, Z.; Yao, H.; Deng, X.; Liu, F.; Wei, Z. Acoustic quantitative analysis of ferromanganese nodules and cobalt-rich crusts distribution areas using EM122 multibeam backscatter data from deep-sea basin to seamount in Western Pacific Ocean. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2020, 161, 103281. [Google Scholar] [CrossRef]
- Kawabe, M.; Fujio, S. Pacific ocean circulation based on observation. J. Oceanogr. 2010, 66, 389–403. [Google Scholar] [CrossRef]
- Gordon, A.L.; Visbeck, M.; Huber, B. Export of Weddell Sea deep and bottom water. J. Geophys. Res.-Oceans 2001, 106, 9005–9017. [Google Scholar] [CrossRef]
- Kawabe, M.; Fujio, S.; Yanagimoto, D.; Tanaka, K. Water masses and currents of deep circulation southwest of the Shatsky Rise in the western North Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 1675–1687. [Google Scholar] [CrossRef]
- Johnson, H.P.; Hautala, S.L.; Bjorklund, T.A.; Zarnetske, M.R. Quantifying the North Pacific silica plume. Geochem. Geophys. Geosyst. 2006, 7, Q05011. [Google Scholar] [CrossRef]
- Kawabe, M. Deep Water Properties and Circulation in the Western North Pacific. In Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1993; pp. 17–37. [Google Scholar]
- Kawabe, M.; Taira, K. Flow distribution at 165° E in the Pacific Ocean. In Biogeochemical Processes and Ocean Flux in the Western Pacific; Sakai, H., Nozaki, Y., Eds.; Terra Scientific Publishing Company (TERRAPUB): Tokyo, Japan, 1995; pp. 629–649. [Google Scholar]
- Kawabe, M.; Fujio, S.; Yanagimoto, D. Deep-water circulation at low latitudes in the western North Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 631–656. [Google Scholar] [CrossRef]
- Kawabe, M.; Yanagimoto, D.; Kitagawa, S.; Kuroda, Y. Variations of the deep western boundary current in Wake Island Passage. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 2005, 52, 1121–1137. [Google Scholar] [CrossRef]
- Kawabe, M.; Yanagimoto, D.; Kitagawa, S. Variations of deep western boundary currents in the Melanesian Basin in the western North Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 942–959. [Google Scholar] [CrossRef]
- Kato, F.; Kawabe, M. Volume transport and distribution of deep circulation at 165°W in the North Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 2077–2087. [Google Scholar] [CrossRef]
- Guo, B.; Wang, W.; Shu, Y.; He, G.; Zhang, D.; Deng, X.; Wang, J. Observed Deep Anticyclonic Cap Over Caiwei Guyot. J. Geophys. Res. Ocean. 2020, 125, e2020JC01625. [Google Scholar] [CrossRef]
- Yeo, I.A.; Howarth, S.A.; Spearman, J.; Cooper, A.; Crossouard, N.; Taylor, J.; Murton, B.J. Distribution of and Hydrographic Controls on Ferromanganese Crusts: Tropic Seamount, Atlantic. Ore Geol. Rev. 2019, 114, 103131. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, H.; Zhang, H.; Pulyaeva, I.; Liu, J. Two main formation episodes of ferromanganese crusts in the Pacific Ocean. Acta Geol. Sinica 2006, 80, 12. [Google Scholar]
- Rolf, G.L.; Todd, D.M. Topographically induce mixing around shallow seamount. Science 1997, 276, 1831–1833. [Google Scholar]
- Lavelle, J.W.; Lozovatsky, I.D.; Smith, D.C. Tidally induced turbulent mixing at Irving Seamount-Modeling and measurements. Geophys. Res. Lett. 2004, 31, L10308. [Google Scholar] [CrossRef]
- Rudnick, D.L.; Boyd, T.J.; Brainard, R.E.; Sanford, T.B. From tides to mixing along the Hawaiian ridge. Science 2003, 301, 355–357. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks; influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 1989, 21, 169–200. [Google Scholar]
- Manheim, F.T.; Lane-Bostwick, C.M. Cobalt in ferromanganese crusts as a monitor of hydrothermal dischargeon the Pacificsea floor. Nature 1988, 335, 59–62. [Google Scholar] [CrossRef]
- Von Stackelberg, U. Manganese Nodules of the Peru Basin. In Handbook of Marine Mineral Deposits; Cronan, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 197–238. [Google Scholar]
- Glasby, G.P.; Mountain, B.; Vineesh, T.C.; Banakar, V.; Rajani, R.; Ren, X. Role of hydrology in the formation of Co-rich Mn crusts from the equatorial N Pacific, Equatorial S Indian Ocean and the NE Atlantic Ocean. Resour. Geol. 2020, 60, 165–177. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Urabe, T. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Hein, J.R.; Dong, Y.; Wang, M.; Ren, X.; Chu, F. A possible link between seamount sector collapse and manganese nodule occurrence in the abyssal plains, NW Pacific Ocean. Ore Geol. Rev. 2021, 138, 104378. [Google Scholar] [CrossRef]
- Halbach, P.; Hebisch, U.; Scherhag, C. Geochemical variations of ferromanganese nodules and crusts from different provinces of the Pacific Ocean and their genetic control. Chem. Geol. 1981, 34, 3–17. [Google Scholar] [CrossRef]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef]
- Halbach, P.; Segl, M.; Puteanus, D.; Mangini, A. Co-fluxes and growth rates in ferromanganese deposits from central Pacific seamount areas. Nature 1983, 304, 716–719. [Google Scholar] [CrossRef]
- Josso, P.; Rushton, J.; Lusty, P.; Matthews, A.; Chenery, S.; Holwell, D.; Murton, B. Late cretaceous and Cenozoic paleoceanography from north-East Atlantic ferromanganese crust microstratigraphy. Mar. Geol. 2020, 422, 106122. [Google Scholar] [CrossRef]
- Piper, D.Z. Rare earth elements in ferromanganese nodules and other marine phases. Geochim. Cosmochim. Acta 1974, 38, 1007–1022. [Google Scholar] [CrossRef]
- Amakawa, H.; Ingri, J.; Masuda, A.; Shimizu, H. Isotopic compositions of Ce, Nd and Sr in ferromanganese nodules from the Pacific and Atlan tic oceans, the Baltic and Barents Seas, and the Gulf of Bothni a. Earth Planet. Sci. Lett. 1991, 105, 554–565. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A.; Dulski, P. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Menendez, A.; James, R.H.; Roberts, S. Controls on the distribution of rare earth elements in deep-sea sediments in the North Atlantic Ocean. Ore Geol. Rev. 2017, 87, 100–113. [Google Scholar] [CrossRef]
- Giovanoli, R. Vernadite is random-stacked birnessite-A discussion of the paper by F. V. Chukhrov et al.: Contributions to the mineralogy of authigenic manganese phases from marine manganese deposits. Miner. Depos. 1980, 15, 251–253. [Google Scholar]
- Villalobos, M.; Lanson, B.; Manceau, A.; Toner, B.; Sposito, G. Structural model for the biogenic Mn oxide produced by Pseudomonas putida. Am. Miner. 2006, 91, 489–502. [Google Scholar] [CrossRef]
- Grangeon, S.; Lanson, B.; Lanson, M.; Manceau, A. Crystal structure of Ni-sorbed synthetic vernadite: A powder X-ray diffraction study. Mineral. Mag. 2008, 72, 1279–1291. [Google Scholar] [CrossRef]
- Feng, X.H.; Tan, W.F.; Liu, F.; Wang, J.B.; Ruan, H.D. Synthesis of todorokite at atmospheric pressure. Chem. Mat. 2004, 16, 4330–4336. [Google Scholar] [CrossRef]
- Post, J.E.; Heaney, P.J.; Hanson, J. Synchrotron X-ray diffraction study of the structure and dehydration behavior of todorokite. Am. Miner. 2003, 88, 142–150. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, K.D. Tunnel cation and water structures of todorokite: Insights into metal partitioning and isotopic fractionation. Geochim. Cosmochim. Acta 2022, in press. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of phosphatization on the geochemical and mineralogical composition of marine ferromanganese crusts. Geochim. Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Kuhn, T.; Madureira, P.; Wegorzewski, A.V.; Mirao, J.; Lunar, R. Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods. Minerals 2019, 9, 439. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Grangeon, S.; Webb, S.M.; Heller, C.; Kuhn, T. Mineralogical transformations in polymetallic nodules and the change of Ni, Cu and Co crystal-chemistry upon burial in sediments. Geochim. Cosmochim. Acta 2022, 282, 19–37. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Q.S.; Chen, Z.; Gonzalez, F.J.; Hein, J.R.; Zhang, J.; Zhong, L.F. Tectonic and paleoceanographic conditions during the formation of ferromanganese nodules from the northern South China Sea based on the high-resolution geochemistry, mineralogy and isotopes. Mar. Geol. 2019, 410, 146–163. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Müller, D.; Zhang, L.; Deng, J.; Yang, L.Q.; Wang, Q.F. Mineral systems: Their advantages in terms of developing holistic genetic models and for target generation in global mineral exploration. Geosyst. Geoenviron. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Smoot, N.C. Orthogonal Intersections of Megatrends in the Western Pacific Ocean Basin: A Case Study of the Mid-Pacific Mountains. Geomorphology 1999, 30, 323–356. [Google Scholar] [CrossRef]
- Bischoff, J.L.; Piper, D.Z.; Leong, K. The aluminosilicate fraction of North Pacific manganese nodules. Geochim. Cosmochim. Acta 1981, 45, 2047–2063. [Google Scholar] [CrossRef]
- Hem, J.D. Rates of manganese oxidation in aqueous systems. Geochim. Cosmochim. Acta 1981, 45, 1369–1374. [Google Scholar] [CrossRef]
- Wise, W.S. Minerals. Zeolites. In Encyclopedia of Geology; Selley, R.C., Cocks, L.R.M., Plimer, I.R., Eds.; Elsevier: Oxford, UK, 2005; pp. 591–600. [Google Scholar]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Wu, G.H.; Zhou, H.Y.; Zhang, H.S.; Ling, H.F.; Ma, W.L.; Zhao, H.Q.; Chen, J.L.; Liu, J.H. New index of ferromanganese crusts reflecting oceanic environmental oxidation. Sci. China Earth Sci. 2007, 50, 371–384. [Google Scholar] [CrossRef]
- Aplin, A.; Michard, A.; Albarede, F. 143Nd/144Nd in Pacific ferromanganese encrustations and nodules. Earth Planet. Sci. Lett. 1986, 81, 7–14. [Google Scholar] [CrossRef]
- Banakar, V.K.; Borole, D.V. Depth profiles of 230Th excess transition metals and mineralogy of ferromanganese crusts of the Central Indian basin and implications for paleoceanographic influence on crust genesis. Chem. Geol. 1991, 94, 33–44. [Google Scholar] [CrossRef]
- Liu, Q.; Huo, Y.Y.; Wu, Y.H.; Bai, Y.; Yuan, Y.; Chen, M. Bacterial community on a guyot in the northwest Pacific Ocean influenced by physical dynamics and environmental variables. J. Geophys. Res.-Biogeosci. 2019, 124, 2883–2897. [Google Scholar] [CrossRef]
- Halbach, P.; Puteanus, D. The influence of the carbonate dissolution rate on the growth and composition of Co-rich ferromanganese crusts from Central Pacific seamount areas. Earth Planet. Sci. Lett. 1984, 68, 73–87. [Google Scholar] [CrossRef]
- Byrne, R.H. Inorganic speciation of dissolved elements in seawater: The influence of pH on concentration ratios. Geochem. Trans. 2002, 3, 11–16. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Kuhn, T. Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 2020, 1, 158–169. [Google Scholar] [CrossRef]
- Schnitker, D. North Atlantic oceanography as possible cause of Antarctic glaciation and eutrophication. Nature 1980, 284, 615–616. [Google Scholar] [CrossRef]
- Kennett, J.P. Paleo-oceanography: Global ocean evolution. Rev. Geophys. Space. Phys. 1983, 21, 1258–1274. [Google Scholar]
- Halbach, P.E.; Jahn, A.; Cherkashov, G. Marine co-rich ferromanganese crust deposits: Description and formation, occurrences and distribution, estimated worldwide resources. In Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer: Cham, Germany, 2007; pp. 1–535. [Google Scholar]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Trategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- Dymond, J.; Lyle, M.; Finney, B.; Piper, D.Z.; Murphy, K.; Conard, R.; Pisoas, N. Ferromanganese nodules from the MANOP Sites H, S, and R-Control of mineralogical and chemical composition by multiple accretionary processes. Geochim. Cosmochim. Acta 1984, 48, 931–949. [Google Scholar] [CrossRef]
- Lyle, M.; Heath, G.R.; Robbins, J.M. Transport and release of transition elements during early diagenesis: Sequential leaching of sediments from MANOP sites M and H, Part, I. pH5 acetic acid leach. Geochim. Cosmochim. Acta 1984, 48, 1705–1715. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, Q.; Chen, M.; Zhang, R.; Yang, W.; Zheng, M.; Qiu, Y. Enhanced but highly variable bioturbation around seamounts in the northwest Pacific. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2019, 156, 103190. [Google Scholar] [CrossRef]
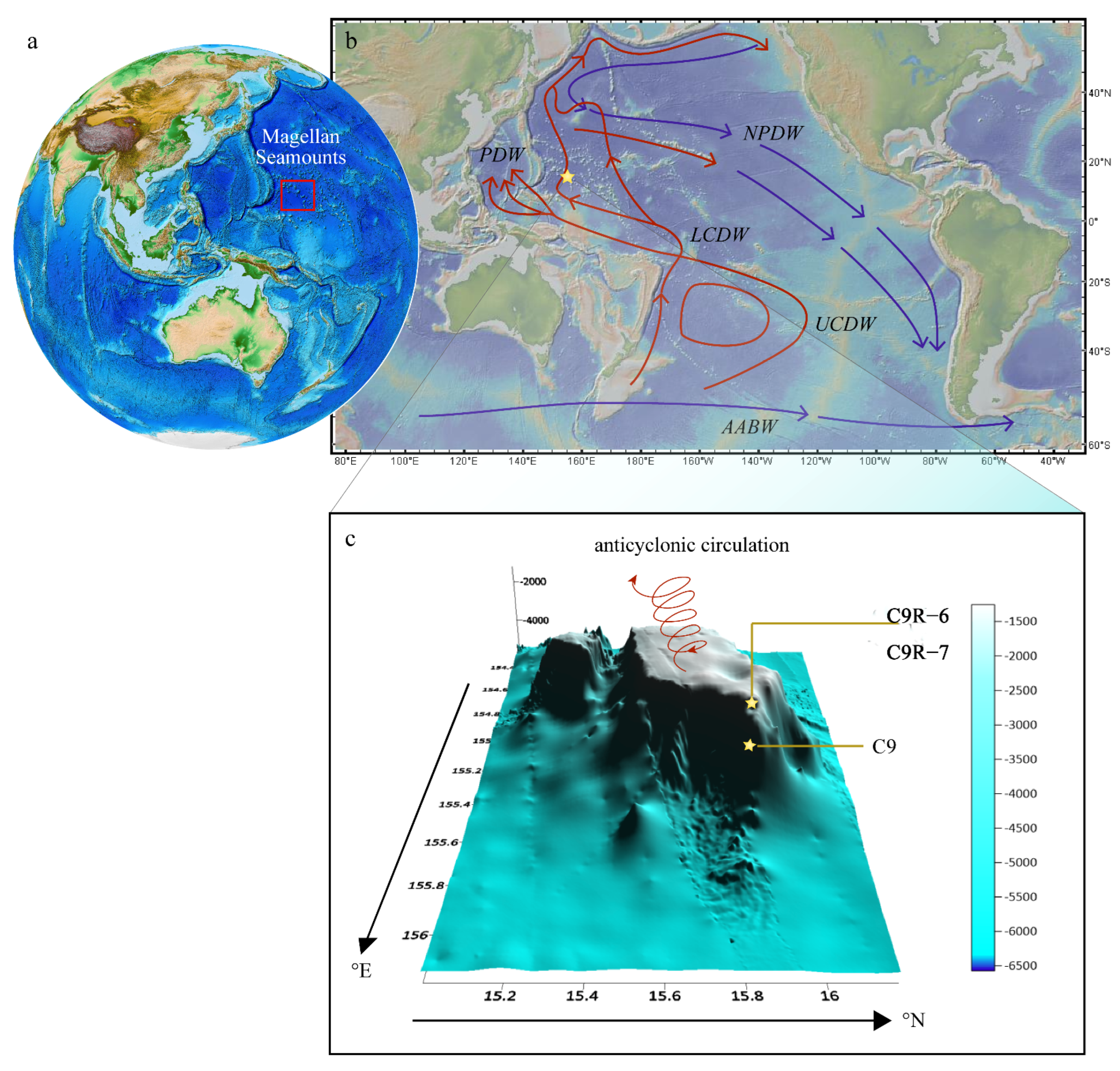














| Cruise ID | Water Depth | Latitude (N) | Latitude (E) |
|---|---|---|---|
| HOBAB5-C9 | 3116 m | 15°51′10.883″ | 155°35′15.620″ |
| HOBAB5-C9R-6 | 1650.1 m | 15°51′08.672″ | 155°29′17.412″ |
| HOBAB5-C9R-7 | 1608.4 m | 15°51.16997′ | 155°29.20822′ |
| Sample | Mn(wt%)) | Fe | Al | Si | Mn/Fe | Si/Al | Co/(Cu + Ni) | Co/(Fe + Mn) | Ca | P | Mg | K | Na | Ti | Growth Rate | Li (ppm) | Be | Sc | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9R-6-1 | 21.6 | 18.2 | 1.20 | 5.77 | 1.18 | 4.81 | 1.38 | 175 | 2.20 | 0.42 | 1.27 | 0.61 | 2.41 | 0.96 | 0.85 | 3.88 | 4.59 | 2.02 | 441 |
| C9R-6-2 | 20.5 | 19.4 | 1.50 | 5.75 | 1.06 | 3.83 | 0.98 | 136 | 2.13 | 0.43 | 1.32 | 0.70 | 2.4 5 | 1.05 | 1.30 | 6.67 | 4.89 | 3.22 | 455 |
| C9R-6-3 | 20.8 | 19.0 | 1.50 | 5.08 | 1.09 | 3.39 | 0.75 | 139 | 2.32 | 0.51 | 1.38 | 0.65 | 2.00 | 1.09 | 1.24 | 11.35 | 4.81 | 4.05 | 460 |
| C9R-6-4 | 23.4 | 17.4 | 1.10 | 3.69 | 1.35 | 3.35 | 0.85 | 137 | 2.66 | 0.49 | 1.28 | 0.61 | 1.84 | 1.32 | 1.28 | 4.98 | 5.63 | 3.80 | 527 |
| C9R-6-5 | 20.4 | 19.3 | 1.34 | 5.18 | 1.06 | 3.87 | 1.07 | 171 | 2.02 | 0.43 | 1.36 | 0.54 | 1.76 | 1.09 | 0.89 | 7.77 | 4.58 | 3.67 | 481 |
| C9R-6-6 | 21.0 | 18.2 | 1.44 | 5.44 | 1.15 | 3.78 | 0.89 | 150 | 2.15 | 0.40 | 1.37 | 0.53 | 1.76 | 1.10 | 1.10 | 9.69 | 3.63 | 2.32 | 474 |
| C9R-7-1 | 15.9 | 11.8 | 1.20 | 5.83 | 1.35 | 4.86 | 0.97 | 139 | 1.92 | 0.78 | 1.18 | 0.40 | 1.92 | 0.91 | 1.25 | 7.67 | 5.46 | 9.94 | 555 |
| C9R-7-2 | 14.7 | 12.5 | 2.22 | 4.90 | 1.17 | 2.21 | 1.68 | 208 | 1.86 | 0.72 | 1.32 | 0.63 | 1.79 | 1.05 | 0.64 | 3.08 | 4.70 | 7.43 | 595 |
| C9R-7-3 | 22.0 | 18.5 | 0.980 | 4.84 | 1.19 | 4.96 | 1.36 | 147 | 2.32 | 0.46 | 1.23 | 0.54 | 2.41 | 0.97 | 1.13 | 2.38 | 4.92 | 2.69 | 577 |
| C9R-7-4 | 22.0 | 18.4 | 1.05 | 4.53 | 1.20 | 4.31 | 1.15 | 147 | 2.30 | 0.42 | 1.23 | 0.522 | 1.99 | 1.11 | 1.14 | 3.48 | 5.47 | 5.43 | 607 |
| C9R-7-5 | 24.8 | 17.5 | 0.700 | 3.68 | 1.41 | 5.28 | 1.40 | 191 | 2.47 | 0.44 | 1.28 | 0.47 | 2.20 | 0.95 | 0.73 | 1.54 | 5.18 | 1.51 | 595 |
| C9R-7-6 | 25.2 | 17.4 | 0.660 | 3.40 | 1.45 | 5.12 | 1.40 | 196 | 2.50 | 0.43 | 1.24 | 0.49 | 2.17 | 0.95 | 0.70 | 0.87 | 4.11 | 0.97 | 543 |
| Sample le | Co | Ni | Cu | Zn | Ga | Rb | Sr | Zr | Nb | Mo | Cd | Cs | Ba | Hf | Ta | W | Tl | Pb | |
| C9R-6-1 | 6962 | 4300 | 741 | 437 | 6.46 | 7.47 | 1026 | 507 | 55.1 | 318 | 5.16 | 0.75 | 1102 | 10.2 | 0.74 | 85.7 | 47.3 | 1740 | |
| C9R-6-2 | 5417 | 4430 | 1071 | 528 | 8.51 | 9.89 | 1017 | 626 | 74.5 | 284 | 5.45 | 1.15 | 1335 | 14.5 | 1.00 | 83.4 | 44.8 | 1578 | |
| C9R-6-3 | 5556 | 5759 | 1664 | 646 | 9.53 | 9.58 | 1037 | 664 | 78.8 | 284 | 6.70 | 1.08 | 1493 | 16.3 | 1.06 | 82.8 | 42.4 | 1727 | |
| C9R-6-4 | 5589 | 5094 | 1485 | 672 | 8.75 | 8.09 | 1255 | 758 | 79.2 | 353 | 6.78 | 0.85 | 1894 | 16.8 | 1.34 | 111 | 48.7 | 1556 | |
| C9R-6-5 | 6769 | 5346 | 1002 | 582 | 9.18 | 9.17 | 1127 | 629 | 75.8 | 281 | 6.16 | 1.01 | 1351 | 14.2 | 0.95 | 78.9 | 36.0 | 1800 | |
| C9R-6-6 | 5872 | 5469 | 1152 | 600 | 9.71 | 9.89 | 1090 | 618 | 65.2 | 290 | 6.60 | 1.08 | 1366 | 13.6 | 0.91 | 82.5 | 36.0 | 1661 | |
| C9R-7-1 | 3841 | 3250 | 704 | 535 | 6.86 | 15.8 | 1254 | 628 | 62.3 | 336 | 3.27 | 1.61 | 1424 | 11.3 | 0.57 | 67.7 | 84.4 | 1412 | |
| C9R-7-2 | 5639 | 3000 | 357 | 452 | 5.33 | 7.29 | 1315 | 527 | 46.3 | 393 | 3.96 | 0.58 | 1095 | 7.3 | 0.48 | 69.9 | 66.5 | 1670 | |
| C9R-7-3 | 5961 | 3816 | 566 | 556 | 9.15 | 8.20 | 1480 | 683 | 61.8 | 376 | 6.68 | 0.76 | 1519 | 12.5 | 0.79 | 102 | 23.7 | 1715 | |
| C9R-7-4 | 5932 | 4375 | 792 | 650 | 10.1 | 9.85 | 1596 | 807 | 76.6 | 384 | 6.96 | 0.96 | 1813 | 15.9 | 0.93 | 109 | 29.4 | 1745 | |
| C9R-7-5 | 8091 | 5075 | 709 | 558 | 8.52 | 5.51 | 1518 | 642 | 66.6 | 459 | 7.19 | 0.42 | 1463 | 11.11 | 0.80 | 127 | 30.2 | 1957 | |
| C9R-7-6 | 8327 | 5201 | 731 | 484 | 7.91 | 4.32 | 1371 | 567 | 59.1 | 429 | 6.72 | 0.31 | 1416 | 9.90 | 0.72 | 127 | 33.4 | 1999 | |
| Sample | Bi | Th | U | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | |
| C9R-6-1 | 27.9 | 19.2 | 11.9 | 255 | 717 | 41.4 | 180 | 38.1 | 9.97 | 44.8 | 7.27 | 40.4 | 147 | 9.61 | 26.0 | 4.28 | 25.7 | 4.21 | |
| C9R-6-2 | 34.7 | 13.5 | 10.8 | 235 | 752 | 39.0 | 172 | 34.9 | 8.84 | 44.1 | 6.92 | 39.9 | 166 | 9.69 | 27.0 | 4.40 | 27.0 | 4.60 | |
| C9R-6-3 | 38.7 | 11.4 | 11.1 | 248 | 846 | 38.5 | 169 | 35.2 | 8.68 | 44.8 | 6.74 | 38.6 | 182 | 9.39 | 26.3 | 4.37 | 26.5 | 4.51 | |
| C9R-6-4 | 48.2 | 8.51 | 11.7 | 246 | 998 | 42.4 | 185 | 38.3 | 10.6 | 48.3 | 7.15 | 41.7 | 202 | 10.2 | 28.0 | 4.57 | 27.9 | 4.70 | |
| C9R-6-5 | 30.2 | 17.9 | 12.0 | 268 | 840 | 43.7 | 194 | 40.2 | 11.0 | 47.9 | 7.69 | 43.6 | 178 | 10.4 | 28.5 | 4.74 | 28.1 | 4.63 | |
| C9R-6-6 | 37.8 | 16.5 | 11.2 | 238 | 861 | 42.2 | 184 | 38.2 | 9.87 | 46.0 | 7.28 | 40.8 | 164 | 9.75 | 26.8 | 4.59 | 27.2 | 4.63 | |
| C9R-7-1 | 15.2 | 11.9 | 10.7 | 242 | 553 | 44.1 | 191 | 39.8 | 9.77 | 46.2 | 7.28 | 41.8 | 192 | 9.75 | 25.3 | 4.02 | 25.5 | 3.92 | |
| C9R-7-2 | 16.3 | 21.2 | 12.0 | 239 | 643 | 46.6 | 200 | 43.0 | 10.3 | 48.3 | 7.61 | 43.3 | 187 | 9.85 | 25.2 | 4.02 | 25.0 | 3.80 | |
| C9R-7-3 | 38.1 | 24.7 | 12.4 | 280 | 777 | 63.8 | 274 | 57.8 | 13.6 | 65.5 | 10.4 | 57.5 | 210 | 12.9 | 35.7 | 5.86 | 34.9 | 5.70 | |
| C9R-7-4 | 45.5 | 20.9 | 13.2 | 302 | 923 | 66.4 | 285 | 61.3 | 14.8 | 68.7 | 10.8 | 58.5 | 227 | 13.6 | 37.4 | 6.16 | 36.9 | 5.83 | |
| C9R-7-5 | 41.0 | 19.2 | 14.7 | 303 | 854 | 58.1 | 252 | 53.6 | 13.8 | 60.7 | 9.90 | 54.7 | 200 | 12.7 | 34.3 | 5.62 | 33.4 | 5.57 | |
| C9R-7-6 | 40.0 | 17.0 | 15.3 | 309 | 877 | 51.1 | 227 | 48.1 | 12.4 | 55.2 | 8.79 | 50.1 | 180 | 11.9 | 32.4 | 5.37 | 31.2 | 5.06 | |
| Sample | Mn (wt%) | Fe | Mn/Fe | Al | Ca | P | Co/(Cu + Ni) | Co/(Fe + Mn) | K | Ti | Mg | Na | Li (ppm) | Be | Sc | V | Co | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9-1 | 20.4 | 9.52 | 2.14 | 1.33 | 2.13 | 0.46 | 0.79 | 195.56 | 0.48 | 1.42 | 1.26 | 1.71 | 3.21 | 4.52 | 5.52 | 493 | 5851 | 4871 |
| C9-2 | 18.5 | 9.10 | 2.03 | 1.36 | 2.04 | 0.51 | 0.73 | 197.73 | 0.50 | 1.34 | 1.20 | 1.62 | 3.56 | 4.88 | 6.38 | 491 | 5457 | 5047 |
| C9-3 | 26.0 | 15.8 | 1.65 | 0.880 | 2.60 | 0.32 | 0.83 | 163.05 | 0.58 | 1.32 | 1.29 | 1.99 | 2.00 | 3.86 | 3.42 | 388 | 6806 | 5807 |
| C9-4 | 24.0 | 14.6 | 1.65 | 1.58 | 2.58 | 0.35 | 0.63 | 136.06 | 0.87 | 1.15 | 1.24 | 1.88 | 3.93 | 5.05 | 3.90 | 366 | 5255 | 5545 |
| C9-5 | 24.8 | 14.8 | 1.68 | 1.41 | 2.89 | 0.45 | 0.64 | 125.38 | 0.73 | 1.17 | 1.27 | 1.91 | 3.66 | 4.96 | 3.77 | 370 | 4973 | 5390 |
| Sample | Cu | Zn | Ga | Rb | Sr | Zr | Nb | Mo | Cd | Cs | Ba | Hf | Ta | W | Tl | Pb | ||
| C9-1 | 2532. | 639 | 6.97 | 4.93 | 1195 | 481 | 44.8 | 519 | 7.28 | 0.23 | 1392 | 6.24 | 0.73 | 72.4 | 103 | 1359 | ||
| C9-2 | 2461 | 698 | 7.60 | 5.79 | 1168 | 503 | 47.9 | 548 | 6.98 | 0.26 | 1470 | 7.54 | 0.86 | 74.3 | 115 | 1400 | ||
| C9-3 | 2427 | 472 | 6.60 | 3.70 | 898 | 447 | 39.9 | 343 | 5.21 | 0.19 | 1491 | 10.4 | 1.0 | 83.7 | 95.2 | 1333 | ||
| C9-4 | 2833 | 487 | 8.06 | 6.29 | 802 | 477 | 42.3 | 318 | 5.25 | 0.25 | 1575 | 12.1 | 0.95 | 77.2 | 78.4 | 1204 | ||
| C9-5 | 2439 | 461 | 8.48 | 5.17 | 801 | 464 | 38.9 | 315 | 4.96 | 0.20 | 1589 | 11.4 | 0.94 | 77.7 | 73.3 | 1243 | ||
| Sample | U | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | ||
| C9-1 | 10.2 | 203 | 875 | 42.0 | 173 | 37.0 | 8.88 | 43.1 | 6.22 | 33.1 | 114 | 7.62 | 19.2 | 3.04 | 18.7 | 2.92 | ||
| C9-2 | 10.0 | 203 | 915 | 43.3 | 177 | 38.1 | 9.08 | 44.1 | 6.39 | 33.8 | 113 | 7.64 | 19.5 | 3.08 | 18.9 | 2.97 | ||
| C9-3 | 10.5 | 265 | 1110 | 47.8 | 199 | 44.1 | 10.8 | 46.4 | 7.47 | 40.1 | 98.4 | 8.61 | 22.5 | 3.62 | 21.6 | 3.35 | ||
| C9-4 | 7.45 | 229 | 1113 | 42.0 | 176 | 36.6 | 9.46 | 42.7 | 6.26 | 33.0 | 109 | 7.18 | 18.9 | 2.98 | 18.0 | 2.83 | ||
| C9-5 | 7.76 | 249 | 1113 | 44.7 | 188 | 39.6 | 10.4 | 46.4 | 6.91 | 36.0 | 119 | 7.95 | 20.6 | 3.26 | 19.7 | 3.09 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zeng, Z. The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean. J. Mar. Sci. Eng. 2022, 10, 1275. https://doi.org/10.3390/jmse10091275
Wang L, Zeng Z. The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean. Journal of Marine Science and Engineering. 2022; 10(9):1275. https://doi.org/10.3390/jmse10091275
Chicago/Turabian StyleWang, Linzhang, and Zhigang Zeng. 2022. "The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean" Journal of Marine Science and Engineering 10, no. 9: 1275. https://doi.org/10.3390/jmse10091275
APA StyleWang, L., & Zeng, Z. (2022). The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean. Journal of Marine Science and Engineering, 10(9), 1275. https://doi.org/10.3390/jmse10091275






